Abstract
BACKGROUND:
Surveys originating from universities appear to have higher response rates than those from commercial sources. In light of the growing scrutiny placed on physician-industry relations, the present study aimed to determine the impact of the pharmaceutical industry versus university sponsorship on response to a postal survey completed by Canadian hepatitis C virus (HCV) care providers.
PATIENTS AND METHODS:
In the present controlled trial, 229 physicians and nurses involved in HCV treatment were randomly assigned to receive a survey with sponsorship from a pharmaceutical company or university. The primary outcome was the proportion of completed surveys returned. The secondary outcomes included the response rate after the first mailing and the number of days taken to respond.
RESULTS:
One hundred fifteen participants were randomly assigned to receive the pharmaceutical industry survey and 114 were assigned to receive the university survey. The final response rate was 72.9% (167 of 229), which did not differ between the industry and university groups (RR=0.91; 95% CI 0.78 to 1.07). Nurses (OR=2.20; 95% CI 1.08 to 4.48) and participants from an academic centre (OR=3.14; 95% CI 1.64 to 6.00) were more likely to respond. The response rate after the first mailing (RR=0.85; 95% CI 0.68 to 1.07) and the median number of days taken to respond (21 days in both groups; P=0.20) did not differ between the industry and university groups.
CONCLUSIONS:
Pharmaceutical industry sponsorship does not appear to negatively impact response rates to a postal survey completed by Canadian HCV care providers.
Keywords: Clinical trial, Data collection, Motivation, Questionnaires, Research design, Survey
Abstract
HISTORIQUE :
Les sondages émanant du milieu universitaire semblent générer des taux de réponse plus élevés que les sondages émanant de l’in-dustrie. À la lumière de la surveillance accrue dont font l’objet les rapports entre le corps médical et l’industrie, la présente étude visait à déterminer l’impact sur le taux de réponse selon qu’un sondage postal à l’intention des professionnels de la santé canadiens experts de l’hépatite C (HCV) émanait de l’industrie pharmaceutique ou du milieu universitaire.
SUJETS ET MÉTHODES :
Lors du présent essai clinique, 229 médecins et infirmières experts du HCV ont été assignés aléatoirement à un sondage émanant soit d’une société pharmaceutique, soit d’une université. Le paramètre principal était la proportion de questionnaires dûment complétés qui ont été retournés au sondeur. Les paramètres secondaires incluaient le taux de réponse après le premier envoi postal et le nombre de jours écoulés jusqu’à l’obtention de la réponse.
RÉSULTATS :
Cent quinze participants ont été assignés aléatoirement au sondage de l’industrie pharmaceutique et 114, au sondage du milieu universitaire. Le taux de réponse final a été de 72,9 % (167 sur 229) et n’a révélé aucune différence entre les deux groupes (RR = 0,91; IC 95 %, 0,78 à 1,07). Les infirmières (RRR = 2,20; IC 95 %, 1,08 à 4,48) et les participants d’un centre universitaire (RRR = 3,14; IC 95 %, 1,64 à 6,00) étaient plus susceptibles de répondre. Le taux de réponse après le premier envoi postal (RR = 0,85; IC 95 %, 0,68 à 1,07) et le nombre médian de jours écoulés avant l’obtention de la réponse (21 jours dans les deux groupes, P = 0,20) ont été semblables pour les deux groupes.
CONCLUSION :
Le fait qu’il émane de l’industrie pharmaceutique ne semble pas exercer d’impact négatif sur les taux de réponse à un sondage postal adressé à des professionnels de la santé canadiens du domaine du HCV.
Postal surveys are commonly used for data collection in health services research. Advantages include limited expense, an increased likelihood of eliciting responses to sensitive questions and the ability to collect data from large, geographically dispersed populations (1). However, nonresponse to postal surveys can introduce bias and decreases the effective sample size. Ensuring a high response rate to initial mailings reduces the costs associated with repeat mailings and other methods of follow-up. As a result, numerous studies (2,3) have examined methods to increase survey response rates. Effective strategies include the use of monetary incentives, mailing by recorded delivery, inclusion of stamped return envelopes, contacting participants before and after delivery, and the use of short, interesting, attractive and personalized questionnaires. Surveys including sensitive questions or a choice to opt out are less likely to be returned. Another factor that appears to impact response rates is the origin of the survey. Questionnaires originating from a university appear to be more likely to be returned than those originating from other sources (eg, industry) (2,3).
Relationships between the pharmaceutical industry and physicians have come under scrutiny due to the potential conflicts of interest they engender. Physicians’ commitments to patient care, avoidance of bias in medical decision-making and scientific integrity now regularly come up against financial conflicts of interest (4,5). Pharmaceutical companies are committed to research and product development, but their ultimate responsibility is to their shareholders. As a result of these disparate responsibilities, a variety of physician, industry and government groups have developed guidelines regulating these interactions (5). The relationship between the industry and physicians involved in the care of hepatitis C virus (HCV)-infected patients, specifically, is impacted by fierce competition between two companies marketing rival products (6).
In view of these issues and the necessity of identifying means of enhancing survey response rates, we undertook a randomized trial comparing pharmaceutical industry versus university sponsorship among physicians and nurses involved in the care of HCV-infected patients across Canada. We hypothesized that university sponsorship would be associated with improved response rates due to skepticism about the motives of industry sponsors.
PATIENTS AND METHODS
Study population and setting
The study population consisted of all physicians and nurses involved in two phase IV clinical trials assessing peginterferon alpha-2a and ribavirin in patients with chronic HCV. These trials, referred to as the Expanded Access Program-2 (EAP-2) and the Ribavirin Access Program (RAP), involved 1632 patients treated at 72 academic and community centres across Canada. The sponsor of these trials (Hoffmann-La Roche Ltd, Canada) provided contact information for the participants, but had no role in the study’s design, conduct, analysis or funding, or the decision to submit the manuscript for publication. The study was approved by the Conjoint Health Research Ethics Board at the University of Calgary (Calgary, Alberta).
Survey content
The present study was part of a larger study designed to assess the relationship between physician and nursing volume and experience and the success of antiviral therapy for chronic HCV. Participants received surveys addressing various issues, including their training, practice setting, HCV patient volume and experience treating HCV-infected patients with interferon-based regimens. Different surveys were sent to physicians and nurses (Appendixes 1 and 2). The one-page surveys were piloted locally and took approximately 3 min to complete. Where information regarding gender, practice setting and specialty were incomplete (among both respondents and nonrespondents), data were obtained from the Web sites of the appropriate provincial physician governing bodies. Where necessary, these data were supplemented by the investigators based on personal knowledge of the relatively small HCV-treating community in Canada.
Effect of sponsorship on response rates
The effect of sponsorship on response rates was assessed in a randomized trial. Participants were allocated to one of two groups using a computerized random number generator with stratification by qualifications (physician versus nurse). The first group (referred to as the ‘university group’) received a package including the questionnaire and a cover letter on university letterhead signed by two investigators (Dr Myers and Dr Lee). All references to the product of interest used generic terminology (ie, peginterferon alpha-2a and ribavirin) and the sponsor was not mentioned. The second group (referred to as the ‘industry group’) received a cover letter containing logos of both the university and the sponsor. The letter was signed by the same investigators plus a senior executive of the sponsor. All references to the product of interest used the trade name (ie, Pegasys RBV, Hoffman-La Roche Ltd, Canada) and the sponsor was mentioned twice in the body of the letter. The content and layout of the questionnaires and letters were identical in the two groups. All packages included a self-addressed, stamped envelope for return of the survey. Nonrespondents were sent a reminder e-mail three weeks following the initial mailing to reiterate the purpose and confidentiality of the study. An additional mailing was sent to nonrespondents after six weeks. Data collection was discontinued 12 weeks after the first mailing.
Statistical analysis
The primary outcome of the study was the final survey response rate. The impact of the study group on response was examined using the Mantel-Haenszel (MH) test with adjustment for qualifications to take into account the stratified randomization. An a priori sample size calculation was not performed because the study population was limited by the number of investigators and nurses involved in the trials from which they were drawn. Other potential predictors of response included gender, region within Canada (west versus east), practice setting (academic versus community) and physician specialty (hepatologist versus nonhepatologist). Between groups comparisons used the Mann-Whitney U test, Fisher’s exact test and logistic regression analyses. Independent predictors of response were examined using multiple logistic regression, including study group, qualifications and variables significant (P<0.05) in the univariate analysis.
As secondary outcomes, the initial response rate (receipt of the response before the second mailing) and the number of days taken to respond were examined. Kaplan-Meier curves were constructed to examine the cumulative probability of response; study groups were compared using the log-rank test. P≤0.05 were considered significant. STATA 8.0 software (StataCorp LP, USA) was used for all analyses.
RESULTS
Participants and survey responses
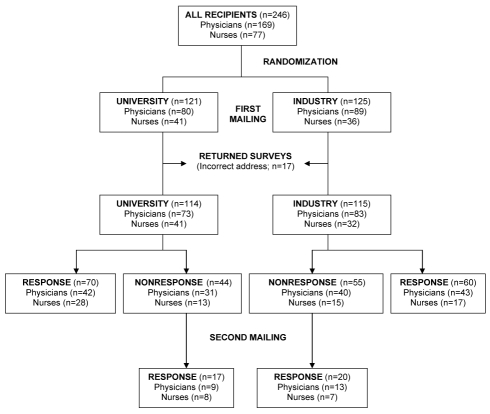
A total of 246 surveys were mailed – 121 surveys to the university group and 125 surveys to the industry group (Figure 1). Seventeen (6.9%) were returned because the respondent had relocated, leaving 229 available for analysis (university, n=114; industry, n=115). The study groups were similar with regard to age, gender, region within Canada, practice setting and physician speciality (Table 1). The majority of the physicians were male (82.7%) and the majority of nurses were female (94.5%). Physician specialities included hepatology (26.9%), gastroenterology (48.1%), infectious diseases (14.7%) and other or unknown (10.3%) specialities. Nearly one-half of surveyed physicians practiced in academic centres (46.8%) and western Canada (44.9%). The median interval between graduation from medical or nursing school and survey completion was 25 years (range 10 to 58 years) among physicians (available in 140 of 156 [89.7%]) and 25 years (range four to 48 years) among nurses (available in 58 of 73 [79.5%]).
Figure 1).
Flow chart demonstrating survey responses according to study group
TABLE 1.
Characteristics of the survey recipients according to study group and response
|
All survey recipients (n=229) |
Survey responders (n=167) |
|||
|---|---|---|---|---|
| Characteristic | University survey (n=114) | Industry survey (n=115) | University survey (n=87) | Industry survey (n=80) |
| Physicians (n=156) | ||||
| Age, years (range)* | N/A | N/A | 48 (35–68) | 49 (36–64) |
| Male, n (%) | 59/73 (80.8) | 70/83 (84.3) | 44/51 (86.3) | 46/56 (82.1) |
| Specialty, n (%) | ||||
| Hepatology | 20/73 (27.4) | 22/83 (26.5) | 16/51 (31.4) | 16/56 (28.6) |
| Gastroenterology | 33/73 (45.2) | 42/83 (50.6) | 24/51 (47.0) | 27/56 (48.2) |
| Infectious diseases | 11/73 (15.1) | 12/83 (14.5) | 9/51 (17.7) | 9/56 (16.1) |
| Other/unknown | 9/73 (12.3) | 7/83 (8.4) | 2/51 (3.9) | 4/56 (7.1) |
| Academic centre, n (%) | 33/73 (45.2) | 40/83 (48.2) | 31/51 (60.8) | 29/56 (51.8) |
| Western Canada, n (%) | 33/73 (45.2) | 37/83 (44.6) | 25/51 (49.0) | 25/56 (44.6) |
| Nurses (n=73), n (%) | ||||
| Male | 2/41 (4.9) | 2/32 (6.3) | 1/36 (2.8) | 1/24 (4.2) |
| Academic centre | 17/41 (41.5) | 15/32 (46.9) | 16/36 (44.4) | 12/24 (50.0) |
| Western Canada | 19/41 (46.3) | 10/32 (31.3) | 18/36 (50.0) | 9/24 (37.5) |
Age available in 98 of 156 (62.8%) of responding physicians only. N/A Not available
Of the 229 survey recipients, 167 responded before study closure (final response rate 72.9%). The response rate did not differ between the university and industry groups (76.3% versus 69.6%; RR=0.91; 95% CI 0.78 to 1.07) (Table 2). There was no evidence of effect modification by qualifications (MH χ2 test of homogeneity 0.62; P=0.43). Similar results were obtained in the analysis of initial response rates (university versus industry RR=0.85; 95% CI 0.65 to 1.07; MH χ2 0.36; P=0.55).
TABLE 2.
Survey response rates according to study group and qualifications
| Outcome | University survey (n=114) | Industry survey (n=115) | RR (95% CI)* | P* |
|---|---|---|---|---|
| Final response rate, n (%) | ||||
| Overall | 87/114 (76.3) | 80/115 (69.6) | 0.91 (0.78 to 1.07) | 0.29 |
| Physicians | 51/73 (69.9) | 56/83 (67.5) | 0.97 (0.78 to 1.20) | 0.86 |
| Nurses | 36/41 (87.8) | 24/32 (75.0) | 0.85 (0.69 to 1.06) | 0.22 |
| Initial response rate, n (%) | ||||
| Overall | 70/114 (59.2) | 60/115 (52.2) | 0.85 (0.68 to 1.07) | 0.18 |
| Physicians | 42/73 (57.5) | 43/83 (51.8) | 0.90 (0.68 to 1.20) | 0.52 |
| Nurses | 28/41 (68.3) | 17/32 (53.1) | 0.78 (0.53 to 1.13) | 0.23 |
| Time to response, median days (range) | ||||
| Overall | 21 (2–80) | 21 (14–80) | – | 0.20 |
| Physicians | 21 (2–71) | 21 (14–71) | – | 0.59 |
| Nurses | 14 (3–80) | 18 (14–80) | – | 0.21 |
For comparison between the university and the industry group
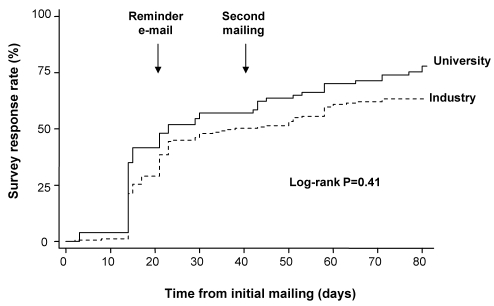
The timing of survey responses according to study group is illustrated in Figure 2. There was no difference between the university and industry groups with adjustment according to physician versus nurse status (log-rank P=0.41). The median interval between the initial mailing and response was 21 days in both groups (P=0.20; Table 2). The median time to response was similar between nurses (15 days; range three to 80 days) and physicians (21 days; two to 71 days; P=0.49).
Figure 2).
Kaplan-Meier plot demonstrating timing of survey responses according to study group. There was no difference between the university and industry groups with adjustment according to qualifications (physician versus nurse; log-rank P=0.41)
Predictors of survey response
A logistic regression analysis examining predictors of survey response is outlined in Table 3. In univariate analysis, nurses (P=0.02) and survey recipients in an academic setting (P=0.001) were more likely to respond. The response rate of physicians was 68.6% (107 of 156) versus 82.2% (60 of 73) among nurses. The response rate among recipients in an academic setting was 83.8% (88 of 105) versus 63.7% (79 of 124) in those from a community setting. Physician specialty (hepatologist versus nonhepatologist) did not impact response rates (P=0.22). In multivariate analysis, being a nurse (OR=2.20; 95% CI 1.08 to 4.48) and being in an academic setting (OR=3.14; 95% CI 1.64 to 6.00) were independent predictors of response. The study group was not significant in either analysis.
TABLE 3.
Univariate and multivariate predictors of survey response
| Variable | Univariate analysis OR (95% CI) | P | Multivariate analysis OR (95% CI) | P |
|---|---|---|---|---|
| Industry versus university survey | 0.71 (0.39 to 1.28) | 0.25 | 0.70 (0.38 to 1.30) | 0.26 |
| Nurse versus physician | 2.11 (1.06 to 4.21) | 0.03 | 2.20 (1.08 to 4.48) | 0.03 |
| Male versus female | 0.63 (0.34 to 1.15) | 0.13 | – | – |
| Academic versus community setting | 2.95 (1.56 to 5.57) | 0.001 | 3.14 (1.64 to 6.00) | 0.001 |
| Western versus eastern Canada | 1.56 (0.85 to 2.84) | 0.15 | – | – |
| Hepatologist versus nonhepatologist* | 1.66 (0.74 to 3.74) | 0.22 | – | – |
Only physicians
DISCUSSION
In the present randomized, controlled trial, we did not find a significant impact of pharmaceutical industry versus university sponsorship on response to a postal survey completed by Canadian physicians and nurses involved in HCV treatment. We hypothesized that industry sponsorship would reduce response rates due to skepticism regarding the motives of pharmaceutical companies. In our study, the overall response rate (approximately 73%) was very good; all of the subgroup-specific rates exceeded 67%, a rate generally considered acceptable for studies of this kind (1). By way of comparison, the mean response rate in eight similar studies (7–15) published in The Canadian Journal of Gastroenterology (including many of the same recipients) within the past five years was 58% (range 40% to 86%).
Our results are at odds with a Cochrane systematic review (2,3) assessing the impact of survey origin on response rates. In this meta-analysis of 14 randomized trials, including over 20,000 participants, the odds of a response fell by approximately one-quarter when the survey originated from a commercial versus university source. The reasons for this discrepancy are likely multifactorial. Whereas our study included only health care providers, all but one of the trials in this review (2,3) included laypersons, a population with potentially different opinions of surveys originating from commercial organizations. In the single trial (16) involving only physicians, the odds of a response were actually lower in the group receiving a university versus a cancer agency-sponsored survey (OR=0.67; 95% CI 0.48 to 0.93). To our knowledge, our study is the first of its kind to assess the impact of pharmaceutical industry sponsorship, specifically, on survey responses from health care providers. In fact, the nature of the commercial organization may have an impact on response rates. Whereas we assessed sponsorship by a pharmaceutical company, the trials in the Cochrane review (2,3) included marketing agencies, research firms, government and financial organizations. Study participants may be more likely to view questionnaires from such organizations as ‘surveys masquerading as research’ (17). Finally, we may have failed to detect a difference between industry and university sponsorship due to the simplicity of the surveys. A quick perusal by recipients of the industry survey would have revealed the absence of a hidden marketing agenda.
Although we did not observe a statistically significant impact of pharmaceutical industry sponsorship on response rates, there was a trend toward reduced responses in all subgroups receiving the industry survey. Moreover, the Kaplan-Meier curve showing timing of survey response rates (Figure 2) suggests divergence between the two groups approximately two weeks following the initial mailing. Anecdotally, several recipients of the industry survey expressed concern regarding its confidentiality and the motives of the sponsor. Some thought that the survey was a marketing exercise aimed at assessing prescribing patterns in a highly competitive marketplace (6). Although we cannot exclude a type II error as the cause of our negative result, we would argue that the practical significance of the observed difference in response rates (6%) is negligible, and is likely outweighed by the financial benefits of industry sponsorship for survey studies that can be costly, with large sample sizes and multiple mailings.
We also examined predictors of survey response among the HCV-treating community in Canada. Nurses and recipients practicing in academic centres were more likely to respond. We hypothesize that this relates predominantly to workload. Physicians today, particularly those practicing in the community, are overtaxed by patient demands and have limited time to complete the myriad of questionnaires they typically receive. For example, general practitioners receive an average of 16 to 24 research requests annually, approximately 80% of which are commercial and satisfaction surveys (18). Academic physicians may be more likely to respond if there were fewer time constraints and/or a greater appreciation for research. We could not assess the association between physician performance of independent research and response due to a lack of information among nonrespondents. However, and as expected, a higher proportion of academic respondents performed independent research than nonacademics (75% versus 43%), supporting the hypothesis that interest in research may explain part of the observed differences across practice settings. Other factors, such as gender, hepatology training and geographic location, were not significant in our analysis.
Several unmeasured factors may have affected survey response rates in our study. First, due to the small size of the HCV-treating community in Canada, most of the survey recipients had personal knowledge of the investigators. Thus, the high overall response rate may reflect the recipients’ sense of obligation to respond. Most importantly, the content of the survey must be considered; it has been shown that questionnaires containing sensitive questions are less likely to be returned (2,3,19). The primary purpose of our questionnaire was to assess the relationship between the volume and experience of the health care provider and the success of antiviral therapy for chronic HCV. The recipients were informed that survey responses would be linked with clinical trial data to assess this ‘volume-outcome’ relationship (20). One may expect reluctance of recipients with poorer outcomes of HCV treatment to return the survey. If the volume-outcome relationship observed in other disciplines proves true for HCV treatment, this may explain the lower rates of survey response among community-based physicians, who generally treat fewer patients with HCV than academic physicians (7). Another related factor that may explain the relatively high overall response rate is the study’s association with the aforementioned phase IV trials, which are ongoing. Considering their involvement in these trials, the study participants may be more receptive to the pharmaceutical industry than the broader population of HCV care providers, thus potentially limiting the generalizability of the results. This factor was likely exaggerated by the scheduling of an investigator meeting in a desirable location within the study interval, perhaps illustrating the influence of pharmaceutical companies on the behaviour of health care providers.
SUMMARY
In the present randomized, controlled trial, pharmaceutical industry sponsorship was not associated with a reduced rate of response to a postal questionnaire among Canadian HCV care providers. As research funding from granting agencies dwindles, investigators in this field should not be reluctant to obtain financial support from the pharmaceutical industries for fear of limiting survey response rates.
Acknowledgments
The authors thank Reg Dias, Maresa Fernandes, Tarang Manchanda and Nisha Narang (Hoffmann-La Roche Ltd, Canada) for providing contact information for the survey recipients, and all the respondents for taking time to complete the survey. Dr Myers is supported by a Clinical Investigator Award from the Alberta Heritage Foundation for Medical Research. Dr Shaheen is supported by a grant from the Canadian Liver Foundation.
APPENDIX 1 Survey form for nurses
APPENDIX 2 Survey form for physicians
REFERENCES
- 1.Bryman A. Self-completion questionnaires. In: Bryman A, editor. Social Research Methods. Oxford: Oxford University Press; 2001. pp. 128–39. [Google Scholar]
- 2.Edwards P, Roberts I, Clarke M, et al. Increasing response rates to postal questionnaires: Systematic review. BMJ. 2002;324:1183. doi: 10.1136/bmj.324.7347.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edwards P, Roberts I, Clarke M, et al. Methods to increase response rates to postal questionnaires. Cochrane Database Syst Rev. 2003;(4):MR000008. doi: 10.1002/14651858.MR000008.pub3. [DOI] [PubMed] [Google Scholar]
- 4.Brennan TA, Rothman DJ, Blank L, et al. Health industry practices that create conflicts of interest: A policy proposal for academic medical centres. JAMA. 2006;295:429–33. doi: 10.1001/jama.295.4.429. [DOI] [PubMed] [Google Scholar]
- 5.Studdert DM, Mello MM, Brennan TA. Financial conflicts of interest in physicians’ relationships with the pharmaceutical industry – self-regulation in the shadow of federal prosecution. N Engl J Med. 2004;351:1891–900. doi: 10.1056/NEJMlim042229. [DOI] [PubMed] [Google Scholar]
- 6.Herper M.Forbes Magazine: Roche and Schering’s desperate duel<http://www.forbes.com/2002/11/15/cx_mh_1115roche_print.html> (Version current at December 13, 2006)
- 7.Bain VG, Wong WW, Greig PD, Yoshida EM, Canadian Association of Gastroenterology Hepatology and the Canadian gastroenterologist: Interest, attitudes and patterns of practice: Results of a national survey from the Canadian Association of Gastroenterology. Can J Gastroenterol. 2003;17:25–9. doi: 10.1155/2003/709303. [DOI] [PubMed] [Google Scholar]
- 8.Chande N, Ponich T, Gregor J. A survey of Canadian gastroenterologists about the use of methotrexate in patients with Crohn’s disease. Can J Gastroenterol. 2005;19:553–8. doi: 10.1155/2005/382379. [DOI] [PubMed] [Google Scholar]
- 9.Ilnyckyj A, Bernstein CN. Sexual abuse in irritable bowel syndrome: To ask or not to ask – that is the question. Can J Gastroenterol. 2002;16:801–5. doi: 10.1155/2002/245256. [DOI] [PubMed] [Google Scholar]
- 10.MacNeil-Covin L, Casson AG, Malatjalian D, Veldhuyzen van Zanten S. A survey of Canadian gastroenterologists about the management of Barrett’s esophagus. Can J Gastroenterol. 2003;17:313–7. doi: 10.1155/2003/648497. [DOI] [PubMed] [Google Scholar]
- 11.McDonald JW, Mahon J, Zarnke K, Feagan B, Simms L, Tucker W. A randomized survey of the preference of gastroenterologists for a Cochrane review versus a traditional narrative review. Can J Gastroenterol. 2002;16:17–21. doi: 10.1155/2002/513758. [DOI] [PubMed] [Google Scholar]
- 12.O’Sullivan S, Bridge G, Ponich T. Musculoskeletal injuries among ERCP endoscopists in Canada. Can J Gastroenterol. 2002;16:369–74. doi: 10.1155/2002/523125. [DOI] [PubMed] [Google Scholar]
- 13.Raza M, Bernstein CN, Ilnyckyj A. Canadian physicians’ choices for their own colon cancer screening. Can J Gastroenterol. 2006;20:281–4. 14. doi: 10.1155/2006/969832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallis TM, Greaven SH, Leddin D. What are we going to do with you? Gastroenterology service providers’ perceptions of “difficult to manage” IBD patients. Can J Gastroenterol. 2002;16:87–93. doi: 10.1155/2002/234518. [DOI] [PubMed] [Google Scholar]
- 15.Wang P, Yi Q, Scully L, Heathcote J, Krahn M. Indications for interferon/ribavirin therapy in hepatitis C patients: Findings from a survey of Canadian hepatologists. Can J Gastroenterol. 2003;17:183–6. doi: 10.1155/2003/498120. [DOI] [PubMed] [Google Scholar]
- 16.Sloan M, Kreiger N, James B. Improving response rates among doctors: Randomized trial. BMJ. 1997;315:1136. doi: 10.1136/bmj.315.7116.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lydeard S. Commentary: Avoid surveys masquerading as research. BMJ. 1996;313:733–4. [Google Scholar]
- 18.Barclay S, Todd C, Finlay I, Grande G, Wyatt P. Not another questionnaire! Maximizing the response rate, predicting non-response and assessing non-response bias in postal questionnaire studies of GPs. Fam Pract. 2002;19:105–11. doi: 10.1093/fampra/19.1.105. [DOI] [PubMed] [Google Scholar]
- 19.Dillman DA, Sinclair MD, Clark JR. Effects of questionnaire length, respondent-friendly design, and a difficult question on response rates for occupant-addressed census mail surveys. Public Opinion Quarterly. 1993;57:289–304. [Google Scholar]
- 20.Halm EA, Lee C, Chassin MR. Is volume related to outcome in health care? A systematic review and methodologic critique of the literature. Ann Intern Med. 2002;137:511–20. doi: 10.7326/0003-4819-137-6-200209170-00012. [DOI] [PubMed] [Google Scholar]