Abstract
BACKGROUND:
Pulmonary aspiration is a life-threatening complication of upper gastrointestinal endoscopy, the incidence of which has not been determined. Endoscopy-related aspiration has not been studied in procedures in which patients swallow a radiolabelled potential aspirate immediately before endoscopy and undergo nuclear scanning postprocedure.
METHODS:
A pilot study was conducted in which 200 MBq of non-absorbable technetium-99m phytate in 10 mL of water was administered orally to 50 patients who were about to undergo endoscopy. Gamma camera images were obtained to ensure that there had been no aspiration before endoscopy. After endoscopy, a repeat scan was performed. Fluid aspirated through the endoscope was also collected and analyzed for radioactivity using a hand-held radiation monitor.
RESULTS:
No evidence of pulmonary aspiration was found in any of the patients studied. The mean estimated percentage of the initially administered radioactivity aspirated through the endoscope was 2.66% (range 0% to 10.3%).
CONCLUSION:
The present pilot study confirms earlier observations that clinically significant aspiration in the context of upper gastrointestinal endoscopy is uncommon. The incidence of aspiration may, however, be different in acutely bleeding patients undergoing endoscopy. For logistic reasons, this group could not be studied.
Keywords: Aspiration, Endoscopy, Gastroscopy
Abstract
HISTORIQUE :
L’aspiration pulmonaire est une complication gravissime de l’endoscopie des voies digestives hautes dont l’incidence n’a pas été déterminée. L’aspiration liée à l’endoscopie n’a pas été étudiée lors d’interventions au cours desquelles les patients risquent d’aspirer une substance radiomarquée administrée par voie orale immédiatement avant l’endoscopie et doivent ensuite subir l’épreuve d’imagerie en médecine nucléaire.
MÉTHODES :
Une étude pilote a été réalisée au cours de laquelle 200 MBq de phytate de technétium (99m) non absorbable dans 10 mL d’eau ont été administrés par voie orale à 50 patients sur le point de subir une endoscopie. Des images ont été obtenues par gamma-caméra pour confirmer l’absence d’aspiration avant l’endoscopie. Après l’endoscopie, une épreuve d’imagerie de contrôle a été réalisée. Du liquide a aussi été recueilli au moyen de l’endoscope et analysé pour dosage de la radioactivité à l’aide d’un moniteur de rayonnement portatif.
RÉSULTATS :
Aucun signe d’aspiration pulmonaire n’a été observé chez les patients étudiés. Le pourcentage moyen estimé de la radioactivité initialement administrée et recueillie au moyen de l’endoscope a été de 2,66 % (entre 0 et 10,3 %).
CONCLUSION :
La présente étude pilote a confirmé les observations antérieures à l’effet qu’il est rare d’assister à une aspiration cliniquement significative lors d’une endoscopie des voies digestives hautes. L’incidence de l’aspiration peut par contre être différente chez des patients en hémorragie qui doivent subir une endoscopie. Pour des raisons logistiques, ce groupe n’a pas pu être étudié.
The risk of aspiration following upper gastrointestinal (GI) endoscopy has received scant attention. The abolition or impairment of the gag reflex with local pharyngeal anesthesia, use of intravenous sedation and the splinting of the upper esophageal sphincter by the endoscope, however, all predispose to aspiration of gastric contents. In addition, endoscopies are sometimes performed with the stomach full of blood or the small bowel full of bowel preparation fluid, further adding to the risk of this life-threatening complication. Easy access to endoscopy (partly due to its perceived lack of complications) means that a considerable number of patients at risk for aspiration pneumonia, such as those who are elderly, immunosuppressed, and victims of cerebrovascular disease and other neurological diseases are able to undergo endoscopy.
In the evaluation of endoscopy-induced aspiration, some studies (1,2) have relied on the performance of chest x-rays (CXRs) before and after endoscopy, with the presence of new pulmonary infiltrate(s) thought to indicate aspiration. However, to depend on CXR evidence alone is unreliable, because new CXR infiltrates may not be related to aspiration but may be related to coexistent pneumonic processes. If general anesthesia and intubation are performed before endoscopy, patients may aspirate during induction or extubation rather than during the endoscopy itself. Moreover, aspiration may occur during endoscopy but may not lead to any radiological changes evident immediately after endoscopy, either because insufficient fluid was aspirated or because new opacities may be hidden by other soft tissue opacities, such as the cardiac shadow or shadows related to pre-existing pulmonary disease. The clinical significance and prevalence of episodes of subclinical ‘microaspiration’ is unknown but may be useful as markers of overt aspiration. Characterization of patients sustaining aspiration during endoscopy may better identify the risk factors associated with aspiration and enable appropriate preventive strategies to be devised. To determine whether there was any unequivocal evidence of aspiration occurring during endoscopy, an open study was conducted in which endoscopy-related aspiration could be determined directly. Patients drank a small amount of radiolabelled fluid before endoscopy and then had nuclear scans taken after endoscopy to see whether any fluid had been aspirated from their stomachs into their lungs. A scan was also performed immediately after drinking the radiolabelled fluid to ensure that none of the fluid had been aspirated during swallowing.
METHODS
Written consent was obtained from all patients. The present study was approved by the Australian Capital Territory Health and Safety Ethics Committee. Clearance by the Australian Capital Territory Radiation Council was also granted. Overall, 50 patients were recruited to the study; the demographic profile of the group is shown in Table 1. Exclusion criteria included women of child-bearing age and individuals younger than 18 years of age. Immediately before endoscopy, with the patient sitting upright and before the administration of either lignocaine spray to the pharynx or intravenous sedation, 200 MBq of technetium-99m phytate in 10 mL of water was taken orally. There is evidence that a small amount of fluid can be safely administered orally before endoscopy (3). This isotope was chosen because its half-life is 6 h and, thus, would still be evident in the lung fields for several hours if significant aspiration were to occur. It is also cheap, readily available and not absorbed enterically. A nuclear scan with particular attention to the lung fields was then performed immediately to exclude aspiration that may have occurred during swallowing. Patients were transferred to the endoscopy unit, where 44 underwent endoscopies and six underwent both an endoscopy and a colonoscopy. Before gastroscopy, each patient received a 1% lignocaine pharyngeal spray. Intravenous midazolam (maximum dose of 5 mg) was administered to all patients, and fentanyl (maximum dose of 100 μg) was administered to all patients except one as initial sedation. In 11 patients, intravenous propofol (total dose between 20 mg and 50 mg, given in aliquots of not more than 30 mg) was administered. Propofol was only administered if the other drugs did not lead to adequate sedation as determined by the endoscopist. Periods of apnea and pulse oximetry levels of less than 90% were recorded. All endoscopies were performed either by an experienced endoscopist (Dr Thomson) or a trainee endoscopist under his supervision. At endoscopy, gastric fluid was aspirated in the usual manner soon after the stomach had been intubated. The volume and radioactivity of the aspirate collected through the endoscope during gastroscopy was recorded. This was performed after each endoscopy by removing the entire aspiration flask from the endoscopy unit and taking it to the x-ray department for analysis. The centre of the flask was placed 10 cm from a hand-held radiation monitor, and the activity was measured in microSieverts per hour. The amount of radiation in megabecquerels was then calculated by dividing this value by 1.6 (1 MBq of technetium-99m equals 1.6 μSv/h at 10 cm). Allowing for a 2 h delay between the time of administration and the time of analysis, this value was then multiplied by 1.25 to correct for decay. This flask was not reused for at least 24 h. Absorbent towels placed under the patients’ heads and shoulders to collect secretions, together with the gloves and gowns worn by the medical and nursing staff during endoscopy, were collected in a plastic bag and sequestered in a room for 24 h, away from the clinical area. None of the attending medical or nursing staff were pregnant or potentially pregnant. The duration of the gastroscopy was recorded. For the first three endoscopies, the radioactivity of the endoscope was measured immediately after the procedure, but this practice was subsequently discontinued because there was no radiation detected.
TABLE 1.
Patient demographics
| Characteristics | Results |
|---|---|
| Number of patients | 50 |
| Mean age, years | 55 |
| Male:female, n | 25:25 |
| Number of outpatients | 46 |
| Duration of endoscopy | 8 min (range 2 min to 20 min) |
| Time between first scan and endoscopy | 38 min (range 4 min to 90 min) |
| Mean volume of fluid aspirated through the endoscope | 41 mL (range 0 mL to 150 mL) |
| Endoscopic findings, n* | |
| Gastritis | 14 |
| Gastroesophageal reflux | 10 |
| Esophageal stricture | 6 (1 malignant) |
| Barrett’s esophagus | 5 |
| Normal | 12 |
| Other | 11 |
*Several patients had more than one positive finding
RESULTS
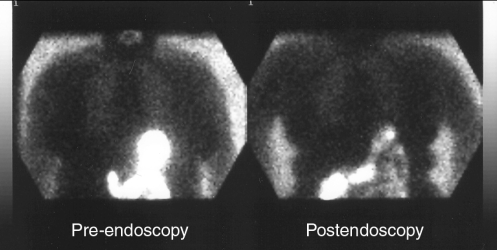
The indications for endoscopy, the endoscopic findings, the volume of fluid aspirated through the endoscope, the duration of endoscopy and the time between the initial scan and the endoscopy are outlined in Table 1. No patient experienced desaturations (less than 90%) during the procedure(s). None of our patients were ‘urgent’ as defined by the need to perform endoscopy within the first 6 h. There were six semiurgent cases (endoscopy required within 24 h), and the remainder were nonurgent cases. Nine patients had significant comorbidities –four had noninsulin-dependent diabetes, three had hepatic cirrhosis, one had chronic obstructive airways disease and one had lymphoma. It had been our intention to include as many urgent endoscopies as possible, but the delays inherent in performing the scans and the absence of facilities open after working hours for such a scan precluded this. On careful review of the scans, no evidence of aspiration was found in any of the patients. A representative study is shown in Figure 1. Even when the sensitivity of the imaging was reduced to 1%, significant amounts of the tracer were visible in the GI tract. This suggests that any aspiration approaching 1% of the administered dose would have been detected in the lung fields. The mean volume of fluid aspirated during endoscopy was 41 mL (range 0 mL to 150 mL) and the mean calculated radioactivity of this fluid was 5.32 MBq (range 0 MBq to 20.6 MBq), 2.66% of the administered dose. No significant thyroid activity was visible on the images, confirming that none of the tracer was absorbed.
Figure 1).
Nuclear scans performed immediately before endoscopy and after endoscopy demonstrating radioactive material in the stomach (left) and subsequently in the small intestine (right). There is an absence of radioactivity in the lung fields in both images
DISCUSSION
Prout and Metreweli (4) first drew attention to the possibility of aspiration occurring during endoscopy in 1972, when they showed that 25% of patients developed CXR changes consistent with aspiration following administration of oral lipiodol in patients undergoing endoscopy. The implications of the study, in terms of aspiration of gastric contents into the lungs, are limited, given the fact that the x-ray contrast medium was given orally and not placed into the stomach. In addition, the endoscopes used were semirigid, the procedures were much longer and the doses of sedative medications correspondingly were much higher. A number of retrospective surveys (5–9) have reported aspiration to be a rare and sometimes fatal complication of endoscopy. Estimates of incidence have varied between one of 9875 procedures (Texas, USA) (7) and one of 184 emergency endoscopies (Paris) (8). All these surveys reported at least one death attributed to aspiration, except the one from Texas (7). It is possible that these surveys underestimated the true incidence of aspiration, because they relied solely on the recall of the participating endoscopists; although equally, some of the episodes attributed to aspiration may have stemmed from other causes of sudden cardiorespiratory deterioration, such as pulmonary emboli or myocardial infarction, particularly because no diagnostic criteria for aspiration were given in any of the studies.
It was not until 1991 that studies using pre- and postendoscopy CXRs were published. Lipper et al (1) reported that six of 30 intensive care patients with active, severe GI bleeding developed new CXR shadowing in their left lung field within 4 h of endoscopy when compared with a CXR taken in the 12 h before endoscopy. Five of these six patients subsequently developed leukocytosis and fever, and five of the six also developed significant desaturation (less than 90%) during endoscopy. The longer term outcomes of the patients were not reported. In another pre- and postendoscopy CXR study involving a total of 220 GI bleeders in an intensive care unit, Rudolph et al (2) reported a 14% incidence of new CXR changes following endoscopy for GI bleeding in an intensive care unit. Interestingly, there was no evidence that securing the airway by intubation led to a decrease in the rate of aspiration, other than possibly reducing the rate of fatal, massive aspiration.
The present pilot study, using a very sensitive technique, failed to show any evidence of aspiration in the cohort of 50 patients. This may have been related to the fact that none of our procedures were particularly prolonged (maximum gastroscopy time was 20 min) and that none of the patients had had major upper GI bleeding. It had been our intention to study such cases during working hours but the logistic delays inherent in this approach made this impossible; as well, the nuclear medicine department was geographically remote from the endoscopy area in our hospital (The Canberra Hospital, Australia), and was relatively free of resuscitation personnel and equipment. Therefore, it was decided that the transfer of acutely bleeding patients would have entailed an unacceptable risk. In addition, the preparation of the radiolabelled colloid required advanced notice and would not have normally been performed outside of working hours. The fact that small quantities of radiation (as much as 10% of the administered dose) were detected in the fluid aspirated at endoscopy suggests that the method used has the potential to demonstrate aspirated material, particularly if the process could be streamlined and patients with acute upper GI bleeding could be studied.
SUMMARY
The present study confirms the safety of sedation practices in endoscopy, even in patients at moderate risk who undergo routine or semiurgent procedures. It is also in accordance with earlier observations that clinically significant aspiration is probably much more common in patients who have had very recent major upper GI bleeding. It would be interesting to perform a similar study in patients undergoing longer procedures, such as endoscopic retrograde cholangiopancreatography, or in those undergoing endoscopic ultrasound examinations, in which a good deal of fluid is often injected into the gastric lumen to aid in visualization.
Acknowledgments
The assistance of the nursing staff in the endoscopy unit and the technologists in the nuclear medicine department at The Canberra Hospital is gratefully acknowledged.
REFERENCES
- 1.Lipper B, Simon D, Cerrone F. Pulmonary aspiration during emergency endoscopy in patients with upper gastrointestinal hemorrhage. Crit Care Med. 1991;19:330–3. doi: 10.1097/00003246-199103000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Rudolph SJ, Landsverk BK, Freeman ML. Endotracheal intubation for airway protection during endoscopy for severe upper GI hemorrhage. Gastrointest Endosc. 2003;57:58–61. doi: 10.1067/mge.2003.46. [DOI] [PubMed] [Google Scholar]
- 3.Greenfield S, Webster G, Brar A, Kuan A, Beck E, Vicary R. Drinking before endoscopy is safe. Gut. 1995;36(Suppl 1):T151. [Google Scholar]
- 4.Prout BJ, Metreweli C. Pulmonary aspiration after fibre-endoscopy of the upper gastrointestinal tract. Br Med J. 1972;4:269–71. doi: 10.1136/bmj.4.5835.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schiller KF, Cotton PB, Salmon PR. The hazards of digestive fibre-endoscopy: A survey of British experience. Gut. 1972;13:1027. [PubMed] [Google Scholar]
- 6.Mandelstam P, Sugawa C, Silvis SE, Nebel OT, Rogers BH. Complications associated with esophagogastroduodenoscopy and with esophageal dilation. Gastrointest Endosc. 1974;23:16–9. doi: 10.1016/s0016-5107(76)73568-5. [DOI] [PubMed] [Google Scholar]
- 7.Davis RE, Graham DY. Endoscopic complications: The Texas experience. Gastrointest Endosc. 1979;25:146–9. doi: 10.1016/s0016-5107(79)73405-5. [DOI] [PubMed] [Google Scholar]
- 8.Noel D, Delage Y, Liguory C, Coffin J-C, Bodin F. Hemorragies digestives d’origine haute chez les sujets de plus de 65 ans Apport de l’endoscopie. Nouv Presse Med. 1979;8:589–91. [PubMed] [Google Scholar]
- 9.Gilbert DA, Silverstein FE, Tedesco FJ. National ASGE survey on upper gastrointestinal bleeding: Complications of endoscopy. Dig Dis Sci. 1981;26(7 Suppl):55S–9S. doi: 10.1007/BF01300808. [DOI] [PubMed] [Google Scholar]