Abstract
BACKGROUND AND AIM:
Helicobacter pylori treatment success rates have varied. A systematic review of the success rate of anti-H pylori therapy in Canada was performed.
METHODS:
All clinical trials containing Canadian data on the success rate of H pylori treatment were identified using MEDLINE searches, through review of references of retrieved studies and by contacting key investigators. Both randomized and open-label trials were included. Treatment effect size was calculated using a variation of Cochran’s Q method.
RESULTS:
Seventeen papers met the inclusion criteria. Both triple therapies consisting of a proton pump inhibitor (PPI), clarithromycin and either amoxicillin or metronidazole performed well, achieving a success rate of 84% and 82%, respectively. The cure rate of PPI-amoxicillin + metronidazole was 76%. Quadruple therapy consisting of a PPI, bismuth, metronidazole and tetracycline, given for seven to 10 days, achieved a success rate of 87%.
CONCLUSION:
Both PPI-based triple therapy and quadruple therapy perform well in Canada for the treatment of H pylori infection.
Keywords: Amoxicillin, Bismuth, Canada, Clarithromycin, Eradication, Helicobacter pylori, Meta-analysis, Metronidazole, Proton pump inhibitors, RCT, Treatment
Abstract
HISTORIQUE ET BUT :
Les taux de réussite des traitements contre Helicobacter pylori ont varié. C’est pourquoi une revue systématique des taux de réussite des traitements anti-H. pylori au Canada a été réalisée.
MÉTHODES :
Tous les essais cliniques comprenant des données canadiennes sur les taux de réussite du traitement anti-H. pylori ont été recensés au moyen du réseau Medline, d’une revue des bibliographies des études et par contact avec les principaux investigateurs. Tant les essais randomisés que les essais ouverts ont été inclus. La taille de l’effet du traitement a été calculée à l’aide d'une version modifiée de la méthode Q de Cochran.
RÉSULTATS :
Dix-sept articles répondaient aux critères d’inclusion. Les trithérapies comportant un inhibiteur de la pompe à protons (IPP), la clarithromycine et soit l’amoxicilline, soit le métronidazole, ont donné de bons résultats avec un taux de réussite de 84 % et de 82 %, respectivement. Le taux de guérison obtenu avec IPP-amoxicilline + métronidazole a été de 76 %. La quadrithérapie comportant un IPP, du bismuth, du métronidazole et de la tétracycline administrée pendant sept à dix jours a donné lieu à un taux de réussite de 87 %.
CONCLUSION :
La trithérapie et la quadrithérapie à base d’IPP ont donné de bons résultats au Canada pour le traitement de l'infection à H. pylori.
Helicobacter pylori is causally associated with gastritis, duodenal and gastric ulcers, and gastric cancer (1,2). Cure of the infection may also improve symptoms in a small proportion of patients presenting with dyspepsia (3). There is a consensus that all patients known to be infected should be offered treatment (4,5). In Canada, the current recommended first-line therapy is proton pump inhibitor (PPI)-based triple therapy with clarithromycin and either amoxicillin or metronidazole (3). Quadruple therapy consisting of a PPI, bismuth, metronidazole and tetracycline (PPI-BMT) is the best tested second-line therapy and has also been recommended as an alternative first-line regimen (4–6). However, for quadruple therapy there are concerns about patient compliance due to the higher number of pills in the regimen. The primary objective of the present meta-analysis was to determine the success rate of H pylori therapies in Canada. The secondary objective was to determine whether there is a difference in adherence to therapy between triple and quadruple therapies.
METHODS
A search was conducted using PubMed in January 2005. Search terms included ‘Canada’ and ‘Canadian’, in combination with variations of ‘Helicobacter pylori’ and treatment key words eradication, cure and treatment. The names of Canadian investigators in the Helicobacter field and current H pylori regimen drug names were also used as search terms. Selected Canadian authors were consulted to ensure no eligible studies were missed. Additionally, a manual reference review of retrieved studies was conducted.
Included studies had to be clinical trials containing Canadian data on H pylori eradication rates, in which one of the main objectives was to assess cure rates of infection in adults. Both randomized controlled trials (RCTs) and open-label or single-regimen trials were included.
Studies were reviewed independently by each author. The following data were extracted – study type (eg, RCT or open-label), type of patient enrolled (eg, those with ulcers or previous eradication attempts), H pylori testing methods, treatment regimen composition, and intent to treat and per protocol eradication rates, with 95% CIs where available. For multinational trials, the corresponding author was contacted to obtain the results of Canadian patients enrolled.
Treatment regimens were grouped into six categories – dual (two antibiotics), bismuth dual (bismuth + one antibiotic), PPI dual (PPI + one antibiotic), bismuth triple (bismuth + two antibiotics), PPI triple (PPI + two antibiotics) and bismuth quadruple (bismuth + PPI + two antibiotics) therapies. PPI triple therapies were further divided into PPI-clarithromycin + amoxicillin (PPI-CA), PPI-clarithromycin + metronidazole (PPI-CM) and PPI-amoxicillin + metronidazole (PPI-AM). Bismuth quadruple therapy consisted of PPI-BMT.
Trials were compared for eradication rates using Einarson’s (7) random effects model for point estimates of single groups, which is based on the method of DerSimonian and Laird (8), which was adapted from Cochran (9). Adherence to therapy was examined where data were available. Adherence was defined as taking anywhere between 75% and 100% of the study medication, but most commonly as taking greater than 75% of the study medication. Regimens were examined to determine whether there was a difference in adherence between triple and quadruple therapy regimens.
RESULTS
The initial search produced 205 papers, 13 of which were retrieved. One hundred ninety-two citations were excluded because they did not report on primary treatment results in Canada. Additional searching, manual reference reviews and author consultations produced 14 additional papers, resulting in 27 papers being retrieved for initial review. Of these, 10 papers were excluded – one did not present novel data, five did not present H pylori treatment data, three dealt with a pediatric population and Canadian data were unobtainable for one study (10). The key characteristics of the included papers are presented in Table 1. There were one dual therapy, one PPI dual, two bismuth dual, three bismuth triple, eight PPI-CA, nine PPI-CM, five PPI-AM and three PPI-BMT treatment regimens.
TABLE 1.
Key characteristics of papers used in meta-analysis
| References | Type of trial | Active ulcers? | Previous eradication attempts | Duration (days) |
|---|---|---|---|---|
| Veldhuyzen van Zanten et al (11) | RCT, OL | No | Yes | 14 |
| Veldhuyzen van Zanten et al (12) | RCT, OL | No | One | 7 |
| Chiba (13) | RCT, OL | Mix | No | 14 |
| Fallone et al (14) | RCT, DB | Yes | Yes | 10 |
| Bardhan et al (15) | RCT, DB | No | One | 7 |
| Lind et al (16) | RCT, DB | Mix | No | 7 |
| Laine et al (17) | RCT, OL | Yes | No | 10 |
| Zanten et al (18) | RCT, DB | Yes | One | 7 |
| Veldhuyzen van Zanten et al (19) | RCT, DB | No | One | 7 |
| Veldhuyzen van Zanten et al (20) | RCT, DB | No | No | 7 |
| Veldhuyzen van Zanten et al (21) | RCT, DB | Mix | One | 7 |
| Chiba et al (22) | RCT, DB | No | Yes | 7 |
| Chiba and Marshall (23) | RCT, OL | Mix | Yes | 7 |
| O’Morain et al (24) | OL | Mix | Yes | 10 |
| Lahaie et al (25) | OL | Mix | No | 7 |
| Veldhuyzen Van Zanten et al (41) | RCT, DB | No | No | 14 |
| Jacobson et al (42) | OL | Mix | Yes | 7 |
DB Double-blinded; OL Open-label; RCT Randomized controlled trial
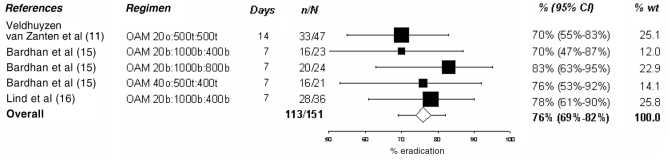
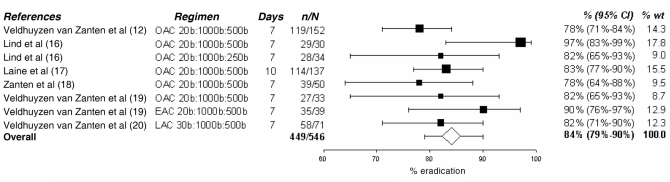
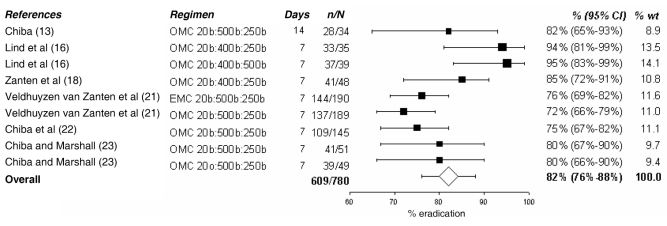
In the present analysis, only PPI triple and quadruple therapies were examined in depth because they are the current standard of care. The tables and figures summarize the various regimens; the doses of medication, their duration and the observed success rate are presented. Dual therapies varied in efficacy between 58% and 66% (11–14) as shown in Table 2. Various PPI-AM regimens were found to have an efficacy of 70% to 83% (11,15,16), with an overall efficacy of 76% (95% CI 69% to 82%) as shown in Figure 1. Six studies (12,16–20) presented PPI-CA regimens with efficacies ranging from 78% to 97%. The overall efficacy, as shown in Figure 2, was 84% (95% CI 79% to 90%). Treatment with a PPI-CM regimen achieved an H pylori cure in 72% to 95% of patients with an overall efficacy of 82% (95% CI 76% to 88%) as shown in Figure 3 (13,16,18,21–23). The overall efficacy of quadruple therapy in three open-label trials was 87% (95% CI 80% to 95%) and is shown in Figure 4 (17,24,25). Two studies using omeprazole-BMT quadruple therapy for 10 days achieved a cure in 88% (17) and 94% (24) of patients, while the third study (25), which used a seven-day course with lower doses (250 mg versus 500 mg) of metronidazole and tetracycline, had an 80% efficacy. There was no significant difference in success rates among PPI-AM, PPI-CM, PPI-CA and PPI-BMT.
TABLE 2.
Dual therapy data
| References | Regimen | Duration (days) | # cure/# ITT (%) | 95% CI |
|---|---|---|---|---|
| Veldhuyzen van Zanten et al (11) | AM 500t:500t | 14 | 31/47 (66) | 51%–79% |
| Veldhuyzen van Zanten et al (12) | RC 400b:500b | 7 | 101/153 (66) | 59%–74% |
| Chiba (13) | OC 20b:250b | 14 | 18/31 (58) | 37%–75% |
| Fallone et al (14) | BM 120b:500t | 10 | 31/47 (66) | 51%–79% |
A Amoxicillin; b Twice a day; B Bismuth; C Clarithromycin; ITT Intent to treat; M Metronidazole; O Omeprazole; R Rifabutin; t Three times a day
Figure 1).
Cure rate with proton pump inhibitor – amoxicillin (A) + metronidazole (M). % wt Percentage of meta-analytic weight given to each regimen; b Twice a day; o Once a day; O Omeprazole; t Three times a day
Figure 2).
Cure rate with proton pump inhibitor – clarithromycin (C) + amoxicillin (A). % wt Percentage of meta-analytic weight given to each regimen; b Twice a day; E Esomeprazole; L Lansoprazole; O Omeprazole
Figure 3).
Cure rate with proton pump inhibitor – clarithromycin (C) + metronidazole (M). % wt Percentage of meta-analytic weight given to each regimen; b Twice a day; E Esomeprazole; o Once a day; O Omeprazole
Figure 4).
Cure rate with proton pump inhibitor – bismuth (B) + metronidazole (M) + tetracycline (T). % wt Percentage of meta-analytic weight given to each regimen; b Twice a day; g Gram; O Omeprazole; q Four times a day
Eight papers (11–13,17,19,20,22,23) provided adherence data for triple therapy and two papers provided adherence data for quadruple therapy (17,24) regimens. Data are presented in Table 3. Adherence to quadruple therapy was high and not different from that of triple therapy.
TABLE 3.
Adherence (adh) to therapy
| Reference | Triple adh/total | % | Quadruple adh/total | % | Adh definition |
|---|---|---|---|---|---|
| Veldhuyzen van Zanten et al (11) | 41/47 | 87 | – | – | >75% |
| Veldhuyzen van Zanten et al (12) | 128/152 | 84 | – | – | 100% |
| Chiba (13) | 31/34 | 91 | – | – | >80% |
| Laine et al (17) | 129/137 | 94 | 126/138 | 91 | >75% |
| Veldhuyzen van Zanten et al (19) | 375/379 | 99 | – | – | >75% |
| Veldhuyzen van Zanten et al (20) | 63/75 | 84 | – | – | 100% |
| Chiba et al (22) | 138/145 | 95 | – | – | >12/14 doses |
| Chiba and Marshall (23) | 94/100 | 94 | – | – | 100% |
| O’Morain et al (24) | 162/170 | 95 | >75% |
DISCUSSION
Treatment of H pylori infection is now recommended for any patient known to be infected. Over the past 10 years, triple therapy consisting of PPI-CA or PPI-CM, given twice daily, has emerged as first-line therapy because it achieves the highest cure rates of the infection (4–6). Quadruple therapy consisting of PPI-BMT also achieves high success rates. However, given that it is a more complex regimen and the number and frequency of pills to be taken by the patient are high, quadruple therapy tends to be reserved as an alternative choice for patients who cannot take the PPI triple therapy because of drug allergies or known resistance to clarithromycin or metronidazole. For all triple therapies, medication is taken twice a day. For PPI-CM, the dose of clarithromycin is 250 mg and metronidazole is 500 mg administered twice a day. A meta-analysis (26) has shown no difference between 250 mg of clarithromycin taken twice a day and 500 mg of clarithromycin taken twice a day. This is in contrast to results from PPI-CA, where a meta-analysis (27) showed that clarithromycin 500 mg taken twice a day, is superior to 250 mg taken twice a day. For PPI-BMT quadruple therapy, the PPI is given twice a day. In Canada, the bismuth preparation used is bismuth subsalicylate (Pepto-Bismol, Procter & Gamble Inc, Canada), two tablets given four times a day. Both the metronidazole and tetracycline dose have varied from 250 mg to 500 mg, two to four times a day. For both metronidazole and tetracycline, the frequency of side effects is said to be higher compared with the frequency using triple therapy, although there is no evidence that it affects adherence to therapy (28). There is some evidence that higher doses of metronidazole are better able to overcome metronidazole resistance (29,30). In contrast with resistance to clarithromycin, metronidazole resistance is not an absolute phenomenon because adding metronidazole to regimens for patients with known metronidazole-resistant strains achieves a higher cure rate compared with the same regimen without metronidazole (30,31).
Consensus guidelines have recommended a target success rate of 80% for an anti-H pylori regimen to be considered (4,5). However, this 80% cure rate was arbitrarily chosen and not based on any scientific rationale. An 80% cure rate may not be achievable in community-based settings. For example, in the Canadian Adult Dyspepsia Empiric Treatment – Helicobacter pylori positive (CADET-Hp) study (22), the rate of cure was 75%. In a recent meta-analysis (28), a small but statistically significant downward trend in cure rates of anti-H pylori treatments was found over time. It is evident from many treatment studies that an 80% cure rate is not consistently achieved (28,32).
In the present systematic review, all published Canadian data from clinical trials evaluating anti-H pylori therapy were collected. The present study confirms that dual therapy with either two antibiotics or a PPI-clarithromycin or bismuthclarithromycin combination therapy achieves success rates that are too low to be recommended. PPI-CA at 84% and PPI-CM at 82% perform well, and their rates were slightly higher than PPI-AM at 76%, although their rates were not statistically significantly different. The number of patients in the combined analysis for PPI-AM is considerably lower than for PPI-CA and PPI-CM. In systematic reviews of global treatment success, PPI-AM has a statistically significant, approximately 10%, lower success rate than PPI-CA or PPI-CM (28).
In Canada, a duration of seven days has been recommended for PPI-CA and PPI-CM. This is in contrast to recommendations from the United States where generally a 10- to 14-day therapy is recommended. There is one large RCT (33) from the United States that showed a similar efficacy for seven- (77%) and 10- (73%) day treatment using rabeprazoleclarithromycin + amoxicillin. Systematic reviews (34,35) have shown that a 10- to 14-day duration of PPI-CA and PPI-CM does improve efficacy by 5% to 7%. However, based on cost-effectiveness data, a seven-day treatment is the preferred option (34). Most Canadian studies have evaluated the PPI omeprazole. Although there are small differences in pH profiles between the different PPIs, a systematic review has not found clinically important differences among the PPIs in achieving cure of H pylori infection when used in triple or quadruple therapies (36).
Quadruple therapy (PPI-BMT) also performs well, achieving a success rate of 86%. The recommended duration of therapy is seven to 14 days, although the total number of patients studied in Canadian trials was relatively small and includes data from open-label studies that did not include a comparative group. In Canada, quadruple therapy has been recommended as an alternative first-line therapy (6). As a second-line treatment, there is convincing evidence that quadruple therapy is superior to an alternate PPI-based triple therapy (28,36,37). For PPI-BMT quadruple therapy, there is evidence based on metaregression that 10- to 14-day treatment is up to 6% more effective than treatments given for four to seven days, although most studies did not compare duration head to head (6).
It has been stated that the side effect profile of PPI-BMT is worse when compared with that of the PPI triple therapies, but this was not confirmed in a systematic review (28). The study showed that side effects of quadruple therapy were similar to the PPI triple therapy, as was adherence to therapy. Whether these results would be replicated in a community-based setting is unknown. Nevertheless, quadruple therapy has been accepted as an alternative first-line therapy option (6).
Our study was limited to adults. Three papers evaluating H pylori treatment in Canadian children have been published (38–40). Only one used an open-label PPI triple regimen (PPI-CM), which cured infection in 14 of 15 (93%, range 68% to 100%) pediatric patients (40). Bismuth-based triple therapies were studied in three papers (14,40,41); however, with the added efficacy demonstrated with the addition of omeprazole, they were not further investigated (6,25,28,42).
It has been well established that resistance of H pylori isolates to antibiotics adversely affects the success rate of therapies. Unfortunately resistance data from across Canada have been very limited. Resistance to metronidazole has been relatively stable at 16% to 20% (43). There are some data that suggests that resistance to clarithromycin is on the rise (44,45). The rate of clarithromycin resistance was less than 3% in 100 studies isolated between 1991 and 1992 (46), but rose to 12% in a nonrandomly selected sample of 200 strains isolated from patients in Halifax, Nova Scotia, between 2000 and 2003 (43). It should be noted that it was not always known in the 200 patients whether the patients had been previously exposed to clarithromycin or other macrolide antibiotics. A recent meta-analysis (28) found that non-nitroimidazole-based regimens were more successful (3% on average) in studies published before 1995, which may reflect an increase in clarithromycin resistance over time. A decrease in treatment efficacy has been documented for strains that are resistant to metronidazole. Resistance is not an absolute phenomenon; it can sometimes be partially overcome even if metronidazole is left in the therapeutic regimen (6,25). In areas where the prevalence of metronidazole resistance was high (greater than 40%), the efficacy of PPI-BMT was reduced by 9% (6). There is, however, convincing evidence that PPI-BMT quadruple therapy is the most efficacious second-line therapy. If indeed clarithromycin resistance is increasing in Canada, current first-line triple therapy will be jeopardized because both PPI-CA and PPI-CM regimens include this antibiotic. For that reason it is important that data are collected to determine the success rate of current anti-H pylori therapies across Canada. Unfortunately to date this is not happening.
Whether a physician will continue to try to eradicate H pylori infections in patients who have failed two treatments is beyond the scope of this discussion and will depend in part on the clinical scenario and the patient’s preference. Several alternative rescue therapies have been evaluated including the combination of PPI-amoxicillin and rifabutin, with which cure rates up to 91% have been reported (47,48). In our own small selected study of 16 patients, the success rate was 63% (10 of 16 patients) (unpublished observations). Several recent studies (47–51) have advocated the use of levofloxacin-based therapy in combination with a PPI and amoxicillin, and reported success rates of 65% to 85%. To date, no Canadian studies have been published evaluating quinolone-based therapies. A potential concern is that primary resistance to quinolones in strains obtained in Halifax is already high at 15% (45). Therefore, before such therapies can be recommended in Canada, efficacy of these regimens needs to be established in Canadian patients.
CONCLUSION
Both triple therapy consisting of PPI-CA and PPI-CM perform well in Canada with high success rates, as does quadruple therapy. The current recommendation for triple therapy is seven days which is most cost-effective, but data from systematic reviews do suggest that increasing the duration to 10 or 14 days slightly improves the success rate. Resistance to antibiotics adversely affects the cure rate of therapy, and Canadian data suggest that clarithromycin resistance may be on the rise. This is a threat to the future success rates of clarithromycin-based triple therapies.
REFERENCES
- 1.Veldhuyzen van Zanten SJ, Sherman PM. Helicobacter pylori infection as a cause of gastritis, duodenal ulcer, gastric cancer and non-ulcer dyspepsia: A systematic overview. CMAJ. 1994;150:177–85. [PMC free article] [PubMed] [Google Scholar]
- 2.Huang JQ, Sridhar S, Chen Y, Hunt RH. Meta-analysis of the relationship between Helicobacter pylori seropositivity and gastric cancer. Gastroenterology. 1998;114:1169–79. doi: 10.1016/s0016-5085(98)70422-6. [DOI] [PubMed] [Google Scholar]
- 3.Moayyedi P, Soo S, Deeks J, et al. Eradication of Helicobacter pylori for non-ulcer dyspepsia. Cochrane Database Syst Rev. 2006;(2):CD002096. doi: 10.1002/14651858.CD002096.pub4. [DOI] [PubMed] [Google Scholar]
- 4.Malfertheiner P, Megraud F, O’Morain C, et al. European Helicobacter Pylori Study Group (EHPSG) Current concepts in the management of Helicobacter pylori infection – the Maastricht 2–2000 Consensus Report. Aliment Pharmacol Ther. 2002;16:167–80. doi: 10.1046/j.1365-2036.2002.01169.x. [DOI] [PubMed] [Google Scholar]
- 5.Hunt R, Fallone C, Veldhuyzen van Zanten S, et al. CHSG 2004 participants Canadian Helicobacter Study Group Consensus Conference: Update on the management of Helicobacter pylori –an evidence-based evaluation of six topics relevant to clinical outcomes in patients evaluated for H pylori infection. Can J Gastroenterol. 2004;18:547–54. doi: 10.1155/2004/326767. [DOI] [PubMed] [Google Scholar]
- 6.Fischbach LA, van Zanten S, Dickason J. Meta-analysis: The efficacy, adverse events, and adherence related to first-line anti-Helicobacter pylori quadruple therapies. Aliment Pharmacol Ther. 2004;20:1071–82. doi: 10.1111/j.1365-2036.2004.02248.x. [DOI] [PubMed] [Google Scholar]
- 7.Einarson TR. Pharmacoeconomic applications of meta-analysis for single groups using antifungal onychomycosis lacquers as an example. Clin Ther. 1997;19:559–69. doi: 10.1016/s0149-2918(97)80140-3. [DOI] [PubMed] [Google Scholar]
- 8.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 9.Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:101–29. [Google Scholar]
- 10.Pare P, Farley A, Romaozinho JM, Bardhan KD, French PC, Roberts PM. Comparison of ranitidine bismuth citrate plus clarithromycin with omeprazole plus clarithromycin for the eradication of Helicobacter pylori. Aliment Pharmacol Ther. 1999;13:1071–8. doi: 10.1046/j.1365-2036.1999.00572.x. [DOI] [PubMed] [Google Scholar]
- 11.Veldhuyzen van Zanten S, Hunt RH, Cockeram A, et al. Adding once-daily omeprazole 20 mg to metronidazole/amoxicillin treatment for Helicobacter pylori gastritis: A randomized, double-blind trial showing the importance of metronidazole resistance. Am J Gastroenterol. 1998;93:5–10. doi: 10.1111/j.1572-0241.1998.005_c.x. [DOI] [PubMed] [Google Scholar]
- 12.Veldhuyzen van Zanten S, Chiba N, Barkun A, et al. A randomized trial comparing seven-day ranitidine bismuth citrate and clarithromycin dual therapy to seven-day omeprazole, clarithromycin and amoxicillin triple therapy for the eradication of Helicobacter pylori. Can J Gastroenterol. 2003;17:533–8. doi: 10.1155/2003/425293. [DOI] [PubMed] [Google Scholar]
- 13.Chiba N. Omeprazole and clarithromycin with and without metronidazole for the eradication of Helicobacter pylori. Am J Gastroenterol. 1996;91:2139–43. [PubMed] [Google Scholar]
- 14.Fallone CA, Loo V, Joseph L, Barkun J, Kostyk R, Barkun AN. Predictors of failure of Helicobacter pylori eradication and predictors of ulcer recurrence: A randomized controlled trial. Clin Invest Med. 1999;22:185–94. [PubMed] [Google Scholar]
- 15.Bardhan K, Bayerdorffer E, Veldhuyzen Van Zanten SJ, et al. The HOMER Study: The effect of increasing the dose of metronidazole when given with omeprazole and amoxicillin to cure Helicobacter pylori infection. Helicobacter. 2000;5:196–201. doi: 10.1046/j.1523-5378.2000.00030.x. [DOI] [PubMed] [Google Scholar]
- 16.Lind T, Veldhuyzen van Zanten S, Unge P, et al. Eradication of Helicobacter pylori using one-week triple therapies combining omeprazole with two antimicrobials: The MACH I Study. Helicobacter. 1996;1:138–44. doi: 10.1111/j.1523-5378.1996.tb00027.x. [DOI] [PubMed] [Google Scholar]
- 17.Laine L, Hunt R, El-Zimaity H, Nguyen B, Osato M, Spenard J. Bismuth-based quadruple therapy using a single capsule of bismuth biskalcitrate, metronidazole, and tetracycline given with omeprazole versus omeprazole, amoxicillin, and clarithromycin for eradication of Helicobacter pylori in duodenal ulcer patients: A prospective, randomized, multicenter, North American trial. Am J Gastroenterol. 2003;98:562–7. doi: 10.1111/j.1572-0241.2003.t01-1-07288.x. [DOI] [PubMed] [Google Scholar]
- 18.Zanten SJ, Bradette M, Farley A, et al. The DU-MACH study: Eradication of Helicobacter pylori and ulcer healing in patients with acute duodenal ulcer using omeprazole based triple therapy. Aliment Pharmacol Ther. 1999;13:289–95. doi: 10.1046/j.1365-2036.1999.00471.x. [DOI] [PubMed] [Google Scholar]
- 19.Veldhuyzen Van Zanten S, Lauritsen K, Delchier JC, et al. One-week triple therapy with esomeprazole provides effective eradication of Helicobacter pylori in duodenal ulcer disease. Aliment Pharmacol Ther. 2000;14:1605–11. doi: 10.1046/j.1365-2036.2000.00911.x. [DOI] [PubMed] [Google Scholar]
- 20.Veldhuyzen van Zanten S, Fedorak RN, Lambert J, Cohen L, Vanjaka A. Absence of symptomatic benefit of lansoprazole, clarithromycin, and amoxicillin triple therapy in eradication of Helicobacter pylori positive, functional (nonulcer) dyspepsia. Am J Gastroenterol. 2003;98:1963–9. doi: 10.1111/j.1572-0241.2003.07583.x. [DOI] [PubMed] [Google Scholar]
- 21.Veldhuyzen Van Zanten S, Machado S, Lee J. One-week triple therapy with esomeprazole, clarithromycin and metronidazole provides effective eradication of Helicobacter pylori infection. Aliment Pharmacol Ther. 2003;17:1381–7. doi: 10.1046/j.1365-2036.2003.01554.x. [DOI] [PubMed] [Google Scholar]
- 22.Chiba N, Van Zanten SJ, Sinclair P, Ferguson RA, Escobedo S, Grace E. Treating Helicobacter pylori infection in primary care patients with uninvestigated dyspepsia: The Canadian adult dyspepsia empiric treatment – Helicobacter pylori positive (CADET-Hp) randomised controlled trial. BMJ. 2002;324:1012–6. doi: 10.1136/bmj.324.7344.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiba N, Marshall CP. Omeprazole once or twice daily with clarithromycin and metronidazole for Helicobacter pylori. Can J Gastroenterol. 2000;14:27–31. doi: 10.1155/2000/916417. [DOI] [PubMed] [Google Scholar]
- 24.O’Morain C, Borody T, Farley A, et al. International multicentre study Efficacy and safety of single-triple capsules of bismuth biskalcitrate, metronidazole and tetracycline, given with omeprazole, for the eradication of Helicobacter pylori: An international multicentre study. Aliment Pharmacol Ther. 2003;17:415–20. doi: 10.1046/j.1365-2036.2003.01434.x. [DOI] [PubMed] [Google Scholar]
- 25.Lahaie R, Farley A, Dallaire C, et al. Bismuth-based quadruple therapy with bismuth subcitrate, metronidazole, tetracycline and omeprazole in the eradication of Helicobacter pylori. Can J Gastroenterol. 2001;15:581–5. doi: 10.1155/2001/305756. [DOI] [PubMed] [Google Scholar]
- 26.Huang J, Hunt RH. The importance of clarithromycin dose in the management of Helicobacter pylori infection: A meta-analysis of triple therapies with a proton pump inhibitor, clarithromycin and amoxycillin or metronidazole. Aliment Pharmacol Ther. 1999;13:719–29. doi: 10.1046/j.1365-2036.1999.00530.x. [DOI] [PubMed] [Google Scholar]
- 27.Gisbert JP, Gonzalez L, Calvet X, et al. Proton pump inhibitor, clarithromycin and either amoxycillin or nitroimidazole: A meta-analysis of eradication of Helicobacter pylori. Aliment Pharmacol Ther. 2000;14:1319–28. doi: 10.1046/j.1365-2036.2000.00844.x. [DOI] [PubMed] [Google Scholar]
- 28.Fischbach LA, Goodman KJ, Feldman M, Aragaki C. Sources of variation of Helicobacter pylori treatment success in adults worldwide: A meta-analysis. Int J Epidemiol. 2002;31:128–39. doi: 10.1093/ije/31.1.128. [DOI] [PubMed] [Google Scholar]
- 29.Dore MP, Leandro G, Realdi G, Sepulveda AR, Graham DY. Effect of pretreatment antibiotic resistance to metronidazole and clarithromycin on outcome of Helicobacter pylori therapy. A meta-analytical approach. Dig Dis Sci. 2000;45:68–76. doi: 10.1023/a:1005457226341. [DOI] [PubMed] [Google Scholar]
- 30.Houben MH, van de Beek D, Hensen EF, Craen AJ, Rauws EA, Tytgat GN. A systematic review of Helicobacter pylori eradication therapy – the impact of antimicrobial resistance on eradication rates. Aliment Pharmacol Ther. 1999;13:1047–55. doi: 10.1046/j.1365-2036.1999.00555.x. [DOI] [PubMed] [Google Scholar]
- 31.van der Hulst RW, van der Ende A, Homan A, Roorda P, Dankert J, Tytgat GN. Influence of metronidazole resistance on efficacy of quadruple therapy for Helicobacter pylori eradication. Gut. 1998;42:166–9. doi: 10.1136/gut.42.2.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laheij RJ, Rossum LG, Jansen JB, Straatman H, Verbeek AL. Evaluation of treatment regimens to cure Helicobacter pylori infection – a meta-analysis. Aliment Pharmacol Ther. 1999;13:857–64. doi: 10.1046/j.1365-2036.1999.00542.x. [DOI] [PubMed] [Google Scholar]
- 33.Vakil N, Schwartz HJ, Lanza FL, et al. A prospective, controlled, randomized trial of 3-, 7-, and 10-day rabeprazole-based triple therapy for H pylori eradication in the United States Gastroenterology 200212265A(Abst) [Google Scholar]
- 34.Calvet X, Garcia N, Lopez T, Gisbert JP, Gene E, Roque M. A meta-analysis of short versus long therapy with a proton pump inhibitor, clarithromycin and either metronidazole or amoxicillin for treating Helicobacter pylori infection. Aliment Pharmacol Ther. 2000;14:603–9. doi: 10.1046/j.1365-2036.2000.00744.x. [DOI] [PubMed] [Google Scholar]
- 35.Ford A, Moayyedi P. How can the current strategies for Helicobacter pylori eradication therapy be improved? Can J Gastroenterol. 2003;17(Suppl B):36B–40B. doi: 10.1155/2003/714124. [DOI] [PubMed] [Google Scholar]
- 36.Vergara M, Vallve M, Gisbert JP, Calvet X. Meta-analysis: Comparative efficacy of different proton-pump inhibitors in triple therapy for Helicobacter pylori eradication. Aliment Pharmacol Ther. 2003;18:647–54. doi: 10.1046/j.1365-2036.2003.01746.x. [DOI] [PubMed] [Google Scholar]
- 37.Hojo M, Miwa H, Nagahara A, Sato N. Pooled analysis on the efficacy of the second-line treatment regimens for Helicobacter pylori infection. Scand J Gastroenerol. 2001;36:690–700. doi: 10.1080/003655201300191941. [DOI] [PubMed] [Google Scholar]
- 38.Sherman P, Shames B, Loo V, Matlow A, Drumm B, Penner J. Omeprazole therapy for Helicobacter pylori infection. Scand J Gastroenterol. 1992;27:1018–22. doi: 10.3109/00365529209028132. [DOI] [PubMed] [Google Scholar]
- 39.Israel DM, Hassall E. Treatment and long-term follow-up of Helicobacter pylori-associated duodenal ulcer disease in children. J Pediatr. 1993;123:53–8. doi: 10.1016/s0022-3476(05)81536-7. [DOI] [PubMed] [Google Scholar]
- 40.Dohil R, Israel DM, Hassall E. Effective 2-wk therapy for Helicobacter pylori disease in children. Am J Gastroenterol. 1997;92:244–7. [PubMed] [Google Scholar]
- 41.Veldhuyzen Van Zanten S, Farley A, Marcon N, et al. Bismuth-based triple therapy with bismuth subcitrate, metronidazole and tetracycline in the eradication of Helicobacter pylori: A randomized, placebo controlled, double-blind study. Can J Gastroenterol. 2000;14:599–602. doi: 10.1155/2000/690307. [DOI] [PubMed] [Google Scholar]
- 42.Jacobson K, Chiba N, Chen Y, et al. Gastric acid secretory response in Helicobacter pylori-positive patients with duodenal ulcer disease. Can J Gastroenterol. 2001;15:29–39. doi: 10.1155/2001/764615. [DOI] [PubMed] [Google Scholar]
- 43.Nash C, Best L, Haldane H, Malatjalian D, Veldhuyzen van Zanten S.Results of PPI-based triple therapies or PPI-based quadruple therapies for cure of H pylori infection in Halifax Can J Gastroenterol 200216Suppl A114A(Abst) [Google Scholar]
- 44.Lahaie RG, Gaudreau C. Helicobacter pylori antibiotic resistance: Trends over time. Can J Gastroenterol. 2000;14:895–9. doi: 10.1155/2000/218256. [DOI] [PubMed] [Google Scholar]
- 45.Best L, Cooper-Lesins G, Haldane D, Spenard J, Fallone C, Veldhuyzen van Zanten SJ. Helicobacter pylori antibiotic resistance in Canadian populations. Gastroenterology. 2004;126:S1293, A189. [Google Scholar]
- 46.Best LM, Haldane DJ, Bezanson GS, Veldhuyzen van Zanten SJ. Helicobacter pylori: Primary susceptibility to clarithromycin in vitro in Nova Scotia. Can J Gastroenterol. 1997;11:298–300. doi: 10.1155/1997/159637. [DOI] [PubMed] [Google Scholar]
- 47.Borody TJ, Pang G, Wettstein AR, et al. Efficacy and safety of rifabutin-containing ‘rescue therapy’ for resistant Helicobacter pylori infection Aliment Pharmacol Ther 200623481–8.(Erratum in Aliment Pharmacol Ther 2006;24:439). [DOI] [PubMed] [Google Scholar]
- 48.Perri F, Festa V, Clemente R, et al. Randomized study of two “rescue” therapies for Helicobacter pylori-infected patients after failure of standard triple therapies. Am J Gastroenterol. 2001;96:58–62. doi: 10.1111/j.1572-0241.2001.03452.x. [DOI] [PubMed] [Google Scholar]
- 49.Bilardi C, Dulbecco P, Zentilin P, et al. A 10-day levofloxacin-based therapy in patients with resistant Helicobacter pylori infection: A controlled trial. Clin Gastroenterol Hepatol. 2004;2:997–1002. doi: 10.1016/s1542-3565(04)00458-6. [DOI] [PubMed] [Google Scholar]
- 50.Gisbert JP, Castro-Fernandez M, Bermejo F, et al. The H pylori Study Group of the Asociacion Espanola de Gastroenterologia Third-line rescue therapy with levofloxacin after two H pylori treatment failures. Am J Gastroenterol. 2006;101:243–7. doi: 10.1111/j.1572-0241.2006.00457.x. [DOI] [PubMed] [Google Scholar]
- 51.Saad RJ, Schoenfeld P, Kim HM, Chey WD. Levofloxacin-based triple therapy versus bismuth-based quadruple therapy for persistent Helicobacter pylori infection: A meta-analysis. Am J Gastroenterol. 2006;101:488–96. doi: 10.1111/j.1572-0241.2006.00637.x. [DOI] [PubMed] [Google Scholar]