Abstract
Changes in renal function were compared in patients receiving oral sodium phosphate (NaP) for colon cleansing and those receiving large-volume polyethylene glycol (PEG) solution to determine whether oral NaP resulted in frequent renal damage that had gone clinically undetected. From 1995 to 2004, a cohort of consecutive patients who had serum creatinine (Cr) drawn immediately before colonoscopy and again after subsequent procedures three months to nine years later (almost 80% of patients between the first and fifth year) were identified. Chronic renal failure (CRF) was defined as an abnormal Cr at repeat measurement or an abnormal Cr clearance as estimated by the Cockroft-Gault equation at the time of repeat Cr measurement. Medications and medical comorbid conditions were recorded. Seven hundred sixty-seven patients (51% female and 49% male; 81% oral NaP and 19% PEG) with normal baseline Cr levels were identified through the endoscopy unit database at the Hotel Dieu Hospital, Queen’s University (Kingston, Ontario). Of these, 55 (7%) developed CRF. Forty-two (6.8%) patients receiving oral NaP developed renal failure compared with 13 patients (8.7%) receiving PEG (Fisher’s exact test; P=0.382), but the magnitude of CRF was small in each group (Cr level lower than 160 μmol/L). Using logistic regression analysis with the choice of preparation, medications and medical comorbid conditions as independent variables, only age and blood pressure were predictive of the development of renal failure (P=0.014 and P=0.001, respectively). Baseline Cr clearance was similiar in both the NaP and PEG groups and the absolute difference after colonoscopy did not differ. The present study concluded that the ingestion of oral NaP for colon cleansing before colonoscopy did not result in frequent renal damage that went clinically undetected.
Keywords: Colon cleansing, Colonoscopy, Polyethylene glycol, Sodium phosphate
Abstract
Les changements de la fonction rénale ont été comparés chez des patients ayant reçu du phosphate de sodium (NaP) oral pour le nettoyage du côlon et chez des patients ayant reçu de forts volumes de solution de polyéthylène glycol (PEG), dans le but de déterminer si le NaP oral a donné lieu à une atteinte rénale fréquente, mais cliniquement passée inaperçue. De 1995 à 2004, une cohorte de patients consécutifs ayant subi un prélèvement pour dosage de la créatinine (Cr) sérique immédiatement avant une coloscopie et à nouveau après des interventions subséquentes, trois mois à neuf ans plus tard (près de 80 % des patients, entre la première et la cinquième année), ont été identifiés. L’insuffisance rénale chronique (IRC) a été définie par un taux de Cr anormal lors d’un contrôle ou par un taux de clairance de la créatinine anormal selon la formule de Cockcroft-Gault au moment du contrôle de Cr. Les médicaments et les comorbidités ont été notés. Sept cent soixante-sept patients (51 % de femmes et 49 % d’hommes : 81 % NaP oral et 19 % PEG) dont les taux de Cr étaient normaux au départ ont été recensés à l’aide de la base de données de l’unité d’endoscopie de l’Hôtel-Dieu, affilié à l’Université Queen’s (Kingston, Ontario). Parmi ces patients, 55 (7 %) ont évolué vers une IRC. Quarante-deux patients (6,8 %) ayant reçu du NaP oral ont présenté une insuffisance rénale, contre 13 patients (8,7 %) ayant reçu le PEG (test exact de Fisher; P = 0,382), mais le degré d’IRC était faible dans chacun des groupes (taux de Cr inférieur à 160 μmol/L). Par analyse de régression logistique et à partir du choix de préparation, des médicaments et des comorbidités comme variables indépendantes, seuls l’âge et la tension artérielle se sont révélés prédicteurs de l’insuffisance rénale (P = 0,014 et P = 0,001, respectivement). La clairance de la Cr de départ était semblable dans les deux groupes (sous NaP et sous PEG) et la différence absolue après la coloscopie n’a pas différé. La présente étude a conclu que la prise orale de NaP pour le nettoyage du côlon avant la coloscopie n’a pas donné lieu à une atteinte rénale fréquente cliniquement passée inaperçue.
Oral sodium phosphate (NaP) is one of the most effective colon cleansing agents for colonoscopy currently available (1–6) and is better tolerated than large-volume polyethylene glycol (PEG) solutions. Although numerous clinical studies (2,7,8) suggest that oral NaP has a good safety profile, it is a small volume, osmotically active solution that causes transient hyperphosphatemia (1,7) and has the potential to induce intravascular volume contraction (1,2,9). There have been reports (2,10) of serious adverse events, including death (11), in a small number of isolated cases with oral NaP, but these adverse events appeared to result from inappropriate dosing, improper patient selection (eg, use in patients with contraindications such as renal failure) and/or poor hydration during the cleansing period (2).
Recently, there have been case reports (12–14) of nephrocalcinosis with renal failure associated with the use of oral NaP. This relationship was first reported in a single case study (14) and subsequently in a case series of 21 patients at a large referral centre (12). These latter cases were identified by reviewing a large renal biopsy database and finding a temporal relationship between nephrocalcinosis and ingestion of oral NaP. The mechanism underlying this relationship is unclear, but these reports raise the concern that there may be more frequent systematic renal damage that went clinically undetected (12,14).
We have a large database of patients who underwent serial colonoscopies for screening and had serum creatinine (Cr) measurements obtained before their colonoscopies. This afforded the opportunity to examine changes in their renal function over time following use of oral NaP. Because some patients received PEG, we could compare changes in renal function following oral NaP use with changes occurring after the use of PEG.
METHOD
The database from the endoscopy unit at the Hotel Dieu Hospital, Queen’s University (Kingston, Ontario), for outpatient colonoscopies performed between 1995 to 2004 was reviewed. Ethics approval was obtained from the Queen’s University School of Medicine Institutional Review Board. All patients who underwent two colonoscopies, three months to nine years apart (with almost 80% of the subsequent procedures between the first and fifth year), and had a serum Cr measured before each colonoscopy were included. These measurements were routinely drawn within two weeks before the procedure in both the initial and follow-up procedure. Patient age, sex, medications, comorbidities and colonic preparation were recorded for each case (Table 1).
TABLE 1.
Patient demographics and risk factors (n=767)
| Variable | Oral NaP n=618 | PEG n=149 | P | Overall |
|---|---|---|---|---|
| Female, n (%) | 324 (52.4) | 64 (42.9) | 0.043 | 388 (50.6) |
| Mean age ± SD, years (range) | 54.1±11.7 (17–85) | 59.2±13.4 (21–88) | <0.001 | 55.1±12.2 (17–88) |
| Medications, n (%) | ||||
| Diuretics | 46 (7.4) | 14 (9.4) | NS | 60 (7.8) |
| Antihypertensives | 43 (7.0) | 38 (25.5) | <0.001 | 81 (10.6) |
| Bisphosphonates | 4 (0.6) | 7 (4.7) | 0.002 | 11 (1.4) |
| NSAIDs | 74 (12.0) | 48 (32.2) | <0.001 | 122 (15.9) |
| 5-ASA | 58 (9.4) | 8 (5.4) | NS | 66 (8.6) |
| Comorbidities, n (%) | ||||
| Diabetes mellitus | 52 (8.4) | 23 (15.4) | 0.013 | 75 (9.8) |
| Coronary artery disease | 57 (9.2) | 28 (18.8) | 0.002 | 85 (11.1) |
| Peripheral vascular disease | 18 (2.9) | 8 (5.4) | NS | 26 (3.4) |
| Kidney stones | 9 (1.5) | 5 (3.4) | NS | 14 (1.8) |
| Chronic kidney disease | 2 (0.3) | 0 (0) | NS | 2 (0.3) |
| Hypertension | 149 (24.1) | 52 (34.9) | 0.009 | 201 (26.2) |
5-ASA 5-Aminosalicylic acid; NaP Sodium phosphate; NS Nonsignificant; NSAIDs Nonsteroidal anti-inflammatory drugs; PEG Polyethylene glycol
Patients received one of two commercially available bowel preparations: oral NaP or oral PEG. For both regimens, patients were instructed to drink clear fluids with their preparation the day before their procedure. The dose of oral NaP prescribed was 45 mL taken at 17:00 and at 22:00. In addition, patients were instructed to drink at least 2 L to 3 L of fluid that day. Patients receiving GoLYTELY (PEG) were required to drink 4 L of preparation in 3 h starting at 18:00 the day before their procedure.
The development of renal insufficiency following ingestion of a cleansing agent was defined in two ways: a serum Cr level above the upper limit of normal at repeat measurement in those patients who had normal baseline serum Cr level (ie, ratio of Cr/baseline Cr greater than one), or an estimated Cr clearance (CrCl) using the Cockroft-Gault equation (15):
Over the course of the present study, the laboratory changed the normal reference value for the upper limit of normal from 80 μmol/L to 110 μmol/L.
Data analysis was performed using SPSS (SPSS Inc, USA) statistical software version 12.0. The comparison of continuous variables was performed using Student’s t test. Categorical variables were compared using the Fisher’s exact test. Multivariate logistic regression was used to determine which variables contributed to the development of renal insufficiency in the study population.
RESULTS
Patient characteristics
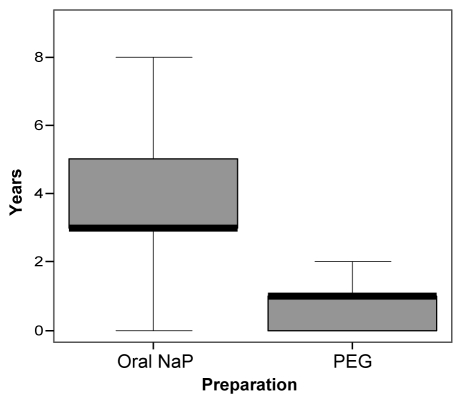
A total of 767 patients (n=618 for oral NaP, n=149 for PEG) with complete data were identified from the database at the Hotel Dieu Hospital between 1995 and 2004. Patients received oral NaP or PEG depending on the preference of the clinician, the patient, or if there was a contraindication to oral NaP. To mitigate against selection bias, 75 PEG patients were identified in 2002 and 2003, at a time when PEG was used almost exclusively in the endoscopy unit. Nonetheless, comparison of their baseline characteristics (Table 1) identified some differences between the groups. There were more PEG patients receiving antihypertensives, bisphosphonates and nonsteroidal anti-inflammatory drugs. Similarly, there were more patients with diabetes, coronary artery disease and hypertension in this group. The mean age of the oral NaP group was slightly younger than the PEG group (54.1±11.7 years versus 59.2±13.4 years, respectively; P<0.001), although the age range was similar. Overall, 50.7% of patients were female. The mean time between colonoscopies in the oral NaP and PEG groups was statistically significant (3.7±1.8 years versus 1.0±1.5 years, respectively). The median time between colonoscopies was three years in the oral NaP group and one year in the PEG group (Figure 1).
Figure 1).
Box plot showing median interval between colonoscopies in the oral sodium phosphate (NaP) and polyethylene glycol (PEG) groups. Bold line represents the median. The median interval was three years (range three months to nine years) in the oral NaP group and one year (range three months to eight years) in the PEG group
Comparison of renal function in oral NaP and PEG groups
Renal function, assessed by serum Cr measurements and estimated CrCl determined at baseline (ie, before the first colonoscopy) and at the second measurement (ie, before the second colonoscopy), was compared for the two groups (Table 2). The baseline mean Cr level was slightly higher in the oral NaP group than in the PEG group, but the difference of 3 μmol/L is minimal and not significant. Moreover, CrCl calculated using the Cockroft-Gault equation was similar between oral NaP and PEG preparations (101.9 mL/min versus 101.1 mL/min, respectively; P=0.807). Repeat Cr measurements were obtained three months to nine years later (almost 80% were between the first and fifth year). After colon cleansing, the mean serum Cr level and CrCl did not differ between the two groups. The change in serum Cr between baseline and repeat measurement was significantly different, favouring stable renal function in the oral NaP group, but these changes were small and clinically insignificant. Furthermore, the mean change in CrCl from baseline to repeat measurement was not statistically significant and remained within the normal range.
TABLE 2.
Renal function of all patients at baseline and at repeat measurement (n=767)
| Preparation | n | Mean ± SD | P | |
|---|---|---|---|---|
| Baseline creatinine, (μmol/L) | Oral NaP | 618 | 78.3±15.7 | 0.027 |
| PEG | 149 | 75.1±15.1 | ||
| Repeat creatinine, (μmol/L) | Oral NaP | 618 | 76.3±17.2 | NS |
| PEG | 149 | 76.0±17.5 | ||
| Baseline creatinine clearance, (mL/min) | Oral NaP | 618 | 101.9±32.7 | NS |
| PEG | 149 | 101.1±33.3 | ||
| Repeat creatinine clearance, (mL/min) | Oral NaP | 618 | 102.8±36.1 | NS |
| PEG | 149 | 99.3±33.2 | ||
| Change in creatinine concentration, (μmol/L) | Oral NaP | 618 | –2.0±11.0 | 0.005 |
| PEG | 149 | 0.9±11.7 | ||
| Change in creatinine clearance, (mL/min) | Oral NaP | 618 | 0.9±18.1 | NS |
| PEG | 149 | –1.8±16.2 |
Only baseline creatinine measurement and changes in creatinine concentrations were significantly different between oral sodium phosphate (NaP) and polyethylene glycol (PEG) groups. However, these changes were small and clinically nonsignificant (NS)
Comparison of the development of renal insufficiency in oral NaP and PEG groups
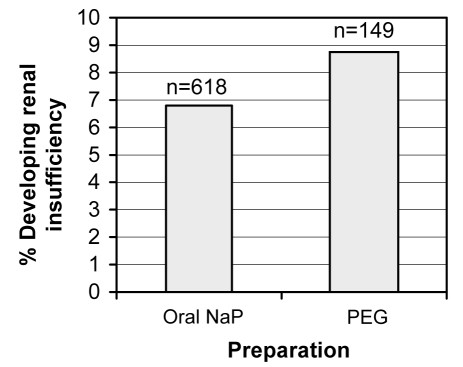
Of the 767 patients identified, 55 developed renal insufficiency based on our criteria. Only a small proportion of patients exhibited abnormal renal function in each group (6.8% of patients in the oral NaP group versus 8.7% of patients in the PEG group; P=0.382), and there was no difference between the groups (Figure 2). All these patients had a normal serum Cr level and estimated CrCl at baseline, and there were no differences between the two groups in mean serum Cr level or CrCl at baseline or following colon cleansing (Table 3).
Figure 2).
Proportion of patients developing renal insufficiency in the oral sodium phosphate (NaP) and polyethylene glycol (PEG) groups as defined by a repeat serum creatinine measurement above the upper limit of normal (6.8% versus 8.7%, respectively; P=0.382). There was no difference in the proportion of patients developing renal insufficiency with regard to the preparation used
TABLE 3.
Renal function in 55 patients developing renal insufficiency at baseline and at repeat measurement
| Preparation | n | Mean ± SD | P | |
|---|---|---|---|---|
| Baseline creatinine, (μmol/L) | Oral NaP | 42 | 93.7±18.0 | NS |
| PEG | 13 | 83.8±16.0 | ||
| Repeat creatinine, (μmol/L) | Oral NaP | 42 | 106.1±22.0 | NS |
| PEG | 13 | 102.3±22.2 | ||
| Baseline creatinine clearance, (mL/min) | Oral NaP | 42 | 78.6±17.0 | NS |
| PEG | 13 | 87.2±21.0 | ||
| Repeat creatinine clearance, (mL/min) | Oral NaP | 42 | 66.2±17.2 | NS |
| PEG | 13 | 71.4±16.5 | ||
| Change in creatinine concentration, (μmol/L) | Oral NaP | 42 | 12.4±15.0 | NS |
| PEG | 13 | 18.5±17.4 | ||
| Change in creatinine clearance, (mL/min) | Oral NaP | 42 | –12.4±13.1 | NS |
| PEG | 13 | –15.8±14.4 |
No significant differences were found between oral sodium phosphate (NaP) and polyethylene glycol (PEG) groups with regard to their renal function both at baseline and at repeat measurement. NS Nonsignificant
When medications were compared in this subset of oral NaP and PEG patients, only antihypertensive medication use was different (11.9% versus 46.2%, respectively; P=0.014). When this group of patients was compared with the cohort of patients who did not develop renal failure, there were significantly more patients in the renal insufficiency group who were on diuretics and antihypertensives. There were also significantly more patients with renal insufficiency who had hypertension or coronary artery disease.
A multiple logistic analysis was performed with development of renal insufficiency (ie, Cr ratio greater than one) as the dependant variable. The following factors were analyzed: preparation used, age, sex, comorbid conditions and medications, as listed in Table 1. The only predictors of the development of renal failure were age (P=0.014) and hypertension (P=0.001). The variables in this model predicted the development of renal insufficiency in 93% of our cases. Preparation used was not an independent predictor of the development of renal insufficiency.
Comparison of the magnitude of renal insufficiency in the oral NaP and PEG groups
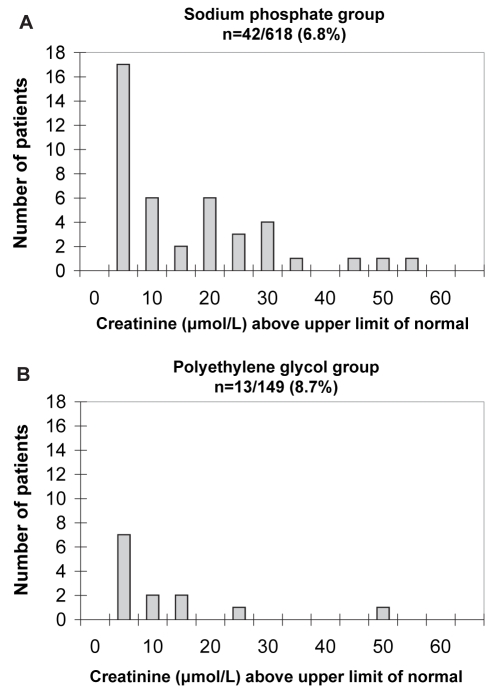
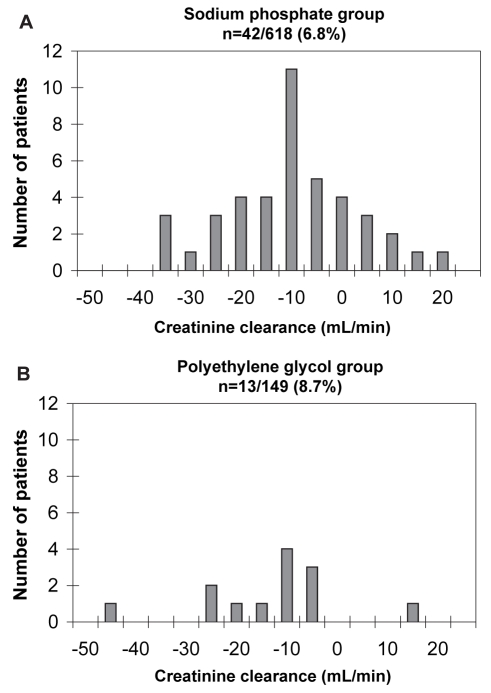
In the subset of patients (n=55) who developed renal insufficiency, the serum Cr increase above the upper limit of normal was small, and the distribution and magnitude did not differ between the oral NaP and PEG groups (Figure 3). Similar results were obtained when the changes in estimated CrCl from baseline were compared for the two groups (Figure 4).
Figure 3).
Elevation of creatinine above the upper limit of normal in oral sodium phosphate (A) and polyethylene glycol (B) groups developing renal insufficiency. The magnitude of the change in creatinine concentration in both groups was small and insignificant (13.9 μmol/L versus 10.3 μmol/L in the oral sodium phosphate and polyethylene glycol groups, respectively; P=0.403)
Figure 4).
Change in the creatinine clearance in oral sodium phosphate (A) and polyethylene glycol (B) groups developing renal insufficiency. The magnitude of the change in creatinine clearance in both groups was small and insignificant (−12.4 mL/min versus −15.8 mL/min in the oral sodium phosphate and polyethylene glycol groups, respectively; P=0.427)
DISCUSSION
The present study examined changes in renal function in patients who had received oral NaP as a colon cleansing agent before colonoscopy and compared them with a group who received PEG. Two major findings emerged from the present study – first, the proportion of patients developing abnormal Cr values over a three-month to nine-year period in a large cohort of patients after receiving oral NaP was small (6.8%) and did not differ from those receiving PEG (8.7%); second, the magnitude of the abnormalities was small (ie, the peak Cr level in both groups was less than 160 μmol/L). Furthermore, Cr measurements were drawn farther apart in the oral NaP group than in the PEG group (median three years versus one year). This would bias the results toward worsening renal function in the oral NaP group given the longer interval to develop renal insufficiency in those patients with predisposing comorbidities (eg, hypertension). Nonetheless, this worsening renal function was not observed in the oral NaP group.
Our analysis of the medications and comorbidities of the oral NaP and PEG groups demonstrated that some differences existed between these groups (Table 1). Given that hypertension and associated medications, coronary artery disease and diabetes mellitus are risk factors for renal failure, it raises the possibility that these differences may confound the findings (ie, increase the rate of renal insufficiency in the PEG group). We did, however, find that the estimated baseline CrCl rates were not different between the two groups, which would argue that renal function at baseline was, in fact, similar between the groups. Nonetheless, given the potential confounders, we performed multivariate analysis to determine independent risk factors. This demonstrated that preparation alone cannot predict the development of renal failure. In view of these findings, as well as the relatively small numbers of patients affected, we concluded that oral NaP does not result in frequent, preparation-specific renal damage that had gone clinically undetected. However, given the limitations of our data set, we cannot exclude the possibility that oral NaP does lead to subclinical renal damage in a very small proportion of patients.
A recent preliminary report (16) provides further support for the notion that oral NaP does not result in widespread, subclinical renal damage. In this study, the relationship between ingestion of oral NaP and coincident use of angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs) on renal function was examined prospectively in 191 patients. They did not find any abnormalities in serum Cr levels following ingestion of oral NaP, regardless of whether the patients were taking ACEIs or ARBs. However, patients were strongly encouraged to drink fluids during the cleansing period, as were patients in our study, to maintain hydration. The hydration status of patients in previous case reports (2,12,14) of renal failure is almost always unknown. It seems prudent to encourage ingestion of fluids, including oral rehydration solutions, given that oral NaP has the potential to induce intravascular volume contraction (17).
Oral hydration may be particularly important in patients with underlying renal disease or in those on medications that may alter renal blood flow. The mechanism linking the recent reports of nephrocalcinosis, renal failure and ingestion of oral NaP (12) is currently unknown. However, most of the patients (16 of 21) were hypertensive, and 14 were either taking ACEIs or ARBs, while four were on diuretics. It is possible that these patients were predisposed because of their medications and that inadequate oral hydration was a link in at least some of these cases. Our multivariate analysis also identified that age and hypertension were independent predictors of renal failure. The variables in our logistic regression model correctly predicted the presence or absence of renal insufficiency in 93% of the cases. These are well-established risk factors for renal disease (5,18,19). However, when taken together with recent reports of nephrocalcinosis and renal failure, it seems prudent to recommend that particularly careful attention be applied to hydration in patients with risk factors and drugs that may compromise renal function.
SUMMARY
The present retrospective study demonstrated that ingestion of oral NaP before colonoscopy had not resulted in widespread renal damage that had gone clinically undetected. Nonetheless, careful attention must be taken with patient selection (contraindicated in patients with renal failure, symptomatic ischemic heart disease, congestive heart failure, ascites and ileus; caution in those at extremes of age and those with hypertension or multiple serious comorbidities) and appropriate dosing of oral NaP (45 mL dose taken twice daily, 6 h to 12 h apart) to prevent renal toxicity. There is growing evidence that adequate hydration (at least 2 L to 3 L of clear fluid or oral rehydration solution throughout the cleansing period) is important during colon cleansing with oral NaP.
Footnotes
CONFLICT OF INTEREST DISCLOSURE: The present investigator-driven study was supported by an unrestricted educational grant from Fleet Laboratories, USA. Dr Vanner has served on the speaker bureau, and both Dr Vanner and Dr Depew have a patent pending with this company on oral rehydration solutions and oral sodium phosphate.
REFERENCES
- 1.Vanner SJ, MacDonald PH, Paterson WG, Prentice RS, Da Costa LR, Beck IT. A randomized prospective trial comparing oral sodium phosphate with standard polyethylene glycol-based lavage solution (Golytely) in the preparation of patients for colonoscopy. Am J Gastroenterol. 1990;85:422–7. [PubMed] [Google Scholar]
- 2.Hookey LC, Depew WT, Vanner S. The safety profile of oral sodium phosphate for colonic cleansing before colonoscopy in adults. Gastrointest Endosc. 2002;56:895–902. doi: 10.1067/mge.2002.129522. [DOI] [PubMed] [Google Scholar]
- 3.Hsu CW, Imperiale TF. Meta-analysis and cost comparison of polyethylene glycol lavage versus sodium phosphate for colonoscopy preparation. Gastrointest Endosc. 1998;48:276–82. doi: 10.1016/s0016-5107(98)70191-9. [DOI] [PubMed] [Google Scholar]
- 4.Cohen SM, Wexner SD, Binderow SR, et al. Prospective, randomized, endoscopic-blinded trial comparing precolonoscopy bowel cleansing methods. Dis Colon Rectum. 1994;37:689–96. doi: 10.1007/BF02054413. [DOI] [PubMed] [Google Scholar]
- 5.Haroun MK, Jaar BG, Hoffman SC, Comstock GW, Klag MJ, Coresh J. Risk factors for chronic kidney disease: A prospective study of 23,534 men and women in Washington County, Maryland. J Am Soc Nephrol. 2003;14:2934–41. doi: 10.1097/01.asn.0000095249.99803.85. [DOI] [PubMed] [Google Scholar]
- 6.Kolts BE, Lyles WE, Achem SR, Burton L, Geller AJ, MacMath T. A comparison of the effectiveness and patient tolerance of oral sodium phosphate, castor oil, and standard electrolyte lavage for colonoscopy or sigmoidoscopy preparation. Am J Gastroenterol. 1993;88:1218–23. [PubMed] [Google Scholar]
- 7.Huynh T, Vanner S, Paterson W. Safety profile of 5-h oral sodium phosphate regimen for colonoscopy cleansing: Lack of clinically significant hypocalcemia or hypovolemia. Am J Gastroenterol. 1995;90:104–7. [PubMed] [Google Scholar]
- 8.Lieberman DA, Ghormley J, Flora K. Effect of oral sodium phosphate colon preparation on serum electrolytes in patients with normal serum creatinine. Gastrointest Endosc. 1996;43:467–9. doi: 10.1016/s0016-5107(96)70287-0. [DOI] [PubMed] [Google Scholar]
- 9.Chan A, Depew W, Vanner S. Use of oral sodium phosphate colonic lavage solution by Canadian colonoscopists: Pitfalls and complications. Can J Gastroenterol. 1997;11:334–8. doi: 10.1155/1997/797486. [DOI] [PubMed] [Google Scholar]
- 10.Hookey LC, Vanner S. Recognizing the clinical contraindications to the use of oral sodium phosphate for colon cleansing: A case study. Can J Gastroenterol. 2004;18:455–8. doi: 10.1155/2004/787515. [DOI] [PubMed] [Google Scholar]
- 11.Tan HL, Liew QY, Loo S, Hawkins R. Severe hyperphosphataemia and associated electrolyte and metabolic derangement following the administration of sodium phosphate for bowel preparation. Anaesthesia. 2002;57:478–83. doi: 10.1046/j.0003-2409.2001.02519.x. [DOI] [PubMed] [Google Scholar]
- 12.Markowitz GS, Stokes MB, Radhakrishnan J, D’Agati VD. Acute phosphate nephropathy following oral sodium phosphate bowel purgative: An underrecognized cause of chronic renal failure. J Am Soc Nephrol. 2005;16:3389–96. doi: 10.1681/ASN.2005050496. [DOI] [PubMed] [Google Scholar]
- 13.Markowitz GS, Nasr SH, Klein P, et al. Renal failure due to acute nephrocalcinosis following oral sodium phosphate bowel cleansing. Hum Pathol. 2004;35:675–84. doi: 10.1016/j.humpath.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Desmeules S, Bergeron MJ, Isenring P. Acute phosphate nephropathy and renal failure. N Engl J Med. 2003;349:1006–7. doi: 10.1056/NEJM200309043491020. [DOI] [PubMed] [Google Scholar]
- 15.Gault MH, Longerich LL, Harnett JD, Wesolowski C. Predicting glomerular function from adjusted serum creatinine. Nephron. 1992;62:249–56. doi: 10.1159/000187054. [DOI] [PubMed] [Google Scholar]
- 16.Balaban DH, Harlan WR, Beavers KL, Moe SM, Thompson WO, Pambianco DJ.Multicenter longitudinal evaluation of the renal effects of sodium phosphate bowel preparation Am J Gastroenterol 2005100A968(Abst) [Google Scholar]
- 17.Barclay RL, Depew WT, Vanner SJ. Carbohydrate-electrolyte rehydration protects against intravascular volume contraction during colonic cleansing with orally administered sodium phosphate. Gastrointest Endosc. 2002;56:633–8. doi: 10.1067/mge.2002.129221. [DOI] [PubMed] [Google Scholar]
- 18.Hunsicker LG, Adler S, Caggiula A, et al. Predictors of the progression of renal disease in the Modification of Diet in Renal Disease Study. Kidney Int. 1997;51:1908–19. doi: 10.1038/ki.1997.260. [DOI] [PubMed] [Google Scholar]
- 19.Ruggenenti P, Perna A, Mosconi L, Pisoni R, Remuzzi G. Urinary protein excretion rate is the best independent predictor of ESRF in non-diabetic proteinuric chronic nephropathies. “Gruppo Italiano di Studi Epidemiologici in Nefrologia” (GISEN) Kidney Int. 1998;53:1209–16. doi: 10.1046/j.1523-1755.1998.00874.x. [DOI] [PubMed] [Google Scholar]