Abstract
Background
Mortality in heart failure (HF) remains high but causes of death are incompletely defined. As HF is heterogeneous syndrome categorized according to ejection fraction (EF), the association between EF and causes of death is important, yet elusive.
Method and Results
Community subjects with HF were classified according to preserved (≥50%) and reduced EF (<50%). Deaths were classified as coronary heart disease (CHD), other cardiovascular and non-cardiovascular. Among 1063 persons with HF, 45% had preserved EF with less cardiovascular risk factors and less coronary disease than those with reduced EF. At 5 years, survival was 45% (95% CI 43%–49%) and 43% of the deaths were non-cardiovascular. The leading cause of death in subjects with preserved EF was non-cardiovascular (49%) vs CHD (43%) for subjects with reduced EF. The proportion of cardiovascular deaths decreased from 69% in 1979–1984 to 40% in 1997–2002 (p=0.007) among subjects with preserved EF contrasting with a modest change among those with reduced EF (77% in to 64%, p=0.08). Advanced age, male sex, diabetes, smoking and kidney disease were associated with an increase risk of all cause and cardiovascular death. After adjustment, preserved EF was associated with a lower risk of cardiovascular death but not all cause death.
Conclusion
Community subjects with HF experience a persistently high mortality and a large proportion of deaths are non-cardiovascular. Subjects with preserved EF have less cardiovascular disease before death, are less likely to experience cardiovascular deaths than those with reduced EF and the proportion of cardiovascular deaths declined over time.
Keywords: HF, ejection fraction, epidemiology, mortality
INTRODUCTION
Despite progress in the management of heart failure (HF),1 the burden of HF, driven largely by the aging of the population is staggering.2 Mortality rates remain quite high with only modest improvements in survival over the past decades.3,4
As HF is a disease of the elderly, subjects with HF have a high prevalence of comorbid conditions,5,6 which can themselves cause death. While several studies examined mortality in HF, there is a paucity of knowledge on cause-specific death, particularly according to EF. Indeed, as stated recently in the Journal of the American College of Cardiology, “no study to date has provided detailed data on the causes of deaths in patients with HF and preserved EF, information that should be of value in the development and testing of treatment for this type of HF.”7 Finally, whether causes of death are changing over time in HF is unknown. This is important as the marginal improvement in survival of HF in the community 3,4 could reflect in part a shift in the distributions of the causes of death with a decrease in cardiovascular deaths, offset by an increase in non-cardiovascular deaths in an elderly population.
HF is a syndrome which encompasses heterogeneous disease processes, customarily categorized according to left ventricular ejection fraction (EF) into HF with preserved versus reduced EF.1 The association between mortality and EF remains controversial,7–18 likely due to differences in time period, study design, sample size and ascertainment of EF across studies.
This study was thus undertaken to address these gaps in knowledge and examine among a geographically defined cohort of subjects with validated HF overall and cause-specific deaths and how their distribution may have changed over time. We sought to evaluate whether these differed according to EF and to identify factors associated with increased mortality.
METHODS
Study Setting
This study was conducted in Olmsted County, Minnesota. Epidemiologic studies in Olmsted County are possible because the county is relatively isolated and only a few providers deliver nearly all health care to local residents. Health care providers in Olmsted County include Mayo Clinic, Olmsted Medical Center, and a handful of private practitioners. Each provider uses a comprehensive medical record system in which the details of every encounter are entered and can be easily retrieved. Medical records are reviewed under the auspices of the Rochester Epidemiology Project, a record-linkage system that allows the indexing of all medical records of Olmsted County residents according to clinical and pathological diagnoses, surgical procedures, and billing information. This indexing system enables the retrieval of all medical records for use in epidemiologic studies and ensures complete capture of all health care related events occurring in Olmsted County for local residents. This centralized system encompasses the medical records of a population representing an estimated 3,600,000 person-years of health care. The potential of this data source has been described elsewhere.19 The appropriate institutional review boards approved all aspects of the study.
The HF Incidence Cohort
The validated incident HF cohort was assembled from a random sample of all potential HF cases in Olmsted County between 1979 and 2002, and thus does not include all cases of HF in the community within the study period. Cases were identified by screening the medical records of all Olmsted County patients from 1979–2002 using the International Classification of Diseases Ninth Revision, Clinical Modification (ICD-9-CM) code 428. Focus on code 428 was based on previously reported yields of all codes relating to HF.4 Nurse abstractors then reviewed the random sample of 2072 cases and validated HF diagnosis according to the Framingham Criteria.4 Persons who had a clinical diagnosis of HF in their medical record prior to 1979 or who were not residents of Olmsted County were excluded. After validation, 1063 cases of heart failure met Framingham criteria and were included in this study. Clinical characteristics including a detailed assessment of comorbidity were collected from the medical record.
Clinical Characteristics
Hypertension was defined by the criteria of the 6th report of the Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure (6th report, 97). Persons were considered hypertensive if 2 or more ambulatory blood pressure readings were greater than or equal to 140 mmHg systolic and/or 90 mmHg diastolic. Physician’s diagnosis of hypertension and treatment with antihypertensive drugs were also considered.
Persons who met standardized criteria of two consecutive fasting glucose levels greater or equal to 140 mg/dl or 1- to 2- hour levels greater or equal to 200 mg/dl obtained using a standard glucose tolerance test prior to HF were diagnosed with diabetes mellitus according to the National Diabetes Data Group (NDDG) recommendations (Anonymous, classification, 1979). Smoking status was categorized as never or ever (past or current). Height and weight measurements reported in the last outpatient visit prior to meeting criteria for HF were abstracted. Body mass index (BMI) was calculated as weight in kg divided by height in meters squared.
Documented coronary disease was defined as the occurrence prior to the index date of HF of a myocardial infarction validated with epidemiological criteria,20 a history of coronary surgery, or the presence of significant coronary disease at angiography. Creatinine clearance was calculated using the last outpatient serum creatinine value prior to the diagnosis of HF in the equation of Cockcroft and Gault: [((140-age) × (weight in kilograms) × (0.85 for women)) / (72 × creatinine (mg/dL)] and used as an estimate of glomerular filtration rate after adjustment for the body surface area.21 Chronic kidney disease was deemed severe when the GFR was less than or equal to 29 ml/min per 1.73m2 while moderate kidney disease was defined by GFR 30–59 ml/min per 1.73m2.22,23
Left ventricular ejection fraction (%) was determined using values collected from any echocardiogram, radionuclide ventriculogram or left ventricular angiogram performed within 90 days of HF diagnosis. The value closest in time to HF diagnosis was used when multiple values were available. When multiple values were measured as part of one test on the same day, the average value was used. Ejection fraction greater than or equal to 50% defined HF with preserved ejection fraction, while reduced ejection fraction was defined as ejection fraction less than 50%.24
Comorbid conditions were categorized as peripheral vascular/cerebrovascular disease, chronic obstructive pulmonary disease, gastrointestinal or liver disease, cancer or rheumatologic disease on the basis of ICD-9 and ICD-10 code groupings defined with the CDC ICD code finder (http://wonder.cdc.gov/wonder/cgi-bin/asp/ICDFinder.asp). In addition, the Charlson Index25 was used to measure the global burden of comorbidity.
Ascertainment of Death and Cause of Death Classification
Follow-up was performed by using all inpatient and outpatient medical records. The ascertainment of death included several procedures. In addition to the deaths noted during clinical care, all death certificates for Olmsted County residents are obtained each year from the county office. The Mayo Clinic registration office records the obituaries and notices of death in the local newspapers. Finally, data on all Minnesota deaths are obtained from the State of Minnesota every year.
Assignment of the cause of death relied on the underlying cause of death listed on the death certificate and was classified into 3 categories including coronary heart disease (CHD), other cardiovascular, and non-cardiovascular cause of death based upon ICD-9 and ICD-10 codes. The categories of cardiovascular deaths, including ischemic heart diseases and other cardiovascular diseases, were adapted from the classification used by the American Heart Association.26
The procedures in place in Olmsted County to complete death certificates differ from procedures in most other locations.27 The coroner (chief medical examiner) or a pathologist member of the staff of the Mayo Clinic completes the death certificates of over 75% of Olmsted County residents irrespectively of whether or not an autopsy is performed. The entire medical record is reviewed prior to assigning the cause of death. Death certificates for in-hospital decedents were not verified manually. However, it is less subject to misclassification than outpatient events due to the availability of the medical record.28 When an autopsy is performed, its findings are taken into account to complete the death certificate and take precedent over the clinical information. Infrequently, the oncologist assigns the cause of death for hospice patients and an internist for nursing home patients. Death certificates for patients under the care of physicians not affiliated with the Mayo Clinic are completed by their physicians.
Statistical Analysis
The data are presented as frequency or mean ± standard deviation (SD). Associations between patient characteristics and EF category were examined with logistic regression. Survival was analyzed with the Kaplan-Meier method. Trends in cause of death over time were analyzed with logistic regression with a 4-level categorical year variable as the predictor variable with levels representing 1979–1984, 1985–1990, 1991–1996, and 1997–2002. Trends in baseline characteristics over time were analyzed with linear regression for continuous variables and with the Mantel-Haenszel chi-square test for categorical variables. Proportional hazards regression was used to examine the association between death and baseline characteristics. First order interactions between EF and baseline characteristics were examined and reported when present. Missing values did not exceed 5% for any variable used in the regression analyses except for EF which was missing in 39% of the cases. Multiple imputation was used to impute missing values using the Markov Chain Monte Carlo method and assuming the missing data mechanism was missing at random. This means that the probability of missingess may depend on data that are observed, but not on values that are missing. The model used to impute EF included demographic variables, cardiovascular risk factors, and comorbidities. Five complete datasets were created for analyses. The relative efficiency from using five imputed datasets is 93%, which is desirable for estimating model parameters. Each of the complete datasets was analyzed using standard statistical analyses. Results were combined, with standard errors obtained using the rules given by Rubin.29 A p-value of 0.05 was selected for the threshold of statistical significance except when testing for interactions when a p-value of 0.10 was used. Analyses were performed using SAS statistical software, version 8 (SAS Institute Inc., Cary, NC).
Statement of Responsibility
The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
RESULTS
Clinical characteristics
One thousand sixty-three persons with incident HF diagnosed between 1979–2002 were included in the study. Their mean age was 76 ± 12 years and 46% were men. Most subjects had a prior diagnosis of hypertension or were smokers. Comorbid conditions were common (Table 1). Mean age increased over time (p=0.04) as did number of comorbidities (p<0.001).
Table 1.
Clinical characteristics of subjects with HF according to ejection fraction
| Overall | EF < 50% | EF ≥ 50% | p-value | |
|---|---|---|---|---|
| Demographics | ||||
| Age (years), mean ± SD | 76.4 ± 12.4 | 75.0 ± 12.7 | 78.2 ± 11.9 | 0.001 |
| Male, % | 46 | 54 | 37 | <0.001 |
| Cardiovascular risk factors | ||||
| Hypertension, % | 68 | 67 | 68 | 0.76 |
| Diabetes mellitus, % | 19 | 23 | 13 | 0.005 |
| Diabetes mellitus (insulin dependent), % | 3 | 4 | 2 | 0.01 |
| Diabetes mellitus (non-insulin dependent), % | 15 | 18 | 10 | |
| Smoker ever, % | 53 | 59 | 46 | 0.005 |
| BMI (kg/m2), mean ± SD | 27.1 ± 5.6 | 27.3 ± 5.7 | 26.8 ± 5.4 | 0.41 |
| Comorbidity | ||||
| Documented coronary disease*, % | 35 | 41 | 27 | 0.003 |
| Peripheral vascular or cerebrovascular disease, % | 37 | 40 | 33 | 0.13 |
| Severe chronic kidney disease, % | 8 | 9 | 8 | 0.60 |
| Moderate chronic kidney disease, % | 50 | 51 | 50 | |
| Chronic obstructive pulmonary disease, % | 22 | 21 | 23 | 0.44 |
| Gastro-intestinal or liver disease, % | 23 | 22 | 25 | 0.24 |
| Cancer, % | 18 | 17 | 20 | 0.27 |
| Rheumatologic disease, % | 8 | 7 | 10 | 0.12 |
Documented coronary disease defined as prior myocardial infarction, coronary artery bypass grafting or angiographically defined significant coronary artery disease.
Forty-five percent of subjects had HF with preserved EF. These subjects were older and more often women. Subjects with preserved EF had a lesser global burden of cardiovascular risk factors, as they were equally likely to be overweight and hypertensive but less likely to have diabetes mellitus or to have a smoking history compared to their counterparts with reduced EF.
Congruent with the prevalence of cardiovascular risk factors, the antemortem prevalence of documented coronary disease was markedly lower among subjects with preserved EF. This was particularly noticeable for myocardial infarction, which was recorded among 16% of patients with preserved EF contrasting with 28% among persons with reduced EF (p<0.001). The total comorbidity burden as measured by the Charlson Index did not differ according to EF. Among subjects with preserved EF, 52% had a Charlson Index of 2 or more while 56% of subjects with reduced EF had a Charlson Index of 2 or more (p=0.34).
All-cause and cardiovascular deaths
After a median follow-up of 4.3 years (minimum=0 years, maximum=27.7 years), 917 deaths were noted, corresponding to a 5-year survival of 45% (95% CI 43%–49%). Overall, 525 (57%) of deaths were categorized as cardiovascular. A total of 330 CHD deaths occurred representing 36% of all deaths and 63% of cardiovascular deaths. Of the 392 (43%) non-cardiovascular deaths, the most common causes of death were due to pulmonary disease (28%) and cancer (25%) followed by central nervous system disease (12%), gastrointestinal disease or genitourinary disease (12%), and diabetes mellitus or endocrine disorders (9%).
Over time, a shift in the distribution of causes of death occurred. The proportion of deaths occurring within 5 years of incident HF that were categorized as cardiovascular decreased from 74% in 1979–1984 to 51% in 1997–2002 (p<0.001).
Advanced age, male sex, diabetes mellitus, history of smoking, and chronic kidney disease were associated with increased risk of all cause mortality and cardiovascular mortality (Table 2). In particular, severe chronic kidney disease was associated with more than a two-fold increase in the risk of overall and cardiovascular death adjusting for other clinical characteristics.
Table 2.
Predictors of death among subjects with HF in the community
| Models not including ejection fraction | Models including ejection fraction | |||
|---|---|---|---|---|
| Predictor Variable | HR (95% CI) | p-value | HR (95% CI) | p-value |
| All Cause Death | ||||
| Age (per year increase) | 1.057 (1.049, 1.064) | <0.001 | 1.058 (1.050, 1.065) | <0.001 |
| Male sex | 1.28 (1.10, 1.49) | 0.001 | 1.27 (1.09, 1.48) | 0.002 |
| Diabetes mellitus | 1.44 (1.21, 1.72) | <0.001 | 1.44 (1.21, 1.71) | <0.001 |
| Smoking | 1.38 (1.18, 1.61) | <0.001 | 1.39 (1.19, 1.62) | <0.001 |
| Documented coronary disease | 0.93 (0.80, 1.08) | 0.32 | 0.93 (0.80, 1.07) | 0.29 |
| Severe chronic kidney disease | 2.23 (1.75, 2.83) | <0.001 | 1.96 (1.51, 2.54) | <0.001 |
| Ejection fraction ≥ 50% | 0.88 (0.74, 1.04) | 0.15 | ||
|
| ||||
| Cardiovascular Death | ||||
| Age (per year increase) | 1.055 (1.044, 1.065) | <0.001 | 1.055 (1.044, 1.065) | <0.001 |
| Male sex | 1.26 (1.03, 1.54) | 0.026 | 1.22 (1.00, 1.48) | 0.054 |
| Diabetes mellitus | 1.47 (1.17, 1.85) | <0.001 | 1.46 (1.16, 1.82) | <0.001 |
| Smoking | 1.27 (1.04, 1.55) | 0.020 | 1.24 (1.01, 1.52) | 0.039 |
| Documented coronary disease | 1.15 (0.95, 1.40) | 0.16 | 1.12 (0.93, 1.35) | 0.23 |
| Severe chronic kidney disease | 2.38 (1.76, 3.23) | <0.001 | 2.10 (1.52, 2.88) | <0.001 |
| Ejection fraction ≥ 50% | 0.71 (0.57, 0.89) | 0.006 | ||
Coronary disease defined as prior myocardial infarction, coronary artery bypass grafting or angiographically defined significant coronary artery disease.
Death in HF according to ejection fraction
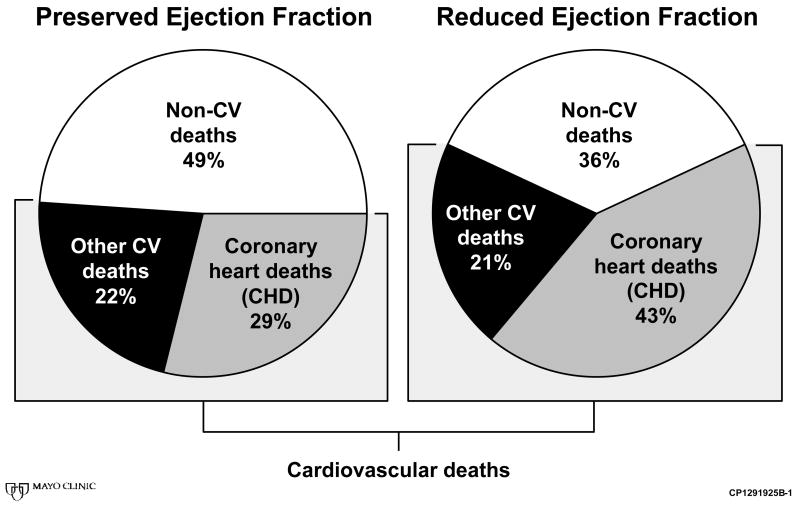
Among subjects with preserved EF, death was most commonly attributed to non-cardiovascular causes (49% of all deaths, Figure 1). Non-cardiovascular deaths included deaths most commonly due to pulmonary disease (29%) and cancer (23%) followed by central nervous system disease (14%), gastrointestinal or genitourinary disease (11%), and diabetes mellitus or endocrine disorders (7%). Coronary heart disease (CHD) and other cardiovascular deaths occurred in 29% and 22% of cases, respectively.
Figure 1.
Causes of Death by Ejection Fraction
In contrast, among subjects with reduced EF, the leading cause of death was CHD (43% of deaths) while 36% of deaths were attributed to non-cardiac causes. These included deaths most commonly due to cancer (28%) and pulmonary disease (27%), followed by gastrointestinal or genitourinary disease (14%), central nervous system disease (10%), and diabetes mellitus or endocrine disorders (10%).
Over time, a shift in the distribution of causes of death occurred among those with preserved EF. The proportion of deaths occurring within 5 years of incident HF that were categorized as cardiovascular decreased from 69% in 1979–1984 to 40% in 1997–2002 (p=0.007). By contrast, among subjects with reduced EF, the temporal decrease in the proportion of cardiovascular deaths was quite modest and not statistically significant (77% in 1979–1984 to 64% in 1997–2002, p=0.08).
EF was not associated with all cause death (hazard ratio for preserved versus reduced EF 0.95, 95% CI 0.81–1.11, p = 0.52). Adjustment for age, sex, diabetes mellitus, smoking, documented coronary disease and chronic kidney disease did not unmask any significant association between EF and death (Table 2). Conversely, preserved EF was univariately associated with markedly lower risk of cardiovascular death (hazard ratio 0.76, 95% CI 0.61–0.94, p=0.014). This association remained after adjustment for age, sex, diabetes mellitus, smoking, documented coronary disease and chronic kidney disease.
DISCUSSION
In the community, all subjects with HF experience high mortality irrespective of EF and the frequency of non-cardiovascular deaths is high. Subjects with preserved EF have less documented coronary disease. Accordingly, cardiovascular deaths were less frequent among subjects with preserved EF. Age, male sex, diabetes, smoking, and kidney disease were important indicators of an increased risk of overall and cardiovascular death while reduced EF was associated with an increased risk of cardiovascular death but not all cause death.
Few studies reported on cause-specific deaths in HF. Studies that did underscored that non-cardiac causes of death were frequent in HF,30 estimated at nearly one-third in a cohort of hospitalized patients from Canada.
The present community-based findings support and extend previous findings by demonstrating that HF patients have a poor survival and that the frequency of non-cardiovascular deaths in this cohort, including both outpatients and hospitalized subjects, is higher than previously reported,30 accounting for nearly half of all deaths. Factors associated with worse survival include advanced age, male sex, preexisting diabetes, smoking history, and chronic kidney disease.16,31,32 In our cohort, heart failure patients were becoming older and had increasing comorbidity over the study period. This underscores the importance of the identification and management of comorbid diseases among all HF patients. Indeed, as noncardiac comorbidities are highly prevalent in patients with HF in the community, further improvement in the survival of patients with HF may be hindered by comorbid conditions, which interfere with HF management strategies and adversely affect outcomes.33
HF is a disease of the elderly, typically with a similar distribution across sexes or a slight female preponderance. Community studies have consistently indicated a high prevalence, even predominance, of preserved EF among subjects with HF.7 As HF is a syndrome, its pathogenesis differs by EF34 and the mechanisms of HF with preserved EF, while remaining controversial, are likely related to impaired myocardial relaxation and reduced LV compliance, leading to impaired left ventricular filling.34–36 Within this context, examining the cause of death in HF can enhance our understanding of the pathophysiology of the disease.7
Herein, patients with preserved EF were less likely to have a history of diabetes, smoking, or documented coronary disease, compared to those with reduced EF. Over a long follow-up period, EF was not associated with mortality. These data extend a prior report from our group showing no difference in short-term mortality according to EF.18 Additionally, the SENIORS study of older adults with heart failure showed no difference in all cause mortality between patients with preserved or reduced EF.37 Other studies, however, indicated that preserved EF was associated with better survival.7,8,14,15 These conflicting results likely reflect differences in study design, sample size and cause of death ascertainment and distributions. These methodological considerations are important as they determine the applicability of these results to different populations and thus the clinical usefulness of such data.
To this end, in the Digitalis Investigation Group (DIG) trial, EF was associated with increased mortality.32 These data present some striking differences compared to the present community study. Indeed, participants to the DIG trial were more than 10 years younger than the present community population, included one-fourth of women as opposed to half in the present study and 63% of trial enrollees had a history of MI compared to 23% herein. Accordingly, 78% of deaths were cardiovascular in the DIG trial compared to 57% in the present study. Similarly, participants in the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) trial,38 were younger, more likely to be male with a preponderance of coronary disease and most deaths (85%) of cardiovascular cause. Thus, in CHARM like in DIG, the association between EF and overall mortality reflects the characteristics and outcomes of selected clinical trial participants that differ markedly from that of community populations. The discrepancies between clinical trial findings and the present community-based data illustrate the limitations of extrapolating the observations made in clinical trials to the community.39 Among hospitalized patients in Ontario, no association between EF and survival was detected.40 This study however included only subjects who underwent an assessment of EF, which represented only 42% of all patients with HF hospitalized during the time period. Thus, this by design led to a substantial selection bias, which may impact on the external validity of these results amplified by the fact that outpatient subjects with HF were excluded. This underscores in turn the importance and relevance of the present data to the community practice. These findings contribute to resolving the aforementioned controversy on the impact of EF on death in HF by indicating that preserved EF carries a lower risk of cardiovascular, but not overall, death. Patients with preserved EF have fewer comorbid cardiovascular conditions than their counterparts with reduced EF, thus deaths from non-cardiac causes predominate among subjects with preserved EF. Further, the present study indicates that the proportion of cardiovascular deaths has decreased overtime among subjects with HF and preserved EF, a finding previously not reported, which should be interpreted in light of a previous report from our group indicating that the prevalence of HF with preserved EF increased overtime with no improvement in survival among these patients.8 This may help to explain findings such as those, in the PEP-CHF study, in which older patients with preserved EF had no 1-year mortality benefit with use of perindopril therapy.41 Indeed, the present findings extend data by indicating that, within the context of stable overall survival, the distribution of the causes of death is shifting towards less cardiovascular causes, which has important implications for the understanding of secular trends in HF, and for therapeutic trials for this condition.
Limitations and strengths
As no study will be generalizable to the entire US population, the racial and ethnic composition of the present population may impact the extrapolation of the data to under-represented populations. While the population of the present study consists mainly of white Caucasian subjects, the value of Olmsted County studies lies in the ability to measure in one population the occurrence of disease and subsequent outcomes and provide benchmarks for needed comparisons to other populations. Ascertainment of the cause of death relied on death certificates. The procedure for death certificate completion, as indicated in the method section, is quite standardized. The validity of death certificate to diagnose deaths due to coronary disease in the outpatient setting is quite robust.42 While we cannot exclude that some deaths could be misclassified, it seems unlikely however that misclassification would differ appreciably according to EF such that it should not affect the primary findings of the study. Further, misclassification tends to be less problematic for broad categories of death causes, such as were used herein. We acknowledge that there are limitations to the Framingham criteria for the diagnosis of heart failure.43,44
Our community-based study has notable strengths. The HF cohort, validated using standardized criteria18 includes both inpatient and outpatient data. Our findings address the stated need for more data on the cause of death among subjects with preserved EF7 in a community cohort, which optimizes its applicability to clinical practice. Ejection fraction was directly measured in a larger proportion of subjects than most previous reports.45 Further, multiple imputation was used to impute EF values when EF was not directly measured, thereby enabling to report on the entire experience of all subjects with HF in the community. This methodology provides unbiased estimates and therefore is a better approach to handling missing data than using an indicator variable to represent missing EF which has known biases.46
CONCLUSION
Community subjects with heart failure experience high mortality whether EF is preserved or reduced and the frequency of non-cardiovascular deaths is high. Subjects with preserved EF have a lower burden of cardiovascular comorbidity before death and experience less cardiovascular deaths than subjects with reduced EF. Among subjects with preserved EF, the proportion of non-cardiovascular deaths increases over time. These findings underscore the heterogeneity of HF and have implications for the design and interpretation of interventions aiming at reducing mortality in HF.
Acknowledgments
Funding Sources: Supported in part by grants from the Public Health Service and the National Institutes of Health (AR30582, R01 HL59205, R01 HL72435, RO1 HL64112),
Footnotes
CONFLICT OF INTEREST
There is no conflict of interest.
“This is an un-copyedited author manuscript that was accepted for publication in Circulation: Heart Failure, copyright The American Heart Association. This may not be duplicated or reproduced, other than for personal use or within the “Fair Use of Copyrighted Materials” (section 107, title 17, U.S. Code) without prior permission of the copyright owner, The American Heart Association. The final copyedited article, which is the version of record, can be found at http://circ.ahajournals.org. The American Heart Association disclaims any responsibility or liability for errors or omissions in this version of the manuscript or in any version derived from it by the National Institutes of Health or other parties.”
References
- 1.Hunt SA. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure) J Am Coll Cardiol. 2005;46:e1–82. doi: 10.1016/j.jacc.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 2.Stewart S, MacIntyre K, Capewell S, McMurray JJ. Heart failure and the aging population: an increasing burden in the 21st century? Heart. 2003;89:49–53. doi: 10.1136/heart.89.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levy D, Kenchaiah S, Larson MG, Benjamin EJ, Kupka MJ, Ho KK, Murabito JM, Vasan RS. Long-term trends in the incidence of and survival with heart failure. N Engl J Med. 2002;347:1397–402. doi: 10.1056/NEJMoa020265. [DOI] [PubMed] [Google Scholar]
- 4.Roger VL, Weston SA, Redfield MM, Hellermann-Homan JP, Killian J, Yawn BP, Jacobsen SJ. Trends in heart failure incidence and survival in a community-based population. Jama. 2004;292:344–50. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- 5.Brown AM, Cleland JG. Influence of concomitant disease on patterns of hospitalization in patients with heart failure discharged from Scottish hospitals in 1995. Eur Heart J. 1998;19:1063–9. [PubMed] [Google Scholar]
- 6.Dahlstrom U. Frequent non-cardiac comorbidities in patients with chronic heart failure. Eur J Heart Fail. 2005;7:309–16. doi: 10.1016/j.ejheart.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 7.Hogg K, Swedberg K, McMurray J. Heart failure with preserved left ventricular systolic function; epidemiology, clinical characteristics, and prognosis. J Am Coll Cardiol. 2004;43:317–27. doi: 10.1016/j.jacc.2003.07.046. [DOI] [PubMed] [Google Scholar]
- 8.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–9. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 9.Ahmed A. A propensity matched study of New York Heart Association class and natural history end points in heart failure. Am J Cardiol. 2007;99:549–53. doi: 10.1016/j.amjcard.2006.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pocock SJ, Wang D, Pfeffer MA, Yusuf S, McMurray JJ, Swedberg KB, Ostergren J, Michelson EL, Pieper KS, Granger CB. Predictors of mortality and morbidity in patients with chronic heart failure. Eur Heart J. 2006;27:65–75. doi: 10.1093/eurheartj/ehi555. [DOI] [PubMed] [Google Scholar]
- 11.Senni M, Tribouilloy CM, Rodeheffer RJ, Jacobsen SJ, Evans JM, Bailey KR, Redfield MM. Congestive heart failure in the community: trends in incidence and survival in a 10-year period. Arch Intern Med. 1999;159:29–34. doi: 10.1001/archinte.159.1.29. [DOI] [PubMed] [Google Scholar]
- 12.Senni M, Redfield MM. Heart failure with preserved systolic function. A different natural history? J Am Coll Cardiol. 2001;38:1277–82. doi: 10.1016/s0735-1097(01)01567-4. [DOI] [PubMed] [Google Scholar]
- 13.Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 14.Solomon SD, Anavekar N, Skali H, McMurray JJ, Swedberg K, Yusuf S, Granger CB, Michelson EL, Wang D, Pocock S, Pfeffer MA. Influence of ejection fraction on cardiovascular outcomes in a broad spectrum of heart failure patients. Circulation. 2005;112:3738–44. doi: 10.1161/CIRCULATIONAHA.105.561423. [DOI] [PubMed] [Google Scholar]
- 15.Vasan RS, Larson MG, Benjamin EJ, Evans JC, Reiss CK, Levy D. Congestive heart failure in subjects with normal versus reduced left ventricular ejection fraction: prevalence and mortality in a population-based cohort. J Am Coll Cardiol. 1999;33:1948–55. doi: 10.1016/s0735-1097(99)00118-7. [DOI] [PubMed] [Google Scholar]
- 16.Kearney MT, Fox KA, Lee AJ, Prescott RJ, Shah AM, Batin PD, Baig W, Lindsay S, Callahan TS, Shell WE, Eckberg DL, Zaman AG, Williams S, Neilson JM, Nolan J. Predicting death due to progressive heart failure in patients with mild-to-moderate chronic heart failure. Journal of the American College of Cardiology. 2002;40:1801–8. doi: 10.1016/s0735-1097(02)02490-7. [DOI] [PubMed] [Google Scholar]
- 17.Curtis JP, Sokol SI, Wang Y, Rathore SS, Ko DT, Jadbabaie F, Portnay EL, Marshalko SJ, Radford MJ, Krumholz HM. The association of left ventricular ejection fraction, mortality, and cause of death in stable outpatients with heart failure. Journal of the American College of Cardiology. 2003;42:736–42. doi: 10.1016/s0735-1097(03)00789-7. [DOI] [PubMed] [Google Scholar]
- 18.Bursi F, Weston SA, Redfield MM, Jacobsen SJ, Pakhomov S, Nkomo VT, Meverden RA, Roger VL. Systolic and diastolic heart failure in the community. Jama. 2006;296:2209–16. doi: 10.1001/jama.296.18.2209. [DOI] [PubMed] [Google Scholar]
- 19.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–74. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 20.Roger VL, Killian J, Henkel M, Weston SA, Goraya TY, Yawn BP, Kottke TE, Frye RL, Jacobsen SJ. Coronary disease surveillance in Olmsted County objectives and methodology. J Clin Epidemiol. 2002;55:593–601. doi: 10.1016/s0895-4356(02)00390-6. [DOI] [PubMed] [Google Scholar]
- 21.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 22.Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41:1–12. doi: 10.1053/ajkd.2003.50007. [DOI] [PubMed] [Google Scholar]
- 23.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS. Prevalence of chronic kidney disease in the United States. Jama. 2007;298:2038–47. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 24.Yturralde RF, Gaasch WH. Diagnostic criteria for diastolic heart failure. Prog Cardiovasc Dis. 2005;47:314–9. doi: 10.1016/j.pcad.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 25.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 26.American Heart Association. Heart Disease and Stroke Statistics-2005 Update. Dallas, Texas: 2005. [Google Scholar]
- 27.Targonski P, Jacobsen SJ, Weston SA, Leibson CL, Pfeifer E, Nemetz P, Roger VL. Referral to autopsy: effect of antemortem cardiovascular disease: a population-based study in Olmsted County, Minnesota. Ann Epidemiol. 2001;11:264–70. doi: 10.1016/s1047-2797(00)00220-9. [DOI] [PubMed] [Google Scholar]
- 28.Folsom AR, Gomez-Marin O, Gillum RF, Kottke TE, Lohman W, Jacobs D., Jr Out-of-hospital coronary death in an urban population--validation of death certificate diagnosis. The Minnesota Heart Survey. American Journal of Epidemiology. 1987;125:1012–8. doi: 10.1093/oxfordjournals.aje.a114617. [DOI] [PubMed] [Google Scholar]
- 29.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: J. Wiley & Sons; 1987. [Google Scholar]
- 30.Ackman ML, Harjee KS, Mansell G, Campbell JB, Teo KK, Montague TJ. Cause-specific noncardiac mortality in patients with congestive heart failure--a contemporary Canadian audit. Clinical Quality Improvement Network (CQIN) Investigators. Canadian Journal of Cardiology. 1996;12:809–13. [PubMed] [Google Scholar]
- 31.Kearney MT, Fox KA, Lee AJ, Brooksby WP, Shah AM, Flapan A, Prescott RJ, Andrews R, Batin PD, Eckberg DL, Gall N, Zaman AG, Lindsay HS, Nolan J. Predicting sudden death in patients with mild to moderate chronic heart failure. Heart. 2004;90:1137–43. doi: 10.1136/hrt.2003.021733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones RC, Francis GS, Lauer MS. Predictors of mortality in patients with heart failure and preserved systolic function in the Digitalis Investigation Group trial. J Am Coll Cardiol. 2004;44:1025–9. doi: 10.1016/j.jacc.2004.05.077. [DOI] [PubMed] [Google Scholar]
- 33.Braunstein JB, Anderson GF, Gerstenblith G, Weller W, Niefeld M, Herbert R, Wu AW. Noncardiac comorbidity increases preventable hospitalizations and mortality among Medicare beneficiaries with chronic heart failure. J Am Coll Cardiol. 2003;42:1226–33. doi: 10.1016/s0735-1097(03)00947-1. [DOI] [PubMed] [Google Scholar]
- 34.Zile MR, Brutsaert DL. New concepts in diastolic dysfunction and diastolic heart failure: Part II: causal mechanisms and treatment. Circulation. 2002;105:1503–8. doi: 10.1161/hc1202.105290. [DOI] [PubMed] [Google Scholar]
- 35.Kass DA. Is heart failure with decent systole due to bad diastole? J Card Fail. 2005;11:188–90. doi: 10.1016/j.cardfail.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 36.Kitzman DW, Little WC, Brubaker PH, Anderson RT, Hundley WG, Marburger CT, Brosnihan B, Morgan TM, Stewart KP. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. Jama. 2002;288:2144–50. doi: 10.1001/jama.288.17.2144. [DOI] [PubMed] [Google Scholar]
- 37.Flather MD, Shibata MC, Coats AJ, Van Veldhuisen DJ, Parkhomenko A, Borbola J, Cohen-Solal A, Dumitrascu D, Ferrari R, Lechat P, Soler-Soler J, Tavazzi L, Spinarova L, Toman J, Bohm M, Anker SD, Thompson SG, Poole-Wilson PA. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS) Eur Heart J. 2005;26:215–25. doi: 10.1093/eurheartj/ehi115. [DOI] [PubMed] [Google Scholar]
- 38.Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J, Yusuf S, Pocock S Investigators C Committees. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme. Lancet. 2003;362:759–66. doi: 10.1016/s0140-6736(03)14282-1. [DOI] [PubMed] [Google Scholar]
- 39.Steg PG, Lopez-Sendon J, Lopez de Sa E, Goodman SG, Gore JM, Anderson FA, Jr, Himbert D, Allegrone J, Van de Werf F. External validity of clinical trials in acute myocardial infarction. Arch Intern Med. 2007;167:68–73. doi: 10.1001/archinte.167.1.68. [DOI] [PubMed] [Google Scholar]
- 40.Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y, Liu PP. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355:260–9. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- 41.Cleland JG, Tendera M, Adamus J, Freemantle N, Polonski L, Taylor J. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J. 2006;27:2338–45. doi: 10.1093/eurheartj/ehl250. [DOI] [PubMed] [Google Scholar]
- 42.Goraya TY, Jacobsen SJ, Belau PG, Weston SA, Kottke TE, Roger VL. Validation of death certificate diagnosis of out-of-hospital coronary heart disease deaths in Olmsted County, Minnesota. Mayo Clin Proc. 2000;75:681–7. doi: 10.4065/75.7.681. [DOI] [PubMed] [Google Scholar]
- 43.Di Bari M, Pozzi C, Cavallini MC, Innocenti F, Baldereschi G, De Alfieri W, Antonini E, Pini R, Masotti G, Marchionni N. The diagnosis of heart failure in the community. Comparative validation of four sets of criteria in unselected older adults: the ICARe Dicomano Study. J Am Coll Cardiol. 2004;44:1601–8. doi: 10.1016/j.jacc.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 44.Schellenbaum GD, Rea TD, Heckbert SR, Smith NL, Lumley T, Roger VL, Kitzman DW, Taylor HA, Levy D, Psaty BM. Survival associated with two sets of diagnostic criteria for congestive heart failure. Am J Epidemiol. 2004;160:628–35. doi: 10.1093/aje/kwh268. [DOI] [PubMed] [Google Scholar]
- 45.Go AS, Yang J, Ackerson LM, Lepper K, Robbins S, Massie BM, Shlipak MG. Hemoglobin level, chronic kidney disease, and the risks of death and hospitalization in adults with chronic heart failure: the Anemia in Chronic Heart Failure: Outcomes and Resource Utilization (ANCHOR) Study. Circulation. 2006;113:2713–23. doi: 10.1161/CIRCULATIONAHA.105.577577. [DOI] [PubMed] [Google Scholar]
- 46.Greenland S, Finkle WD. A critical look at methods for handling missing covariates in epidemiologic regression analyses. Am J Epidemiol. 1995;142:1255–64. doi: 10.1093/oxfordjournals.aje.a117592. [DOI] [PubMed] [Google Scholar]