Abstract
OBJECTIVES:
To assess the cost-effectiveness of photodynamic therapy (PDT) and esophagectomy (ESO) relative to surveillance (SURV) for patients with Barrett’s esophagus (BE) and high-grade dysplasia (HGD).
METHODS:
A Markov decision tree was constructed to estimate costs and health outcomes of PDT, ESO and SURV in a hypothetical cohort of male patients, 50 years of age, with BE and HGD. Outcomes included unadjusted life-years (LYs) and quality-adjusted LYs (QALYs). Direct medical costs (2003 CDN$) were measured from the perspective of a provincial ministry of health. The time horizon for the model was five years (cycle length three months), and costs and outcomes were discounted at 3%. Model parameters were assigned unique distributions, and a probabilistic analysis with 10,000 Monte Carlo simulations was performed.
RESULTS:
SURV was the least costly strategy, followed by PDT and ESO, but SURV was also the least effective. In terms of LYs, the incremental cost-effectiveness ratios were $814/LY for PDT versus SURV and $3,397/LY for ESO versus PDT. PDT dominated ESO for QALYs in the base-case. The incremental cost-effectiveness ratio of PDT versus SURV was $879/QALY. In probabilistic analysis, PDT was most likely to be cost-effective at willingness-to-pay (WTP) values between $100/LY and $3,500/LY, and ESO was most likely to be cost-effective for WTP values over $3500/LY. For quality-adjusted survival, PDT was most likely to be cost-effective for all WTP thresholds above $1,000/QALY. The likelihood that PDT was the most cost-effective strategy reached 0.99 at a WTP ceiling of $25,000/QALY.
CONCLUSIONS:
In male patients with BE and HGD, PDT and ESO are cost-effective alternatives to SURV.
Keywords: Barrett’s esophagus, High-grade dysplasia, Photodynamic therapy
Abstract
BUT :
L’étude avait pour but d’évaluer la rentabilité de la thérapie photodynamique (TPD) et de l’oesophagectomie (OE) par rapport à la surveillance (SURV) chez des patients porteurs d’un œsophage de Barrett (OB) avec dysplasie de degré élevé (DDE) de malignité.
MÉTHODE :
Un arbre de décision markovien a été élaboré afin de permettre l’estimation des coûts et des résultats cliniques de la TPD, de l’OE et de la SURV dans une cohorte hypothétique d’hommes âgés de 50 ans et porteurs d’un OB avec DDE de malignité. Les résultats comprenaient le nombre d’années de vie (AV) non redressées et d’années de vie pondérées par la qualité de vie (AVPQ). Les coûts médicaux directs ($CAN 2003) étaient présentés du point de vue d’un ministère provincial de la Santé. L’horizon temporel du modèle était de cinq ans (durée des cycles : trois mois), et les coûts ainsi que les résultats ont été actualisés à un taux de 3 %. Il y a eu distribution unique des paramètres du modèle et nous avons procédé à une analyse probabiliste fondée sur 10 000 simulations produites selon la méthode Monte Carlo.
RÉSULTATS :
La SURV s’est révélée la stratégie la moins coûteuse, suivie de la TPD et de l’OE, mais elle s’est révélée aussi la moins efficace. En ce qui concerne le nombre d’AV, les rapports coût-efficacité différentiels étaient de 815 $/AV pour la TPD par rapport à la SURV et de 3397 $/AV pour l’OE par rapport à la TPD. Quant au nombre d’AVPQ, la TPD dominait l’OE dans le scénario de référence. Le rapport coût-efficacité différentiel de la TPD par rapport à la SURV était de 879 $/AVPQ. Dans l’analyse probabiliste, la TPD était vraisemblablement rentable à des valeurs de la disposition à payer (DP) variant de 100 à 3500 $/AV; quant à l’OE, elle était vraisemblablement rentable à des valeurs supérieures à 3500 $/AV. Pour ce qui est de la survie pondérée par la qualité, la TPD était vraisemblablement rentable à tous les seuils de la DP supérieurs à 1000 $/AVPQ. La probabilité que la TPD soit la stratégie la plus rentable atteignait 0,99 à un plafond de la DP de 25 000 $/AVPQ.
CONCLUSIONS :
La TPD et l’OE constituent, chez les hommes porteurs d’un OB avec DDE de malignité, des solutions de rechange rentables à la surveillance.
Barrett’s esophagus (BE) is marked by the presence of metaplastic columnar epithelium in the distal esophagus and is an established risk factor for esophageal adenocarcinoma (1). Patients with BE have a 30- to 120-fold increased RR of developing esophageal cancer in comparison with the general population (2). Because a stepwise progression from metaplasia through dysplasia to carcinoma has been proposed, patients with BE are advised to undergo regular endoscopic surveillance (SURV) with biopsy to detect either dysplasia or adenocarcinoma at a treatable stage (3).
If esophageal biopsies reveal high-grade dysplasia (HGD), the likelihood of synchronous adenocarcinoma or progression to adenocarcinoma is increased (4–7). The standard therapy for patients with BE and HGD is distal esophagectomy (ESO) (8,9). However, ESO is associated with significant perioperative morbidity and mortality (10,11), and can lead to long-term complications, such as dumping syndrome and dysphagia, that impair health-related quality of life (12).
Techniques for endoscopic ablation of dysplastic epithelium have been advocated as alternatives to ESO in patients with BE with HGD. Of these, photodynamic therapy (PDT) is the best studied and the only approach approved by the United States Food and Drug Administration (13). PDT involves intravenous infusion of a photosensitizing agent, such as porfimer sodium (Photofrin, Axcan Pharma Inc, Canada), that accumulates in dysplastic tissue, causing tissue destruction when activated by an endoscopic light source (14). PDT has proven to be efficacious in the eradication of BE with HGD and in the prevention of cancer (15,16). It has the theoretical advantages of reduced morbidity, negligible mortality and lower upfront costs compared with ESO.
An alternate, more conservative approach to BE patients with HGD is intensive, endoscopic SURV with biopsy after thorough investigation, including endoscopic ultrasound, to exclude synchronous adenocarcinoma (17). While this approach risks progression to advanced adenocarcinoma, it can entirely defer or avoid the morbidity and mortality of ESO.
Each of these approaches to managing patients with BE and HGD offers tradeoffs in terms of costs and health benefits. In the absence of rigorous, prospective, comparative trials, decision analysis can estimate the expected outcomes of alternative strategies as well as identify key determinants of efficacy and cost-effectiveness. A decision analytical model was constructed and probabilistic sensitivity analysis was used to assess the incremental cost-effectiveness of ESO, PDT and continued endoscopic SURV in patients with BE and HGD.
METHODS
General
A Markov model was constructed using Data 3.5 software (TreeAge Software Inc, USA) to simulate the management of a hypothetical cohort of 50-year-old men with BE and HGD. The incremental cost-effectiveness of three alternative strategies was assessed: immediate ESO, PDT and intense endoscopic SURV. Health outcomes were measured in unadjusted life-years (LYs) and quality-adjusted life-years (QALYs).
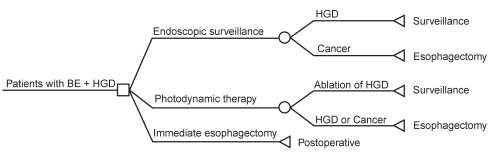
A simplified version of the Markov model is provided in Figure 1. The model allowed the study cohort to move among health states relevant to the management of BE with HGD using predefined transition probabilities. For each cycle spent in a given health state, patients accrued specific costs and gained QALYs proportionate to the utility weight assigned to that health state. The cycle length was three months, and the model’s time horizon was five years. The model considered direct medical costs from the perspective of a Canadian provincial ministry of health.
Figure 1).
Simplified Markov model for management of Barrett’s esophagus (BE) with high-grade dysplasia (HGD)
Model cohort
The model cohort was comprised of men, 50 years of age, with BE and newly diagnosed HGD. The cohort was assumed to be asymptomatic, treatment naive (except for chronic acid suppression) and fit for ESO. Before model entry, the cohort was assumed to have undergone thorough investigation, including endoscopic ultrasound, to exclude synchronous adenocarcinoma.
ESO strategy
In this strategy, the cohort underwent immediate and curative ESO. No further endoscopic SURV was performed.
PDT strategy
In this strategy, patients underwent PDT with porfimer sodium (Photofrin) as described by Overholt (18). The number of PDT sessions required to achieve ablation and the rate of esophageal stricture requiring dilation were estimated from the literature (15,16,19). Patients in whom eradication of HGD was confirmed underwent intense SURV strategy until HGD recurred, cancer developed or the model terminated. Patients who were found to have persistent or recurrent HGD after PDT and those who subsequently progressed to adenocarcinoma underwent ESO as per the ESO strategy.
Endoscopic SURV strategy
In this strategy, the cohort underwent SURV endoscopy with biopsy every three months as per the Seattle protocol (3). Patients who progressed to adenocarcinoma underwent ESO. All others continue to undergo endoscopic SURV indefinitely.
Probability assumptions
For each transition probability, a literature review was conducted to estimate its base-case value and a plausible range for use in sensitivity analysis (Table 1). To estimate the efficacy of PDT, a MEDLINE search was performed in November 2004 using the terms ‘Barrett esophagus’ and ‘photochemotherapy’, with results limited to porfimer sodium (Photofrin). Previously published reviews and proceedings from key conferences were hand-searched for pertinent abstracts and references. Fixed event rates derived from the literature at various time points were fitted to an exponential curve to determine three-month cycle probabilities (20). All costs and outcomes were discounted at an annual rate of 3% per Canadian guidelines (21).
TABLE 1.
Selected base-case parameter values and ranges for probabilistic analysis
| Parameter | Base-case value | Distribution (parameters) | 95% CIs | References |
|---|---|---|---|---|
| Probability of ablating HGD with PDT | 77.0% | Beta (106, 32) | 0.69 to 0.83 | 15,16,39,40 |
| Rate of progression to cancer after PDT (3 months) | 1.7% | Log-normal (0.0058, 0.0014) | 0.009 to 0.025 | 15,16,39,40 |
| Rate of esophageal stricture after PDT | 31.0% | Beta (95, 216) | 0.26 to 0.36 | 15,16,39–42 |
| Rate of progression to cancer from HGD (3 months) | 5.2% | Log-normal (0.0177, 0.04) | 0.029 to 0.073 | 5,7,39,43 |
| Mortality from esophagectomy for HGD | 0.93% | Beta (4, 426) | 0.003 to 0.02 | 6,9,12,44,45 |
| Mortality from esophagectomy for cancer | 2.8% | Beta (29, 1026) | 0.018 to 0.039 | 10,46–48 |
| Health state utility after esophagectomy | 0.80 | Beta (80, 20) | 0.71 to 0.87 | 24,49 |
| Average number of PDT sessions per patient | 1.32 | None | – | 15 |
| Cost of endoscopy with biopsy (includes physician fees and hospital costs) | $244 | Distributions assigned to individual cost components | – | 25,50 |
| Cost of esophagectomy (includes physician fees and hospital costs) | $24,963 | Distributions assigned to individual cost components | – | LHSCCP, 25 |
| Variable cost of PDT per session (includes Photofrin*, centring balloon and optical fibre) | $6,614 | Distributions assigned to individual cost components | – | Direct communication from manufacturer* |
| Amortized fixed cost of PDT light source per session | $101 | Normal (101, 20.2) | – | See text |
| Cost of dilation of PDT-related stricture (includes physician fees and hospital costs) | $418 | Distributions assigned to individual cost components | – | 25,50 |
*Axcan Pharma Inc, Canada. HGD High-grade dysplasia; LHSCCP London Health Sciences Case Cost Program; PDT Photodynamic therapy
Mortality
Survival with BE and HGD and early esophageal adenocarcinoma was estimated from the National Cancer Institute Surveillance Epidemiology and End Results (SEER) database (22). The survival of model cohort subjects with BE and HGD was assumed to equal the SEER program survival for men with histologically confirmed, in situ cancer. The survival of model cohort subjects who developed early adenocarcinoma was assumed to be equal to the weighted mix of localized and in situ esophageal cancer in the SEER program.
Patients in the model who survived ESO for either BE with HGD or cancer assumed the age-specific mortality of the Canadian male population (23). All patients who survived to the model’s five-year time horizon were assigned the predicted life expectancy of a 55-year-old Canadian man (23.38 years) as estimated from Canadian life tables (23).
Health utility weights
The model estimated health outcomes using both unadjusted LYs and QALYs. For the latter, death was assigned a utility weight of zero and the post-ESO state was assigned a utility weight of 0.80, as estimated by Provenzale et al (24). All other health states were assigned a utility value of one (Table 1). The transient disutility of undergoing endoscopy (including PDT) was not considered.
Cost inputs
Direct medical costs were considered from the perspective of a provincial ministry of health (Table 1). Canadian data sources were used wherever possible. American costs were converted to 2003 Canadian costs using prevailing Bank of Canada exchange rates (US$1=CDN$1.34). Standard resource profiles were developed for each health state cycle using expert opinion and consensus. Costs for physician services were obtained from the Ontario Ministry of Health and Long-Term Care Schedule of Benefits (April 2002) (25). Costs for surgical admissions and hospital-based endoscopic procedures were estimated by adapting the London Health Sciences Centre Case Cost Program (LHSCCP) administrative database, which is part of the Ontario Case Costing Initiative. The LHSCCP fully allocates hospital global budget expenditures among standardized functional cost centres such as nursing, clinical laboratory, diagnostic imaging and pharmacy in accordance with Guidelines for Management Information Systems in Canadian Health Care Facilities (26). The LHSCCP assigns each resource or service a variable cost for material supplies, plus a workload-indexed proportion of fixed or overhead costs, such as labour, equipment and capital infrastructure (including depreciation). Total costs were estimated by multiplying resource units by the total unit cost (ie, fixed plus variable).
Resource profiles for performing PDT were adapted from Overholt et al (15). Costs per session of PDT included the facility costs for endoscopy; physician reimbursement fee for endoscopy; and the per patient costs of administering PDT, including 2 mg/kg doses of the sensitizing drug (Photofrin), disposable light-emitting fibre and centring balloon, and laser-light generator. Table 1 shows cost parameters used in the model. Market prices for Photofrin, disposables and laser-light generator were obtained from the manufacturer on October 2003. Cost per use of the light source was amortized assuming a five-year life expectancy with 1000 procedures. Each patient required an average of 1.32 PDT sessions based on available trial data (15). To assess treatment response to PDT, each patient had a follow-up endoscopy at three months, which incurred the costs of the endoscopy and physician reimbursement for the procedure, esophageal biopsy and a subsequent clinic visit. Although Nd:YAG laser therapy and endoscopic ultrasound were used by Overholt et al (15), these procedures were not included in the model, given their limited availability in Canada at the time of study conception. Rates of complications of PDT (eg, esophageal stricture requiring dilation) were estimated from the literature (Table 1). Each event was assigned a standard resource profile for management. Costs incurred from these complications included both treatment costs and physician reimbursement fees for the treatment and clinic follow-up.
Costs for ESO included both facility costs and physician reimbursement for the consultation, surgical procedure and follow-up visits. A routine post-ESO endoscopy was not included in the model. Direct costs from postoperative complications would have been captured by the LHSCCP (see above). Similarly, SURV arm costs included physician reimbursement for the consultation, endoscopy procedure and follow-up visits, and the direct cost per endoscopy. No complication rate was assumed for nontherapeutic endoscopy.
Base-case and incremental cost-effectiveness analysis
Expected lifetime costs, LYs and QALYs were first calculated for each strategy using base-case parameter estimates. General principles of assessing cost-effectiveness among multiple strategies were then applied (27). First it was determined whether any treatment dominated, having both higher costs and lower health benefits by a single strategy (strict dominance) or a linear combination of two strategies (extended dominance). Any dominated strategies were eliminated from further analysis. Among nondominated strategies, incremental cost-effectiveness ratios (ICERs) were calculated as the ratio of the difference in cost to the difference in outcome between two alternatives. Beginning with the least costly treatment, alternatives were compared with the next most costly strategy when calculating ICERs. This process produced an ‘efficiency frontier’ of increasingly more effective but more costly treatment strategies.
Probabilistic sensitivity analysis
To assess the impact of the joint uncertainty of model variables on cost-effectiveness, a probabilistic sensitivity analysis was conducted (28). Here, transition probabilities and cost estimates used in the model were assigned unique distributions (Table 1). Cost parameters were assumed to be normally distributed and transition probabilities were assigned either beta or log-normal distributions. Distribution parameters and 95% CIs for each variable are shown in Table 1. With each model simulation, a value for each parameter was chosen by random selection with a resultant estimate of expected costs and outcomes. Results from 10,000 Monte Carlo simulations were aggregated to define distributions of the expected costs and health outcomes of each strategy (29).
Acceptability curves
The results of the probabilistic sensitivity analysis were also depicted using cost-effectiveness acceptability curves (30). This format is particularly informative when a payer’s willingness-to-pay (WTP) threshold is uncertain. WTP thresholds are the maximum ceiling costs per unit of benefit, below which a payer deems an intervention to be cost-effective (31). Specifically, acceptability curves use Monte Carlo simulation results to estimate the proportion of times that each strategy is the most cost-effective alternative at each WTP threshold.
RESULTS
Base-case analysis
The base-case model results are presented in Table 2. Endoscopic SURV incurred the lowest costs but also yielded the shortest survival, both in unadjusted LYs and QALYs. ESO incurred the highest costs and provided the longest unadjusted LYs but intermediate QALYs. PDT incurred intermediate costs but provided the most QALYs.
TABLE 2.
Results of base-case analysis
| Outcome | Surveillance (SURV) | Photodynamic therapy (PDT) | Esophagectomy |
|---|---|---|---|
| Expected costs | $17,817 | $22,381 | $24,963 |
| Expected LYs | 12.53 | 18.14 | 18.90 |
| Expected QALYs | 11.85 | 17.04 | 15.85 |
| ICER ($/LY) | – | $814 versus SURV | $3,379 versus PDT |
| ICER ($/QALY) | – | $879 versus SURV | Dominated by PDT |
ICER Incremental cost-effectiveness ratio; LYs Life years; QALYs Quality-adjusted life-years
Incremental cost-effectiveness analysis
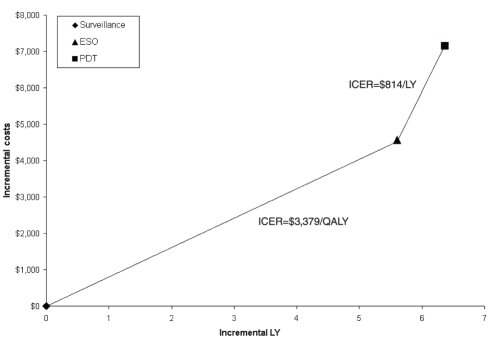
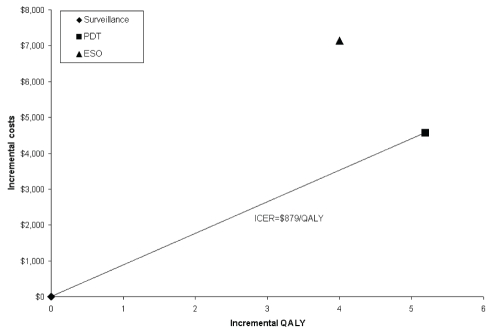
ICERs and cost-effectiveness efficiency frontiers for unadjusted LY and QALY are shown in Table 2 and Figure 2. For the unadjusted LY, all three strategies formed the frontier. The ICER of PDT relative to SURV was $814/LY and that of ESO relative to PDT was $3,397/LY. For QALYs, the efficiency frontier comprised only the SURV and PDT strategies, because ESO was dominated by PDT (Figure 3). The ICER of PDT relative to SURV was $879/QALY.
Figure 2).
Efficiency frontier with base-case results for unadjusted life-years (LY) relative to surveillance (origin). ESO Esophagectomy; ICER Incremental cost-effectiveness ratio; PDT Photodynamic therapy; QALY Quality-adjusted life-years
Figure 3).
Efficiency frontier with base-case results for quality-adjusted life-years (QALY) relative to surveillance (origin). ESO Esophagectomy; ICER Incremental cost-effectiveness ratio; PDT Photodynamic therapy
Probabilistic sensitivity analysis
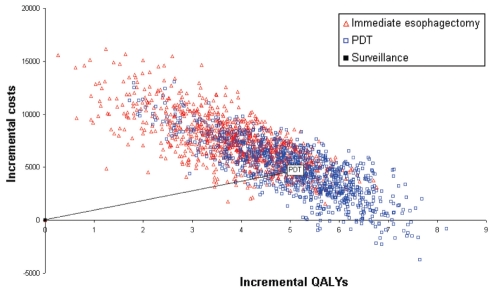
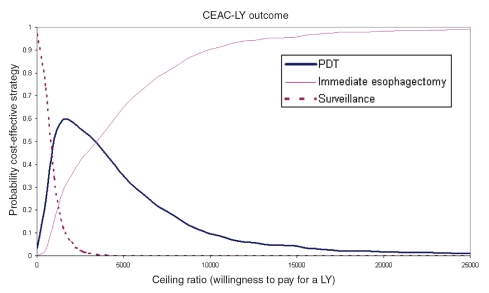
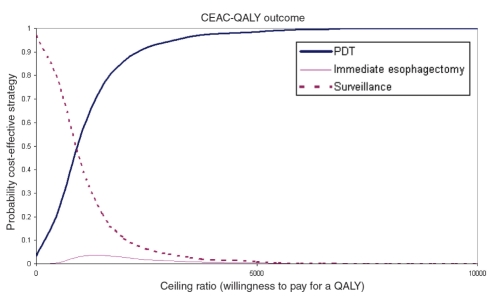
A scatterplot of the incremental cost and QALY pairs for PDT and ESO relative to SURV (reference), generated from the 10,000 Monte Carlo simulations, is shown in Figure 4. The cost-effectiveness acceptability curve for an unadjusted LY (cost per LY) is shown in Figure 5. ESO had the highest probability of being the most cost-effective strategy for WTP thresholds above $3,500/LY. This probability reached 0.99 at a ceiling ratio of $25,000/LY. The cost-effectiveness acceptability curve for a QALY (cost per QALY) is presented in Figure 6. Here, PDT had the highest probability of being the most cost-effective strategy for WTP thresholds above $1,000/QALY. The probability that PDT is the most cost-effective strategy reached 0.99 at a ceiling ratio of $25,000/QALY.
Figure 4).
Scatterplot of incremental costs and effectiveness (quality-adjusted life-years [QALYs]) from 10,000 Monte Carlo simulations. PDT Photodynamic therapy
Figure 5).
Cost-effectiveness acceptability curve for unadjusted life-years (CEAC-LY). PDT Photodynamic therapy
Figure 6).
Cost-effectiveness acceptability curve for quality-adjusted life-years (CEAC-QALY). PDT Photodynamic therapy
DISCUSSION
This cost-effectiveness analysis uses state-of-the-art modelling techniques, including probabilistic sensitivity analysis, to demonstrate that PDT is a cost-effective alternative to endoscopic SURV and immediate ESO in male patients with BE and HGD. In particular, PDT is favoured when survival is adjusted to reflect reduced quality of life after ESO.
Several assumptions made in the design of this model should be acknowledged explicitly and may influence the interpretation of results. First, the model did not allow spontaneous regression of HGD in the setting of BE because there remain few published data on the rate and significance of this phenomenon. Second, rates of progression from HGD to cancer were estimated from observational studies. While diagnostic error is captured implicitly in these data, false-positive error was not modelled explicitly, and we assumed diagnoses of HGD and adenocarcinoma to be accurate. Third, we assumed that all members of the cohort were free of cancer at baseline and remained eligible for surgery throughout the model, neither of which may be clinically realistic. Fourth, patients who underwent ESO for HGD or cancer were deemed cured with no need for further endoscopic SURV, which may not be standard practice at some centres.
ICERs offer meaningful comparisons of costs incurred to achieve additional units of effectiveness among competing strategies, and are informative for decision-makers who must allocate constrained resources (32). In such cases, a management strategy may be adopted if its ICER falls below a predefined WTP threshold. No such threshold is accepted universally, but values between $50,000/LY and $100,000/LY have been proposed and are broadly applied (33). Because all ICERs in our base-case analyses are well below these thresholds, our model suggests that ESO is the most cost-effective strategy for unadjusted LYs and PDT is the most cost-effective strategy for QALYs in patients with BE and HGD.
Fixed estimates of ICER may be misleading, because they do not communicate the inherent uncertainty with which input parameters are estimated. Monte Carlo techniques capture and measure overall uncertainty, which is presented graphically as cost-effectiveness acceptability curves (30). If the exact acceptable WTP threshold is not known, decision-makers may refer to the acceptability curve to identify WTP ranges for which a strategy is most likely to be cost-effective. For the unadjusted LY, our results suggest that PDT is the strategy most likely to be cost-effective for WTP thresholds between $100/LY and $3500/LY, whereas ESO is the most cost-effective at higher WTP thresholds. For QALYs, PDT is the strategy most likely to be cost-effective for all WTP thresholds above $1000/QALY.
Other models have been used to assess the cost-effectiveness of PDT for BE with HGD (34–36). Although our results confirm the conclusions of those analyses in many respects, two important differences warrant mention. First, our study is the only study to assume a non-American perspective. Although Canada and the United States share similar health care standards (37,38), unit costs and practice patterns often vary, and cost-effectiveness cannot be assumed to be transferable. Second, our study is the only analysis of PDT that uses state-of-the-art probabilistic sensitivity analysis to assess simultaneously the joint uncertainty of all model parameters, providing more comprehensive information to decision-makers.
SUMMARY
PDT is a cost-effective alternative to ESO and continued endoscopic SURV for the management of patients with BE and HGD. Assuming reasonable WTP, PDT is the strategy most likely to be cost-effective for gains in QALYs. However, ultimately, management must still be individualized to the patient, considering his or her preferences and comorbidities, as well as local expertise.
Acknowledgments
Unrestricted financial support was provided by Axcan Pharma Inc (Canada).
REFERENCES
- 1.Spechler SJ, Goyal RK. Barrett’s esophagus. N Engl J Med. 1986;315:362–71. doi: 10.1056/NEJM198608073150605. [DOI] [PubMed] [Google Scholar]
- 2.Haggitt RC. Barrett’s esophagus, dysplasia, and adenocarcinoma. Hum Pathol. 1994;25:982–93. doi: 10.1016/0046-8177(94)90057-4. [DOI] [PubMed] [Google Scholar]
- 3.Sampliner RE. Practice guidelines on the diagnosis, surveillance, and therapy of Barrett’s esophagus. The Practice Parameters Committee of the American College of Gastroenterology. Am J Gastroenterol. 1998;93:1028–32. doi: 10.1111/j.1572-0241.1998.00362.x. [DOI] [PubMed] [Google Scholar]
- 4.Pera M, Trastek VF, Carpenter HA, Allen MS, Deschamps C, Pairolero PC. Barrett’s esophagus with high-grade dysplasia: An indication for esophagectomy? Ann Thorac Surg. 1992;54:199–204. doi: 10.1016/0003-4975(92)91370-o. [DOI] [PubMed] [Google Scholar]
- 5.Weston AP, Sharma P, Topalovski M, Richards R, Cherian R, Dixon A. Long-term follow-up of Barrett’s high-grade dysplasia. Am J Gastroenterol. 2000;95:1888–93. doi: 10.1111/j.1572-0241.2000.02234.x. [DOI] [PubMed] [Google Scholar]
- 6.Levine DS, Haggitt RC, Blount PL, Rabinovitch PS, Rusch VW, Reid BJ. An endoscopic biopsy protocol can differentiate high-grade dysplasia from early adenocarcinoma in Barrett’s esophagus. Gastroenterology. 1993;105:40–50. doi: 10.1016/0016-5085(93)90008-z. [DOI] [PubMed] [Google Scholar]
- 7.Schnell TG, Sontag SJ, Chejfec G, et al. Long-term nonsurgical management of Barrett’s esophagus with high-grade dysplasia. Gastroenterology. 2001;120:1607–19. doi: 10.1053/gast.2001.25065. [DOI] [PubMed] [Google Scholar]
- 8.Ruol A, Zaninotto G, Costantini M, et al. Barrett’s esophagus: Management of high-grade dysplasia and cancer. J Surg Res. 2004;117:44–51. doi: 10.1016/j.jss.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 9.Heitmiller RF, Redmond M, Hamilton SR. Barrett’s esophagus with high-grade dysplasia. An indication for prophylactic esophagectomy. Ann Surg. 1996;224:66–71. doi: 10.1097/00000658-199607000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karl RC, Schreiber R, Boulware D, Baker S, Coppola D. Factors affecting morbidity, mortality, and survival in patients undergoing Ivor Lewis esophagogastrectomy. Ann Surg. 2000;231:635–43. doi: 10.1097/00000658-200005000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tseng EE, Wu TT, Yeo CJ, Heitmiller RF.Barrett’s esophagus with high grade dysplasia: Surgical results and long-term outcome – An update J Gastrointest Surg 20037164–70.discussion 170–1 [DOI] [PubMed] [Google Scholar]
- 12.Headrick JR, Nichols FC, III, Miller DL, et al. High-grade esophageal dysplasia: Long-term survival and quality of life after esophagectomy Ann Thorac Surg 2002731697–702.discussion 1702–3 [DOI] [PubMed] [Google Scholar]
- 13.McCaughan JS., Jr Photodynamic therapy: A review. Drugs Aging. 1999;15:49–68. doi: 10.2165/00002512-199915010-00005. [DOI] [PubMed] [Google Scholar]
- 14.Overholt B, Panjehpour M, Tefftellar E, Rose M. Photodynamic therapy for treatment of early adenocarcinoma in Barrett’s esophagus. Gastrointest Endosc. 1993;39:73–6. doi: 10.1016/s0016-5107(93)70017-6. [DOI] [PubMed] [Google Scholar]
- 15.Overholt BF, Panjehpour M, Haydek JM. Photodynamic therapy for Barrett’s esophagus: Follow-up in 100 patients. Gastrointest Endosc. 1999;49:1–7. doi: 10.1016/s0016-5107(99)70437-2. [DOI] [PubMed] [Google Scholar]
- 16.Overholt BF, Panjehpour M, Halberg DL. Photodynamic therapy for Barrett’s esophagus with dysplasia and/or early stage carcinoma: Long-term results. Gastrointest Endosc. 2003;58:183–8. doi: 10.1067/mge.2003.327. [DOI] [PubMed] [Google Scholar]
- 17.Sharma P.Review article: Emerging techniques for screening and surveillance in Barrett’s oesophagus Aliment Pharmacol Ther 20042063–70.discussion 95–6 [DOI] [PubMed] [Google Scholar]
- 18.Overholt BF. Results of photodynamic therapy in Barrett’s esophagus: A review. Can J Gastroenterol. 1999;13:393–6. doi: 10.1155/1999/692453. [DOI] [PubMed] [Google Scholar]
- 19.Overholt BF, Panjehpour M. Photodynamic therapy in the management of Barrett’s esophagus with dysplasia. J Gastrointest Surg. 2000;4:129–30. doi: 10.1016/s1091-255x(00)80047-5. [DOI] [PubMed] [Google Scholar]
- 20.Sonnenberg FA, Beck JR. Markov models in medical decision making: A practical guide. Med Decis Making. 1993;13:322–38. doi: 10.1177/0272989X9301300409. [DOI] [PubMed] [Google Scholar]
- 21.Canadian Coordinating Office for Health Technology Assessment . 2nd edn. Ottawa: Canadian Coordinating Office for Health Technology Assessment (CCOHTA); 1997. Guidelines for Economic Evaluation of Pharmaceuticals: Canada. [DOI] [PubMed] [Google Scholar]
- 22.Surveillance, Epidemiology, and End Results (SEER) program (1973–2002), version 50. 20th edn. Bethesda, Maryland: National Cancer Institute; 2002. [Google Scholar]
- 23.Statistics Canada Health Statistics Division Causes of Death, 1995–1997Catalogue No 84-537-XIEOttawa: Minister of Industry; 2002 [Google Scholar]
- 24.Provenzale D, Kemp JA, Arora S, Wong JB. A guide for surveillance of patients with Barrett’s esophagus. Am J Gastroenterol. 1994;89:670–80. [PubMed] [Google Scholar]
- 25.Schedule of Benefits for Physician Services under the Health Insurance Act <http://www.health.gov.on.ca/english/providers/program/ohip/sob/physserv/physserv_mn.html> (Version current at June 26, 2006).
- 26.Guidelines for Management Information Systems in Canadian Health Care Facilities. Ottawa: Canadian Institute for Health Information; 1984. [Google Scholar]
- 27.Drummond MF, O’Brien B, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. 2nd edn. New York: Oxford University Press Inc; 1997. [Google Scholar]
- 28.Briggs AH, Goeree R, Blackhouse G, O’Brien BJ. Probabilistic analysis of cost-effectiveness models: Choosing between treatment strategies for gastroesophageal reflux disease. Med Decis Making. 2002;22:290–308. doi: 10.1177/0272989X0202200408. [DOI] [PubMed] [Google Scholar]
- 29.Critchfield GC, Willard KE. Probabilistic analysis of decision trees using Monte Carlo simulation. Med Decis Making. 1986;6:85–92. doi: 10.1177/0272989X8600600205. [DOI] [PubMed] [Google Scholar]
- 30.Fenwick E, O’Brien BJ, Briggs A. Cost-effectiveness acceptability curves – Facts, fallacies and frequently asked questions. Health Econ. 2004;13:405–15. doi: 10.1002/hec.903. [DOI] [PubMed] [Google Scholar]
- 31.Gafni A. Willingness-to-pay as a measure of benefits. Relevant questions in the context of public decisionmaking about health care programs. Med Care. 1991;29:1246–52. [PubMed] [Google Scholar]
- 32.O’Brien BJ, Briggs AH. Analysis of uncertainty in health care cost-effectiveness studies: An introduction to statistical issues and methods. Stat Methods Med Res. 2002;11:455–68. doi: 10.1191/0962280202sm304ra. [DOI] [PubMed] [Google Scholar]
- 33.Laupacis A, Feeny D, Detsky AS, Tugwell PX. How attractive does a new technology have to be to warrant adoption and utilization? Tentative guidelines for using clinical and economic evaluations. CMAJ. 1992;146:473–81. [PMC free article] [PubMed] [Google Scholar]
- 34.Hur C, Nishioka NS, Gazelle GS. Cost-effectiveness of photodynamic therapy for treatment of Barrett’s esophagus with high grade dysplasia. Dig Dis Sci. 2003;48:1273–83. doi: 10.1023/a:1024146823549. [DOI] [PubMed] [Google Scholar]
- 35.Vij R, Triadafilopoulos G, Owens DK, Kunz P, Sanders GD. Cost-effectiveness of photodynamic therapy for high-grade dysplasia in Barrett’s esophagus. Gastrointest Endosc. 2004;60:739–56. doi: 10.1016/s0016-5107(04)02167-4. [DOI] [PubMed] [Google Scholar]
- 36.Shaheen NJ, Inadomi JM, Overholt BF, Sharma P. What is the best management strategy for high grade dysplasia in Barrett’s oesophagus? A cost effectiveness analysis. Gut. 2004;53:1736–44. doi: 10.1136/gut.2003.033837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rouleau JL, Moye LA, Pfeffer MA, et al. A comparison of management patterns after acute myocardial infarction in Canada and the United States. The SAVE investigators. N Engl J Med. 1993;328:779–84. doi: 10.1056/NEJM199303183281108. [DOI] [PubMed] [Google Scholar]
- 38.Redelmeier DA, Fuchs VR. Hospital expenditures in the United States and Canada. N Engl J Med. 1993;328:772–8. doi: 10.1056/NEJM199303183281107. [DOI] [PubMed] [Google Scholar]
- 39.Overholt B, Lightdale CJ, Wang KK, et al. International, multicenter, partially blinded, randomized study of the efficacy of photodynamic therapy using porfimer sodium for the ablation of high-grade dysplasia in Barrett’s esophagus: Results of 24-month follow-up Gastroenterology 2003124A20(Abst) [Google Scholar]
- 40.Wolfsen HC, Hemminger LL, Wallace MB, Devault KR. Clinical experience of patients undergoing photodynamic therapy for Barrett’s dysplasia or cancer. Aliment Pharmacol Ther. 2004;20:1125–31. doi: 10.1111/j.1365-2036.2004.02209.x. [DOI] [PubMed] [Google Scholar]
- 41.Wang K, Song L, Buttar N, et al. Long-term follow-up after photodynamic therapy (PDT) for Barrett’s esophagus Gastrointest Endosc 200255AB100(Abst) [Google Scholar]
- 42.Beejay U, Ribeiro A, Hourigan L, et al. Photodynamic therapy of high grade dysplasia/intramucosal carcinoma in Barrett’s esophagus – 30 months follow up Gastrointest Endosc 200153AB144(Abst) [Google Scholar]
- 43.Reid BJ, Blount PL, Feng Z, Levine DS. Optimizing endoscopic biopsy detection of early cancers in Barrett’s high-grade dysplasia. Am J Gastroenterol. 2000;95:3089–96. doi: 10.1111/j.1572-0241.2000.03182.x. [DOI] [PubMed] [Google Scholar]
- 44.Ferguson MK, Naunheim KS. Resection for Barrett’s mucosa with high-grade dysplasia: Implications for prophylactic photodynamic therapy. J Thorac Cardiovasc Surg. 1997;114:824–9. doi: 10.1016/S0022-5223(97)70087-4. [DOI] [PubMed] [Google Scholar]
- 45.DeMeester TR, Attwood SE, Smyrk TC, Therkildsen DH, Hinder RA. Surgical therapy in Barrett’s esophagus. Ann Surg. 1990;212:528–540. doi: 10.1097/00000658-199010000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patti MG, Corvera CU, Glasgow RE, Way LW. A hospital’s annual rate of esophagectomy influences the operative mortality rate. J Gastrointest Surg. 1998;2:186–92. doi: 10.1016/s1091-255x(98)80011-5. [DOI] [PubMed] [Google Scholar]
- 47.Begg CB, Cramer LD, Hoskins WJ, Brennan MF. Impact of hospital volume on operative mortality for major cancer surgery. JAMA. 1998;280:1747–51. doi: 10.1001/jama.280.20.1747. [DOI] [PubMed] [Google Scholar]
- 48.Ellis FH, Jr, Heatley GJ, Krasna MJ, Williamson WA, Balogh K. Esophagogastrectomy for carcinoma of the esophagus and cardia: A comparison of findings and results after standard resection in three consecutive eight-year intervals with improved staging criteria. J Thorac Cardiovasc Surg. 1997;113:836–46. 846–8. doi: 10.1016/S0022-5223(97)70256-3. [DOI] [PubMed] [Google Scholar]
- 49.Provenzale D, Schmitt C, Wong JB. Barrett’s esophagus: A new look at surveillance based on emerging estimates of cancer risk. Am J Gastroenterol. 1999;94:2043–53. doi: 10.1111/j.1572-0241.1999.01276.x. [DOI] [PubMed] [Google Scholar]
- 50.Goeree R, O’Brien BJ, Blackhouse G, Marshall J, Briggs A, Lad R. Cost-effectiveness and cost-utility of long-term management strategies for heartburn. Value Health. 2002;5:312–28. doi: 10.1046/j.1524-4733.2002.54145.x. [DOI] [PubMed] [Google Scholar]