Abstract
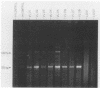
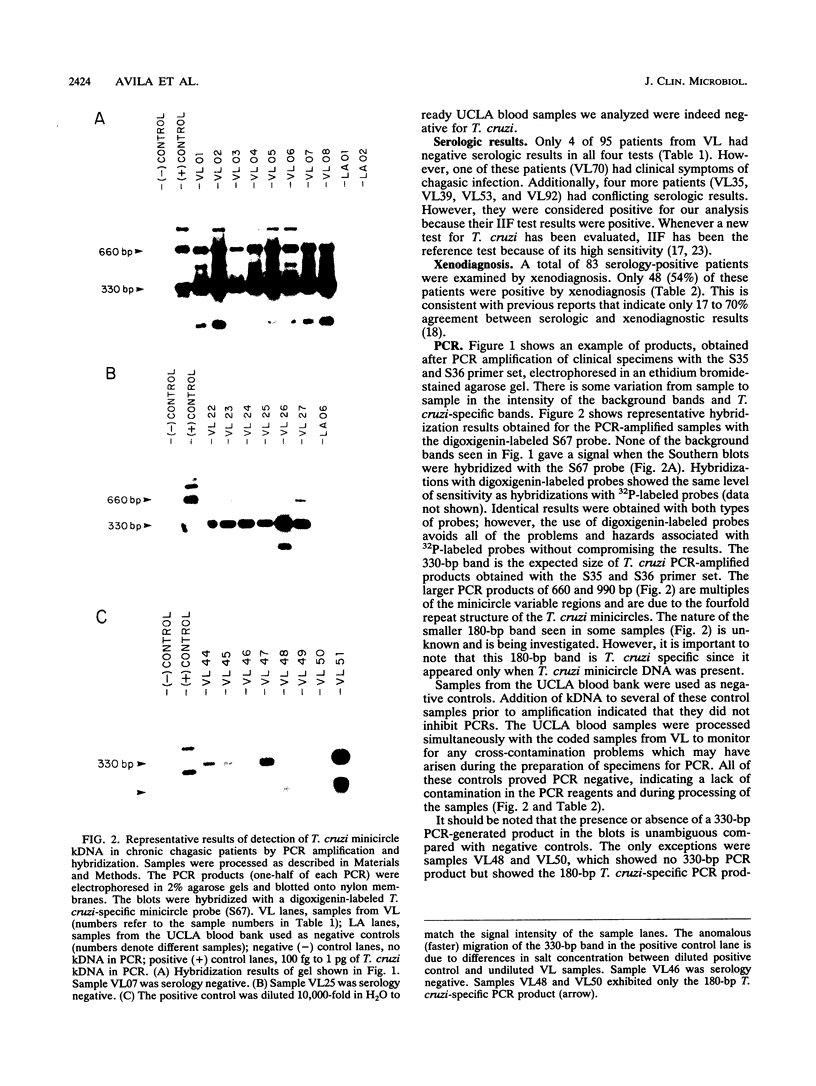
A panel of 114 blood samples from chronic chagasic patients and nonchagasic patients was screened for Trypanosoma cruzi by xenodiagnostic, serologic, and polymerase chain reaction (PCR) amplification tests. Blood samples were preserved in a guanidine-EDTA buffer, and total blood DNA was isolated after chemical nuclease cleavage with 1,10-phenanthroline-copper ion and used as a template for PCR amplification of the conserved and variable regions of T. cruzi minicircle molecules. The PCR products were screened by Southern blot hybridization with a digoxigenin-labeled oligonucleotide probe specific for the conserved region of the minicircle. The method showed a sensitivity of 100% compared with the serologic test. In addition, all of the serology-positive, xenodiagnosis-negative samples were positive by PCR. This demonstrates that PCR amplification of T. cruzi kinetoplast minicircle DNA could replace xenodiagnosis for evaluation of parasitemia in chronic chagasic patients and could serve as a complement for serologic testing in the screening of blood bank donors.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleman M. D., Shulman I. A., Saxena S., Kirchhoff L. V. Use of a questionnaire to identify potential blood donors at risk for infection with Trypanosoma cruzi. Transfusion. 1993 Jan;33(1):61–64. doi: 10.1046/j.1537-2995.1993.33193142312.x. [DOI] [PubMed] [Google Scholar]
- Ashall F., Yip-Chuck D. A., Luquetti A. A., Miles M. A. Radiolabeled total parasite DNA probe specifically detects Trypanosoma cruzi in mammalian blood. J Clin Microbiol. 1988 Mar;26(3):576–578. doi: 10.1128/jcm.26.3.576-578.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila H. A., Sigman D. S., Cohen L. M., Millikan R. C., Simpson L. Polymerase chain reaction amplification of Trypanosoma cruzi kinetoplast minicircle DNA isolated from whole blood lysates: diagnosis of chronic Chagas' disease. Mol Biochem Parasitol. 1991 Oct;48(2):211–221. doi: 10.1016/0166-6851(91)90116-n. [DOI] [PubMed] [Google Scholar]
- Avila H., Goncalves A. M., Nehme N. S., Morel C. M., Simpson L. Schizodeme analysis of Trypanosoma cruzi stocks from South and Central America by analysis of PCR-amplified minicircle variable region sequences. Mol Biochem Parasitol. 1990 Sep-Oct;42(2):175–187. doi: 10.1016/0166-6851(90)90160-n. [DOI] [PubMed] [Google Scholar]
- Breniere S. F., Bosseno M. F., Revollo S., Rivera M. T., Carlier Y., Tibayrenc M. Direct identification of Trypanosoma cruzi natural clones in vectors and mammalian hosts by polymerase chain reaction amplification. Am J Trop Med Hyg. 1992 Mar;46(3):335–341. doi: 10.4269/ajtmh.1992.46.335. [DOI] [PubMed] [Google Scholar]
- Degrave W., Fragoso S. P., Britto C., van Heuverswyn H., Kidane G. Z., Cardoso M. A., Mueller R. U., Simpson L., Morel C. M. Peculiar sequence organization of kinetoplast DNA minicircles from Trypanosoma cruzi. Mol Biochem Parasitol. 1988 Jan 1;27(1):63–70. doi: 10.1016/0166-6851(88)90025-4. [DOI] [PubMed] [Google Scholar]
- Gibson K. M., McLean K. A., Clewley J. P. A simple and rapid method for detecting human immunodeficiency virus by PCR. J Virol Methods. 1991 May;32(2-3):277–286. doi: 10.1016/0166-0934(91)90058-8. [DOI] [PubMed] [Google Scholar]
- Kataoka A., Claesson U., Hansson B. G., Eriksson M., Lindh E. Human papillomavirus infection of the male diagnosed by Southern-blot hybridization and polymerase chain reaction: comparison between urethra samples and penile biopsy samples. J Med Virol. 1991 Mar;33(3):159–164. doi: 10.1002/jmv.1890330304. [DOI] [PubMed] [Google Scholar]
- Kerndt P. R., Waskin H. A., Kirchhoff L. V., Steurer F., Waterman S. H., Nelson J. M., Gellert G. A., Shulman I. A. Prevalence of antibody to Trypanosoma cruzi among blood donors in Los Angeles, California. Transfusion. 1991 Nov-Dec;31(9):814–818. doi: 10.1046/j.1537-2995.1991.31992094668.x. [DOI] [PubMed] [Google Scholar]
- Morel C., Chiari E., Camargo E. P., Mattei D. M., Romanha A. J., Simpson L. Strains and clones of Trypanosoma cruzi can be characterized by pattern of restriction endonuclease products of kinetoplast DNA minicircles. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6810–6814. doi: 10.1073/pnas.77.11.6810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puchhammer-Stöckl E., Popow-Kraupp T., Heinz F. X., Mandl C. W., Kunz C. Establishment of PCR for the early diagnosis of herpes simplex encephalitis. J Med Virol. 1990 Oct;32(2):77–82. doi: 10.1002/jmv.1890320202. [DOI] [PubMed] [Google Scholar]
- Russomando G., Figueredo A., Almirón M., Sakamoto M., Morita K. Polymerase chain reaction-based detection of Trypanosoma cruzi DNA in serum. J Clin Microbiol. 1992 Nov;30(11):2864–2868. doi: 10.1128/jcm.30.11.2864-2868.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmuñis G. A. Trypanosoma cruzi, the etiologic agent of Chagas' disease: status in the blood supply in endemic and nonendemic countries. Transfusion. 1991 Jul-Aug;31(6):547–557. doi: 10.1046/j.1537-2995.1991.31691306255.x. [DOI] [PubMed] [Google Scholar]
- Sturm N. R., Degrave W., Morel C., Simpson L. Sensitive detection and schizodeme classification of Trypanosoma cruzi cells by amplification of kinetoplast minicircle DNA sequences: use in diagnosis of Chagas' disease. Mol Biochem Parasitol. 1989 Mar 15;33(3):205–214. doi: 10.1016/0166-6851(89)90082-0. [DOI] [PubMed] [Google Scholar]
- Tibayrenc M., Kjellberg F., Ayala F. J. A clonal theory of parasitic protozoa: the population structures of Entamoeba, Giardia, Leishmania, Naegleria, Plasmodium, Trichomonas, and Trypanosoma and their medical and taxonomical consequences. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2414–2418. doi: 10.1073/pnas.87.7.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibayrenc M., Ward P., Moya A., Ayala F. J. Natural populations of Trypanosoma cruzi, the agent of Chagas disease, have a complex multiclonal structure. Proc Natl Acad Sci U S A. 1986 Jan;83(1):115–119. doi: 10.1073/pnas.83.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Meirvenne N., Le Ray D. Diagnosis of African and American trypanosomiases. Br Med Bull. 1985 Apr;41(2):156–161. doi: 10.1093/oxfordjournals.bmb.a072043. [DOI] [PubMed] [Google Scholar]