Figure 7.
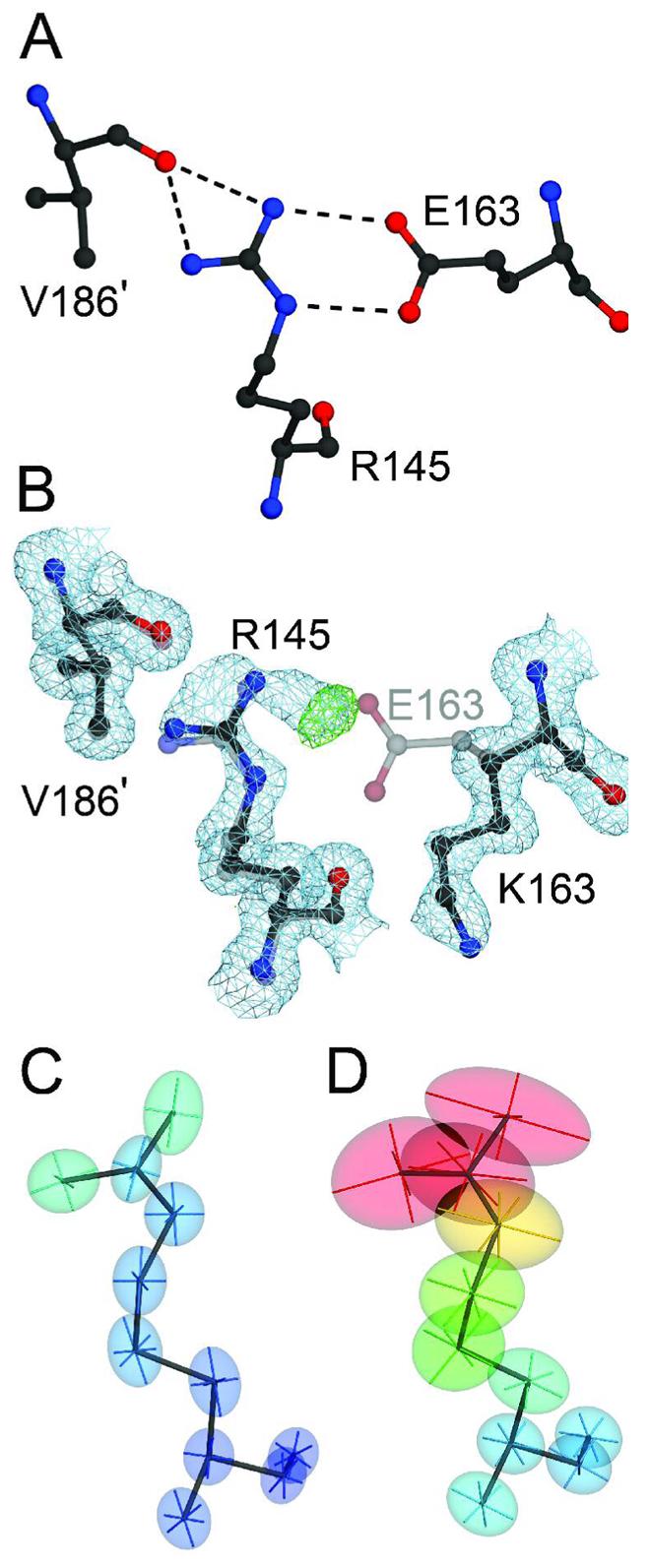
The E163K substitution disrupts a critical salt bridge in DJ-1. Panel A shows the network of hydrogen bonds (dashed lines) that extend from the E163-R145 salt bridge to V186 in the other monomer of the DJ-1 dimer (marked with a prime). Panel B shows a superposition of wild-type (1SOA(5); semi-transparent grey) and the 1.15 Å resolution crystal structure of E163K DJ-1 (dark grey), showing that the substitution of a lysine at position 163 disrupts the salt bridge with R145 and leads to reorientation of the K163 sidechain. Electron density (2mFO-DFC) contoured at 1σ (blue) and positive difference mFO-DFC electron density contoured at 4σ (green) calculated from the E163K DJ-1 model indicate that loss of the E163-R145 salt bridge leads to disorder at Arg145 in E163K DJ-1. The increased mobility of R145 is evident by comparison of the thermal ellipsoids calculated from the refined ADPs of wtDJ-1 (Panel C) and E163K DJ-1 (Panel D). The thermal ellipsoids with their principal axes are shown at the 50% probability level and are colored to indicate the magnitude of the total atomic displacement (blue; 6 Å2, red; 40 Å2). The figure was created with POVscript+(65).
