Abstract
Neurosteroids, such as the progesterone metabolite 3α-OH-5α[β]-pregnan-20-one (THP or [allo]pregnanolone), function as potent positive modulators of the GABAA receptor (GABAR) when acutely administered. However, fluctuations in the circulating levels of this steroid at puberty, across endogenous ovarian cycles, during pregnancy or following chronic stress produce periods of prolonged exposure and withdrawal, where changes in GABAR subunit composition may occur as compensatory responses to sustained levels of inhibition. A number of laboratories have demonstrated that both chronic administration of THP as well as its withdrawal transiently increase expression of the α4 subunit of the GABAR in several areas of the central nervous system (CNS) as well as in in vitro neuronal systems. Receptors containing this subunit are insensitive to benzodiazepine (BDZ) modulation and display faster deactivation kinetics, which studies suggest underlie hyperexcitability states. Similar increases in α4 expression are triggered by withdrawal from other GABA-modulatory compounds, such as ethanol and BDZ, suggesting a common mechanism. Other studies have reported puberty or estrous cycle-associated increases in δ-GABAR, the most sensitive target of these steroids which underlies a tonic inhibitory current. In the studies reported here, the effect of steroids on inhibition, which influence anxiety state and seizure susceptibility, depend not only on the subunit composition of the receptor but also on the direction of Cl- current generated by these target receptors. The effect of neurosteroids on GABAR function thus results in behavioral outcomes relevant for pubertal mood swings, premenstrual dysphoric disorder and catamenial epilepsy, which are due to fluctuations in endogenous steroids.
Keywords: GABAA receptor, Alpha-4, Delta, Neurosteroid, Allopregnanolone, Pregnanolone, THP, GABA, Hippocampus, Anxiety, Panic, Premenstrual syndrome, Premenstrual dysphoric disorder, Puberty, Mood
1. Introduction
Gamma-aminobutyric acid (GABAA) receptors (GABAR) mediate the majority of inhibition in the brain, essential for sculpting patterns of circuit activity, gating relevant sensory signals (Smith & Chapin, 1996), and restoring neuronal inhibition via feedback pathways (Smith, 2003). Behaviorally, these receptors influence mood, relieving anxiety states, and suppress seizure activity (Smith, 2003). The function of these receptors is determined to some extent by their subtype, which is under dynamic control (Mody, 2005) by the principal modulators of GABAergic inhibition in the brain, the neurosteroids.
1.1. Neurosteroids
The endogenous neurosteroid 3α-OH-5α-pregnan-20-one (allopregnanolone or 3α,5α-THP) and its active 5β isomer 3α,5β-THP (collectively referred to here as THP) are metabolites of the ovarian/adrenal steroid progesterone (Compagnone & Mellon, 2000). Similar compounds such as 5α-pregnane-3α,21-diol-20-one (THDOC) are metabolites of the adrenal steroid corticosterone (Compagnone & Mellon, 2000). Unlike classic steroids which act via nuclear receptors with ensuing genomic effects, THP and THDOC act selectively as potent positive modulators of the GABAR (Majewska et al., 1986; Callachan et al., 1987; Gee et al., 1987; Turner et al., 1989), increasing GABA-gated current at physiological concentrations by increasing the open duration and frequency of channel openings (Twyman & Macdonald, 1992). Potentiation of GABA inhibition has also been seen after intravenous administration of its parent compound progesterone (Smith et al., 1987).
1.2. The GABAA receptor
The GABAR mediates most fast inhibition in the central nervous system (CNS; Hevers & Luddens, 1998). This receptor possesses remarkable structural diversity: composed of 5 subunits, the usual stoichiometry of the receptor is 2α, 2β and 1 γ or δ subunit (Chang et al., 1990), but multiple subtypes of subunits exist, creating a vast pool of possible receptor isoforms. These different subunit combinations result in receptors with strikingly different pharmacological and biophysical properties, sometimes resulting in distinctive functional effects on excitability of CNS circuits (Hevers & Luddens, 1998).
The localization pattern of GABAR subtypes reveals highest expression of α1β2γ2 throughout the CNS (Wisden et al., 1992), which can be localized at GABAergic synapses (Nusser et al., 1996) where saturating or near-saturating levels of GABA (1 mM) are released for brief exposures (< 1 ms). Alternatively, α1β2γ2 GABAR can be localized at extrasynaptic sites where they would come into contact with ambient levels of GABA (1 μM or less) (Wu et al., 2003). Other receptor subtypes have a heterogeneous distribution, with α5-containing GABAR localized exclusively to extrasynaptic sites on CA1 hippocampal pyramidal cells, where they underlie a tonic inhibition (Caraiscos et al., 2004). In contrast, α6-containing GABAR are found exclusively on cerebellar granule cells at both synaptic and extrasynaptic sites (Nusser et al., 1998).
The α4 subunit has relatively low expression in the CNS (Wisden et al., 1992), but is concentrated on dentate gyrus granule cells, thalamic relay neurons and to a lesser extent on neurons in cerebral cortex and striatum. This subunit can coexpress with either γ2 or δ (Sur et al., 1999), yielding receptors which are insensitive to modulation by benzodiazepines (BDZ; Wisden et al., 1991; Wafford et al., 1996; Benke et al., 1997) due to an arginine at residue 99. Mutation of the homologous arginine to histidine in the BDZ-insensitive α6, as found in BDZ-sensitive α subunits (i.e., α1, α2, α3, α5) reverses this effect (Wieland et al., 1992), yielding receptors which bind BDZs (Binkley & Ticku, 1991). Recent evidence suggests that α4βγ2 may be localized at the synapse (Hsu et al., 2003; Chandra et al., 2006), as well as extrasynaptically, as shown both by electron microscopy and pharmacological modulation of the tonic current (Liang et al., 2004, 2006).
In contrast, α4β2δ GABAR are exclusively extrasynaptic (Wei et al., 2003) because they lack the γ subunit. α4β2δ GABAR have a high sensitivity to GABA (1 μM = ∼ EC75; Sundstrom-Poromaa et al., 2002) but desensitize little (Brown et al., 2002; Feng et al., 2006), making them ideally suited for an extrasynaptic function. δ-containing GABAR are also the most sensitive to modulation by steroids (Wohlfarth et al., 2002; Brown et al., 2002; Belelli et al., 2002; Bianchi & Macdonald, 2003; Liang et al., 2004), which increase receptor efficacy (Bianchi & Macdonald, 2003). α4β2δ GABAR mediate a tonic inhibitory current in thalamus (Belelli et al., 2005; Cope et al., 2005; Jia et al., 2005) and dentate gyrus (Stell et al., 2003; Mtchedlishvili & Kapur, 2006), which is sensitive to modulation by steroids, such as THP (Mtchedlishvili & Kapur, 2006) and the related steroid THDOC (Stell et al., 2003; Cope et al., 2005). This tonic current is thus the most sensitive target for steroid-induced changes in inhibitory tone of neuronal circuits, as evidenced by the reduced steroid sensitivity of mice lacking expression of the δ subunit (Mihalek et al., 1999; Spigelman et al., 2003; Shen et al., 2007).
1.3. Polarity-dependent effects of steroids
Several reports suggest that steroids such as THDOC increase current gated by δ-GABAR by increasing receptor efficacy (Bianchi et al., 2002), augmenting peak current but accelerating desensitization, to ultimately reduce current amplitude gated by saturating concentrations of GABA. However, recent studies have suggested that physiological concentrations of THP (30 nM) can accelerate the desensitization of α4β2δ GABAR in response to ambient concentrations of 1 μM GABA in a polarity-dependent manner (Shen et al., 2007). THP produced rapid desensitization of outward current (inward Cl- flux), but potentiated inward current at these receptors, suggesting that the ultimate effect of this steroid on neuronal excitability is in part determined by the direction of the Cl- gradient. In areas which normally have high levels of α4β2δ GABAR expression, dentate gyrus and cortex (Wisden et al., 1992), the GABAergic current is inward (Staley & Mody, 1992; Gulledge & Stuart, 2003), and thus inhibition in these areas would be enhanced by THP.
This polarity-dependent decrease in current generated by THP at α4β2δ GABAR was dependent upon a basic residue, arginine 353, in the intracellular loop of α4, which may function as a putative Cl- modulatory site (Shen et al., 2007). Polarity-dependent rates of desensitization have been reported for other GABAR, including the homologous α6 (Bianchi et al., 2002) as well as α5 (Burgard, 1996). This steroid-mediated polarity dependent decrease in inhibition would have important implications for effects of steroids across brain areas which generate outward GABAergic current via α4β2δ GABAR.
1.4. GABAA receptor plasticity
GABAR populations are not static, but rather undergo dynamic modulation when the balance of inhibition is increased in a chronic manner. This review will detail examples of plasticity in the GABAR population which result from fluctuations in naturally occurring or administered steroids such as THP. In particular, the α4 subunit undergoes marked changes in expression as a response to fluctuating levels of steroids. These steroid-induced fluctuations in GABAR isoforms result in alterations in CNS excitability with implications for syndromes such as pubertal mood swings, premenstrual syndrome (PMS), postpartum blues, and the perimenopause when alterations in mood can occur.
2. Steroids and endogenous cycles
2.1. Steroid metabolism
THP is produced systemically from progesterone of ovarian or adrenal origin, but can also be synthesized de novo in the brain from cholesterol via side chain cleavage enzyme which forms pregnenolone, the precursor of all steroid molecules (Compagnone & Mellon, 2000). 3β-Hydroxysteroid dehydrogenase converts pregnenolone to progesterone, which is then converted via neuronal enzymes into THP. Both the 5α-reductase and 3α-hydroxysteroid oxidoreductase (3α-HSD) enzymes are under steroidal control (Compagnone & Mellon, 2000; Belelli & Herd, 2003), and can be inhibited with selective blockers, allowing direct manipulation of THP levels experimentally. A recent study has demonstrated that output cells of cortex, hippocampus, thalamus, and amygdala contain both 5α-reductase and 3α-HSD enzymes for local formation of THP (Agis-Balboa et al., 2006). There are 2 subtypes of 3α-HSD enzymes localized to the cytosol or membrane-bound, which catalyze either the forward reducing reaction to THP or the reverse oxidative pathway, respectively (Mellon & Vaudry, 2001). Regional variations in these subtypes (Mellon & Vaudry, 2001) dictate to some extent the local concentrations of THP in various brain structures. Thus, THP is effectively both a neurosteroid (i.e., “made in the brain”) as well as a neuroactive steroid (i.e., positive GABA modulator).
2.2. Puberty
Levels of THP remain at very low levels throughout postnatal development but increase in the period immediately before the onset of puberty (Fadalti et al., 1999). In the human female, puberty is comprised of 5 stages which extend across a period of several years and culminate in the onset of menstruation. Fully ovulatory periods generally occur months later. Steroids, such as 17β-estradiol and THP, remain relatively low in the early phases of puberty but increase throughout development, with peak levels of THP noted during stages IVand V (Fadalti et al., 1999) prior to the onset of fluctuations induced by the menstrual cycle (see below). High circulating and CNS levels of THP have been noted in the mouse and rat immediately before puberty onset (Mannan & O’Shaughnessy, 1988; Palumbo et al., 1995), followed by a decline (Palumbo et al., 1995; Shen et al., 2007).
2.3. Menstrual cycle
Circulating levels of THP parallel those of its parent compound progesterone across the menstrual cycle; that is, THP levels remain low in the follicular phase of the cycle prior to the midcycle peak in estradiol but are elevated in the midluteal phase for around 10-11 days before declining in the late luteal phase (Schmidt et al., 1994; Rapkin et al., 1997). In this case, THP is formed directly from progesterone, which is produced at high levels from the corpus luteum following ovulation. This pattern of steroid formation is distinct from the rodent (Mannan & O’Shaughnessy, 1988), where the granulosa cells of the ovary form progesterone at high levels prior to ovulation, and following peak levels of estradiol. The rodent has a 4- or 5-day cycle with highest steroid levels on the day of proestrus when circulating levels of progesterone and THP are elevated for 10-12 h in the late PM (Corpechot et al., 1997). Ovulation takes place on the night of proestrus (also known as “behavioral estrus” because it is the time of peak sexual receptivity), and the resulting corpus luteum develops over several days to produce a smaller secondary increase in progesterone (and THP) on the day of diestrus-1. Thus, the human cycle is associated with a much longer period of THP exposure than the rodent, which in contrast exhibits 2 peaks of THP, suggesting that differences in steroid modulation of GABAR expression may result.
In addition to fluctuations in endogenous THP across the estrous cycle, the mouse also exhibits fluctuations in brain THP levels across the circadian cycle (Corpechot et al., 1997). In this case, THP levels peak at the onset of the dark phase, with levels that exceed those seen across the estrous cycle in the light phase (∼ 30-40 nM). This suggests that the mouse may be more dependent upon steroid modulation of GABAergic inhibition than other species.
2.4. Pregnancy
In pregnancy, the highest levels of THP are achieved as a result of increased progesterone production from the corpus luteum during the first 3 months and the placenta thereafter (Paoletti et al., 2006). Circulating levels of THP increase from a low of < 3 ng/mL at the onset to 50 ng/mL by the end of a full-term pregnancy (40 weeks; Paoletti et al., 2006). Progesterone levels increase in a parallel fashion, but smaller increases in precursors such as pregnenolone and 3α-dihydroprogesteone are observed (Gilbert Evans et al., 2005; Paoletti et al., 2006). Circulating and umbilical cord levels of THP peak at delivery, but rapidly decline in the postpartum period. In the rodent peak levels of THP are achieved by day 19 of pregnancy and ∼ 70 nM, 2-fold higher than seen across the circadian cycle (Concas et al., 1999; Zuluaga et al., 2005). Thus, pregnancy represents the most significant exposure to THP in terms of duration and concentration.
2.5. Stress and addictive drugs
In addition to reproductive cycles, acute stress has been shown to increase production of THP in both human and rodent studies (Purdy et al., 1991). In rodents, 45 min of restrain stress or 5 min of CO2 asphyxiation stress increase CNS levels of THP for up to 30 min to 2 hr after the event (Barbaccia et al., 1996, 2001; Higashi et al., 2005). Social isolation stress has also been shown to increase neurosteroid formation in male rodents (Dong et al., 2001). In humans, examination stress (Droogleever Fortuyn et al., 2004) and performance stress (Girdler et al., 2006) were also accompanied by significant elevations in circulating levels of THP in both sexes, although ethnic differences have been reported (Girdler et al., 2006). Recent studies also suggest that drugs such as alcohol (VanDoren et al., 2000), morphine and δ-9-tetrahydrocannabinol (Grobin et al., 2005) increase circulating THP levels as a long-term response to intake of these drugs. Thus, stress may represent an additional mechanism by which neurosteroid levels can be altered over time, irrespective of gender.
2.6. Menopause
As ovarian function declines in the perimenopause, both progesterone and THP levels decrease in the circulation. One study has also shown declining brain levels of the steroid in postmenopausal women, assessed postmortem (Bixo et al., 1997). This is an additional phase of life when long-term alterations in levels of the steroid may alter GABAergic function.
3. Effect of chronic THP on GABAA receptor expression
3.1. Time course of steroid effects on α4 subunit expression
Because exposure to endogenous steroids across natural cycles is normally extended across days to weeks, a number of studies have investigated the effect of chronic steroid exposure on GABAR function. In our laboratory, in vivo treatment of female rats with progesterone or THP increased expression of the normally underexpressed α4 subunit in the hippocampus, an effect which reached significant levels after 48-72 hr (Fig. 1; Gulinello et al., 2001; Hsu et al., 2003). This was a transient event, as continued treatment with the steroid reflected a decrease in the subunit back to control levels after 4-5 days (Gulinello et al., 2001), suggesting that it represented a compensatory response to the continued presence of the GABA-modulatory steroid. Because the α4 subunit is BDZ insensitive (Wisden et al., 1991; Wafford et al., 1996), functional expression of α4-containing GABAR was verified by a near insensitivity of isolated CA1 hippocampal pyramidal cells to BDZ modulation across a range of concentrations (0.01-100 μM lorazepam [LZM]) evaluated using whole-cell patch clamp techniques (Fig. 1; Gulinello et al., 2001). Furthermore, the anxiolytic response to LZM was blunted, as evidenced by reduced open arm entries on the elevated plus maze (Gulinello et al., 2001; Gulinello & Smith, 2003), suggesting that increased expression of α4-containing GABAR has a functional impact on behavior.
Fig. 1.
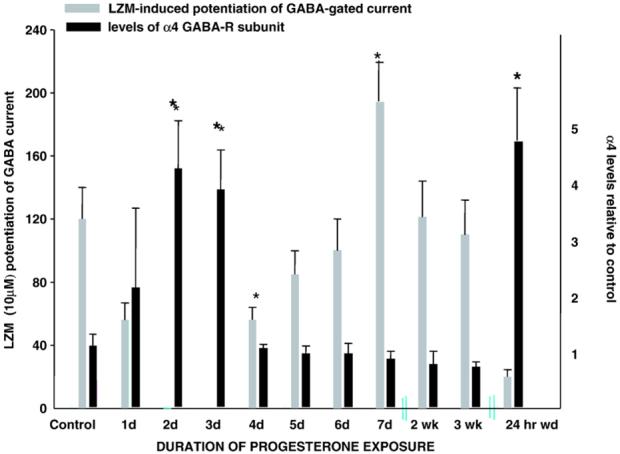
Time course of progesterone effects on GABAR subunit composition and pharmacology. Levels of α4 expression were detected with Western blot techniques on a daily or weekly basis during sustained exposure to progesterone (and thus THP) via subcutaneous capsule for 3 weeks. Levels of α4 expression increased significantly by 2-3 days of progesterone exposure compared to vehicle; then levels recovered to control values across the remainder of the exposure period until progesterone withdrawal (24 hr after capsule removal), when significant increases in α4 expression were again observed (P<0.001). Concomitant pharmacological evaluation of BDZ LZM potentiation of GABA-gated current evaluated in pyramidal neurons acutely isolated from CA1 hippocampus revealed a LZM insensitivity closely correlated with α4 upregulation. *P<0.05 (from Smith, 2003, reprinted with permission).
Our more recent in vitro studies also demonstrate that 48-hr THP exposure of a neuroblastoma cell line, IMR-32, also increases α4 expression (Zhou & Smith, 2007). This in vitro model required prior differentiation of the cells in a serum-free medium to reduce the ambient steroid levels and application of nerve growth factor to enhance developmental of dendritic processes. Other in vitro studies have reported that 7-day administration of the parent compound progesterone increases α4 expression in NT2-N cells (Pierson et al., 2005). Interestingly, 48-hr administration of estradiol also increased α4 expression (Zhou & Smith, 2007), as has been shown for 7-day administration of estradiol to NT2-N cells (Pierson et al., 2005). In the latter study (Pierson et al., 2005) 7-day progesterone also increased α2 and γ3 subunit messenger ribonucleic acid (mRNA) and decreased α5 mRNA, while 7-day estradiol treatment increased α3, β3, and ε mRNA.
Other studies (Grobin & Morrow, 2000) noted decreases in α4 expression after 5-day administration of THP. The discrepancy here may be related to the use of serum-containing medium and length of treatment, which have selective effects on α4 expression. These effects of chronic steroid administration may be dependent upon the developmental stage, as this study (Grobin & Morrow, 2000) utilized developmental day 19.
3.2. Pharmacological changes
A number of studies have suggested that chronic exposure to neuroactive steroids result in altered GABAAR pharmacology, which in some cases was associated with subunit changes. For instance, micromolar concentrations of THP were shown to decrease GABA-gated current recorded from neocortical neurons in culture after 5-day exposure (Yu et al., 1996a, 1996b). In addition, modulation of GABA-gated current by barbiturates and THP was also significantly decreased by this long-term hormone exposure (Yu & Ticku, 1995a; Yu et al., 1996a, 1996b). These changes were accompanied by decreases in the β subunits, generally, and a decrease in the α3 subunit, specifically (Yu et al.,1996a, 1996b). Although basal binding was unaltered by this treatment, heterologous uncoupling between GABA, barbiturate, neurosteroid sites and the BDZ site was observed as a decrease in Emax values (Yu & Ticku, 1995b; Friedman et al., 1996). Heterologous uncoupling for THP had an EC50 of 1.7 μM and was blocked by the GABA site antagonist SR-95531, whereas homologous uncoupling was resistant to this antagonist, suggesting 2 distinct sites of action for the 2 effects of the steroid. Neither depolarization nor voltage-gated Ca++ channels played a role in this phenomenon (Lyons et al., 2001). A more recent study showed that uncoupling was dependent upon receptor activation, as well as transcription (Gravielle et al., 2005).
Other studies have demonstrated significant alteration in GABAAR subunits, which are differentially expressed in subzones of the hippocampal formation by 2-3 day exposure to progesterone (Weiland & Orchinik, 1995). Specifically, THP and progesterone decreased α1 mRNA in CA2, CA3, and dentate gyrus in animals that were pretreated with estradiol. In contrast, progesterone also decreased α2 to a lesser extent in the CA3 region, in estradiol-treated animals, but increased mRNA for γ2 in CA1, CA2, and CA3 areas in animals untreated with estradiol. Other studies (Friedman et al., 1996; Yu & Ticku, 1995b) noted that 48-hr exposure of cultured neurons to THP reduced the ability of GABA to increase BDZ binding (heterologous uncoupling), an outcome which may be due to increased α4 expression.
3.3. Synaptic current
Because α4-containing GABAR may localize to either synaptic or extrasynaptic compartments on the CA1 hippocampal pyramidal cell, we conducted studies to examine miniature inhibitory synaptic currents (mIPSC) after steroid treatment. These represent the unitary response to quantal release of transmitter, and thus more closely reflect the properties of the postsynaptic receptor population. In fact, mIPSCs recorded after 48-hr THP in vivo treatment to female rats exhibited a decreased response to 10 μM LZM (Hsu et al., 2003), which was reflected by about 50% of the recorded population. Other pharmacological characteristics of α4 which coexpress with the γ2 subunit include atypical responsiveness to the BDZ partial inverse agonist RO15-4513 (Wafford et al., 1996). This compound normally reduces GABA-gated current at non-α4 subtypes, but increases current via α4βγ2 GABAR, as reflected by an increase in the decay time of mIPSCs recorded after 48-hr THP treatment.
In addition to pharmacological changes, significant acceleration in the decay time of α4-containing GABAR were also detected following 48-hr THP treatment (Hsu et al., 2003). Specifically, the decay time constant τ-fast was decreased relative to the control currents, while the slow component of decay was unaltered (Fig. 2). This acceleration in the kinetics of synaptic current was prevented when α4 expression was suppressed using intraventricular administration of α4-selective antisense oligonucleotides (Fig. 2; Hsu et al., 2003). Thus, these results suggest that increased expression of α4-containing GABAR subsynaptically accelerates the decay of current. These findings were confirmed in a more recent study employing rapid application techniques to directly assess postsynaptic current kinetics in hippocampal neurons acutely isolated following 48-hr THP exposure (Smith & Gong, 2005). In fact, direct assessment of the kinetics of recombinant α4β2γ2 GABAR expressed in human embryonic kidney (HEK)-293 cells revealed an accelerated τ-fast compared to other GABAR (Fig. 3), including α1β2γ2, α2β2γ2 (Lavoie et al., 1997), α3β2γ2 (Gingrich et al., 1995) and α5β2γ2 (Smith & Gong, 2005). This faster decay would result in less total charge transfer and thus would be expected to reduce inhibition. This possibility is also supported by recent findings in the α4 knock-out mouse (Chandra et al., 2006) where the fast component of decay of mIPSCs recorded from dentate gyrus was slower than in comparable recordings from wild-type mouse.
Fig. 2.
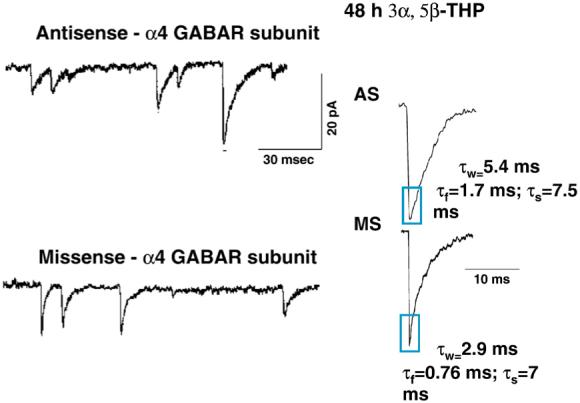
Suppression of α4 expression alters the kinetics of mIPSCs after chronic neurosteroid exposure. For this study, female rats were administered THP for 48 hr (10 mg/kg, i.p.) in conjunction with α4 antisense (or missense) oligonucleotide administered intraventricularly to suppress α4 expression. mIPSCs were recorded from the soma with whole cell patch clamp techniques from CA1 hippocampal pyramidal cells in the slice at near physiological temperature (33-35 °C). Representative traces (left) and averaged currents (right) reveal that THP treatment results in a faster τ-fast of decay, an effect prevented by antisense suppression of α4 expression (n = 30-34 rats; from Hsu et al., 2003, reprinted with permission).
Fig. 3.
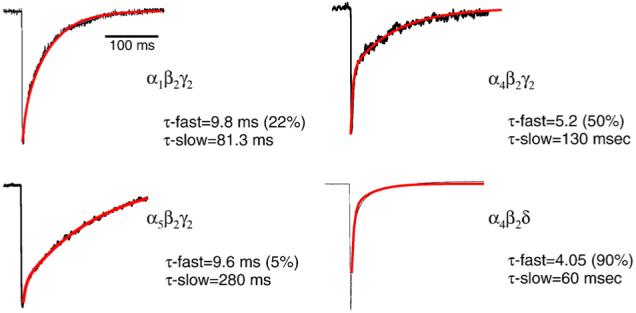
α4-containing GABAR exhibit faster deactivation kinetics than other receptor subtypes. Representative current traces illustrate the different kinetics exhibited by α1β2γ2, α4β2γ2, α4β2δ, and α5β2γ2 recorded from HEK-293 cells using outside-out patch recording techniques and rapid application of saturating concentrations of agonist. Both α4-containing GABAR deactivate with an accelerated τ-fast compared with the other subtypes. These results are representative of those from 6 to 8 cells/group (from Smith & Gong, 2005).
3.4. Extrasynaptic current
Although the previous studies suggest that α4β2γ2 GABAR may express at the synapse, these receptors have also been localized extrasynaptically, both based on electron microscopic findings as well as pharmacological modulation of the tonic current (Liang et al., 2004, 2006). In addition, α4βδ and the homologous α5βδ GABAR are localized exclusively at extrasynaptic sites where they mediate a tonic current (Brickley et al., 2001; Stell & Mody, 2002; Stell et al., 2003; Farrant & Nusser, 2005). Therefore, we directly assessed δ subunit expression levels in CA1 hippocampus after 48-hr THP administration. Our findings suggest that levels of the δ subunit are increased 4- to 5-fold after 48-hr steroid exposure (Shen et al., 2005). δ subunit upregulation was associated with alterations in the pharmacology of the tonic GABAergic current recorded from CA1 pyramidal cells in the hippocampal slice, which showed increased responsiveness to the GABA agonist gaboxadol (THIP) and was completely inhibited by application of La3+ (Shen et al., 2005). Both pharmacological findings are consistent with results from α4βδ recombinant receptors (Brown et al., 2002), suggesting functional expression of this receptor subtype. However, the tonic current was unresponsive to the BDZ agonist LZM as well as the BDZ partial inverse agonist RO15-4513, suggesting that unlike the synaptic current, α4βγ2 GABAR are not expressed extrasynaptically after steroid exposure although they have been noted in control animals (Liang et al., 2004, 2006).
3.5. Compensatory change in α1
The increase in CA1 hippocampal α4 and δ subunit expression after 48-hr THP treatment was accompanied by a compensatory decrease in α1 expression (Shen et al., 2005). This was verified pharmacologically by an insensitivity to the α1-selective type I BDZ agonist zolpidem, which normally enhances tonic current in control hippocampus. This “subunit switch” did not result in alteration of the magnitude of the tonic current.
3.6. Physiological outcome
The functional implications of effects on synaptic and extrasynaptic current are quite different. Synaptic current mediates afferent-selective reduction in neuronal activity, as would be important for sensory gating (Smith & Chapin, 1996) and feedback inhibition mediated by activation of excitatory inputs (Hsu & Smith, 2003). In contrast, alterations in tonic inhibition would exert a greater impact on the general input resistance of the neuron, increasing the threshold for eliciting an action potential (Brickley et al., 2001; Shen et al., 2007).
The physiological outcome of such a switch in subunit composition of extrasynaptic receptors would also be expected to differentially affect neuronal excitability depending on the localization of the receptors. Localization peri-synaptic to inhibitory input would decrease inhibition due to the faster deactivation of α4β2δ GABAR compared to α1β2γ2 (Fig. 3; Smith & Gong, 2005), while sites distant from the synapse would be expected to increase inhibitory tone because α4βδ GABAR do not desensitize as readily as α1βγ2 GABAR (Brown et al., 2002). Relatively low levels of afferent activity would activate synaptic α4βγ2 receptors with rapid deactivation, which would yield a decreased level of inhibition compared to α1-GABAR. Higher levels of afferent activity would activate perisynaptic α4βδ receptors with even faster rates of deactivation, decreasing inhibition compared to α1-GABAR. However, highest levels of afferent activity would activate the distant extrasynaptic receptors, resulting in less desensitization, and thus, relatively higher levels of inhibition compared to comparable effects at α1-containing GABAR.
4. Steroid withdrawal
4.1. Effect of THP withdrawal on α4 subunit expression
As noted above, the timecourse of changes in α4 expression as a response to the continued exposure to THP is complex (Gulinello et al., 2001). In addition to its effect on GABAR plasticity after 48-hr exposure, THP also exhibits withdrawal properties following chronic exposure. Following a 21-day administration of either THP directly (Fig. 4) or its parent compound progesterone to female rats, discontinuation of steroid administration (i.e., “withdrawal”) resulted in a marked 3-fold increase in hippocampal expression of the α4 subunit, assessed by both mRNA and protein levels (Smith et al., 1998a). This effect was accompanied by a change in the pharmacological state of the CA1 pyramidal cells to reflect α4βγ2 expression (Wafford et al., 1996). That is, GABA-gated current was insensitive to modulation by a BDZ agonist (LZM; Fig. 4), reversed the effect of a BDZ antagonist (flumazenil) and a BDZ partial inverse agonist (RO15-4513) to a BDZ agonist (Smith et al., 1998a). Antisense suppression of α4 expression restored the responsiveness to BDZs and resulted in a pharmacological characterization consistent with expression of non-α4 receptors (Smith et al., 1998a). Despite the change in pharmacology, the amplitude of the GABA-gated current was unchanged by steroid withdrawal, however, suggesting that α4 was substituting for other subunits rather than adding to the total pool of functional GABARs. Other studies have noted increases in expression of the α4 subunit following withdrawal from THP using an in vitro system (Follesa et al., 2000).
Fig. 4.
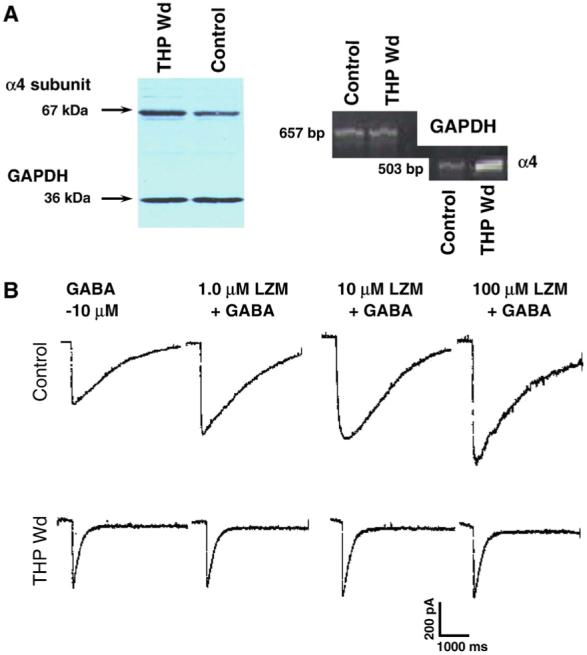
Withdrawal from THP increases hippocampal α4 expression and results in a BDZ insensitivity. (A) 24 hr withdrawal from chronic administration of THP (10 mg/kg, i.p. for 3 weeks) to adult, female rats increased expression of α4 protein (left) and α4 mRNA (right) in hippocampal membranes compared to vehicle-injected controls. (B) This THP withdrawal state produced a relative insensitivity to modulation of GABA (10 μM)-gated current by the BDZ LZM in pyramidal neurons acutely isolated from CA1 hippocampus and recorded with whole-cell patch clamp techniques. In contrast, LZM was effective in potentiating GABA-gated current up to 2-fold in neurons from control rats (n = 6-8 cells/group).
4.2. Kinetics of GABA-gated current
Because initial studies suggested that the kinetics of GABA-gated current were accelerated after steroid withdrawal, our studies examined this directly using a piezo-controlled theta tube to rapidly apply saturating concentrations of agonist to outside-out patches of membrane. As reported for mIPSCs after chronic steroid treatment, GABA-evoked current deactivated more rapidly after steroid withdrawal (Smith & Gong, 2005). τ-fast was significantly accelerated in the majority of the currents recorded, but values for τ-slow exhibited a bi-modal distribution of currents which were accelerated or unchanged from control. These deactivation times approximate those we have shown for recombinant α4β2δ and α4β2γ2 (Fig. 3; Smith & Gong, 2005), respectively, suggesting that the subunit conformation of the receptor underlies this change in kinetics.
The mechanism underlying acceleration in the deactivation rate, and τ-fast, in particular, is thought to be a function of the initial closing of channels in a burst, as we have also demonstrated in theoretical analysis (Smith & Gong, 2005). In fact, the mean open time of α4β2γ2 GABAR is one-half that of α1β2γ2 (Akk et al., 2004), while the mean open time of α4β2δ is considerably faster and lacks the longest open channel time reported for α1β2γ2 (Bianchi & Macdonald, 2003). The physiological outcome of such an acceleration in deactivation would be to decrease the total charge transfer, as seen after the 48-hr steroid paradigm, leading to decreases in inhibition at the circuit level. However, other characteristics of single channel openings, such as burst duration, may also play a role in macroscopic kinetics. Here, α4β2γ2 and α4β2δ GABAR single channel behavior can be readily distinguished, as α4β2γ2 receptor openings occur in well-defined clusters, while α4β2δ receptor openings occur as isolated events (Akk et al., 2004). This difference in single channel burst events may account for the more prolonged τ-slow of α4β2γ2 versus α4β2δ GABAR (Smith & Gong, 2005).
4.3. Hippocampal excitability
Withdrawal from THP also increased excitability of the circuit, assessed using a paired-pulse paradigm (Fig. 5; Hsu & Smith, 2003). To this end, paired stimulations (10 ms apart) to the Schaffer collaterals were used to assess the inhibition generated by inhibitory loops. Steroid withdrawal greatly reduced the level of feedback inhibition (Fig. 5), an effect which was reversed when α4 expression was suppressed using intraventricular antisense selective for the α4 subunit (Hsu & Smith, 2003). This is in contrast to acute administration of THDOC, a similar steroid, which decreases excitability of dentate gyrus granule cells via potentiation of the GABAergic tonic current (Stell et al., 2003).
Fig. 5.
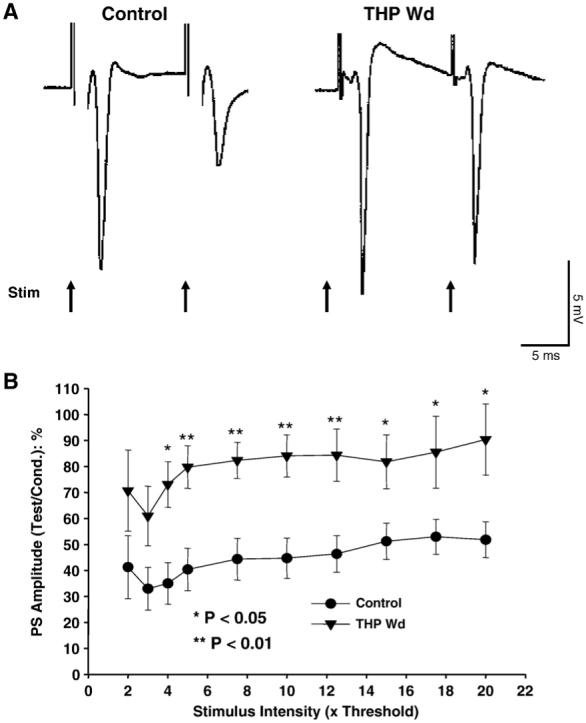
THP withdrawal reduces paired-pulse inhibition. Following 24 hr withdrawal from chronically administered THP (10 mg/kg, i.p. for 3 weeks) to adult, female rats hippocampal slices were prepared, and population EPSPs recorded from the pyramidal cell layer of CA 1 hippocampus in response to 2 stimulating pulses (4× threshold, 10 ms interpulse interval) delivered to the Schaffer collaterals. In contrast to the vehicle-injected controls, which exhibited a highly significant reduction in population spike amplitude in response to the second (test) pulse compared to the first (conditioning) pulse, reduction in PS amplitude in response to the second stimulus was not significantly different from the response to the first stimulus after THP withdrawal. This demonstrates a decrease in feedback inhibition after THP withdrawal. (A) Representative traces. (B) Population data (n = 10-12 slices/group).
4.4. Progesterone withdrawal and the δ subunit
In addition to increasing hippocampal α4βγ2 GABAR, withdrawal from progesterone also upregulated the δ subunit (Sundstrom-Poromaa et al., 2002) which co-expressed with α4 based on coimmunoprecipitation findings. This increase in α4βδ GABAR produced additional pharmacological changes of the GABA-gated current recorded from CA1 hippocampal pyramidalcells. Consistent with findings in recombinant α4βδ receptors (Brown et al., 2002), gaboxadol (THIP) generated a greater maximal current than GABA, which was blocked by La3+ (Sundstrom-Poromaa et al., 2002). This effect was paralleled by an increase in the anxiolytic effectiveness of gaboxadol compared to control rats (Gulinello et al., 2003a). In addition, GABA-gated current was potentiated by low doses of ethanol (Sundstrom-Poromaa et al., 2002), an effect also reflected by an increased behavioral sensitivity to the drug. Recent studies have suggested the α4β2/3δ GABAR possess a unique sensitivity to low doses of ethanol (Sundstrom-Poromaa et al., 2002; Wallner et al., 2003), although conflicting reports exist (Borghese et al., 2006). Increases in δ-subunit expression have also been noted in in vitro systems following progesterone treatment or withdrawal (Mostallino et al., 2006).
4.5. Pregnancy and THP withdrawal
Pregnancy is associated with prolonged increases in circulating levels of progesterone and its THP metabolite (Concas et al., 1999; Gilbert Evans et al., 2005). In order to distinguish between direct actions of THP and other nonspecific effects of pregnancy (i.e., changes in body weight, maternal behavior, effects of the anxiolytic hormone oxytocin, etc.), we used a model of pseudopregnancy (Smith et al., 1998b), which uses HCG and PMSG to stimulate ovarian production of progesterone and THP to pregnancy-like levels (i.e., 70 nM; Paoletti et al., 2006). Using this model, THP withdrawal was induced by removal of the ovaries or with a 5α-reductase blocker to stop formation of THP. Using either of these 2 approaches to end the ovarian production of steroid or to directly induce THP withdrawal, α4 expression was increased in the hippocampus (Smith et al., 1998b), accompanied by a relative BDZ insensitivity of the GABA-gated current. Increases in anxiety also accompanied this “withdrawal” state, suggesting that it may be relevant as a model of postpartum dysphoria (Smith et al., 1998b). Consistent with this, α4 levels are not altered in rodent pregnancy, but are elevated by 7-day postpartum (Concas et al., 1999).
GABAR are also found in the uterus where this form of inhibition plays an important role in controlling uterine contractility during pregnancy. One study (Fujii & Mellon, 2001) examined the changes in GABAR subunits which accompany pregnancy: α1 expression increased and α2 and δ expression decreased, whereas expression of the ð subunit decreased at the onset of labor. These changes were accompanied by altered responsiveness to THP, with the greatest response on day 19 of pregnancy, when THP levels peak, and the least sensitive period at the onset of labor, when uterine contractility is maximal (Fujii & Mellon, 2001).
4.6. Seizure susceptibility
Progesterone withdrawal also increased seizure susceptibility (Smith et al., 1998a), lowering the threshold for seizure induction by picrotoxin or a BDZ inverse agonist and increasing the severity and timecourse of the seizure-like activity. This effect was prevented when α4 expression was suppressed using in vivo antisense treatment, suggesting that increased α4 expression mediates the increase in convulsant activity (Smith et al., 1998a).
Other studies (Reddy et al., 2001; Rogawski, 2003) have investigated the use of progesterone withdrawal as a model of catamenial epilepsy either after progesterone injection or induction of pseudopregnancy via ovarian stimulation (Reddy et al., 2001). In both cases, the threshold for pentylenetetrazole-induced seizures was lowered. Furthermore, the pharmacological changes were consistent with expression of α4βγ2 receptors (Wafford et al., 1996), that is, increased responsiveness to the anticonvulsant effect of pentobarbital (Reddy & Rogawski, 2001).
4.7. Anxiety
The GABAR, as the primary mediator of CNS inhibition, plays a pivotal role in the regulation of anxiety states (Rudolph et al., 1999). In fact, THP, when acutely applied, is a potent anxiolytic when given systemically (Bitran et al., 1995) or locally applied to the dorsal hippocampus (Bitran et al., 1999), a component of the limbic system important in generating mood. In addition, decreases in anxiety are tightly correlated with increased circulating levels of endogenous THP on proestrus (Frye et al., 2000) and can be prevented when THP formation is blocked (Frye & Walf, 2002).
However, most positive GABA modulators exhibit withdrawal states after chronic exposure when increases in anxiety and dysphoria occur. Thus, we studied the effect of progesterone withdrawal on anxiety using a variety of rodent models. In fact, a relatively short period (4-5 days) of progesterone exposure produced increases in anxiety behavior 24 hr following its discontinuation (Gallo & Smith, 1993). This was assessed using light/dark transition and the burying response as indicators of anxiety state. In the first model, transition from a well-lit to a dark chamber indicates an increase in anxiety. In the second, the animal is exposed to an electrified prod, an aversive stimulus, which reflexively results in burying behavior. In this test, the latency to and duration of this response reflects the level of anxiety. Progesterone withdrawal increased anxiety using both measures, an effect which was enhanced when 17β-estradiol was co-administered (Gallo & Smith, 1993). This anxiogenic effect of progesterone withdrawal was prevented when THP formation was prevented by concomitant administration of a 5α-reductase blocker, finasteride, suggesting that it was due to THP. In addition, animals tested following withdrawal from THP administered directly exhibited a 72% decrease in the latency (P<0.05) and a 7-fold increase in the duration (P<0.05) of the burying response compared to vehicle-injected controls.
Later studies used a longer period of progesterone or THP exposure to demonstrate increases in anxiety assessed using the elevated plus maze. In fact, 11-21 day periods of THP exposure, produced by pseudopregnancy induction (Smith et al., 1998b) or progesterone administration, decreased open arm entries on the elevated plus maze, a measure of increased anxiety. This effect was observed in both male and female rats, and was produced by direct block of THP formation despite high circulating levels of progesterone (Smith et al., 1998b). A more recent study has shown that the anxiogenic effect of progesterone withdrawal is correlated with the initial performance state of the animal, with a high level of open arm entries associated with a greater increase in anxiety after progesterone withdrawal (Lofgren et al., 2006).
Another rodent model of anxiety, the acoustic startle paradigm, is often used to assess the level of anxiety in both rodent models and in human conditions (Davis et al., 1982) and is increased following withdrawal from alcohol or chronic stress. It is also significantly increased during the luteal phase in women with premenstrual dysphoric disorder (PMDD; Epperson et al., 2007). The amplitude of this response was significantly increased after progesterone withdrawal in female rats (Gulinello et al., 2003b) but not in males. This sex difference may be related to the fact that male rats exhibit higher baseline levels of startle response (Plappert et al., 2005) or to other gender-related steroids, such as estradiol, which can increase neuronal excitability (Smith, 2003). The acoustic startle also exhibits prepulse inhibition, that is, a reduced response to a 120 dB auditory stimulus following an initial stimulus subthreshold for a startle response (Gulinello et al., 2003b). This prepulse inhibition also exhibited gender differences, as it was decreased following progesterone withdrawal in female rats but not in males. Our most recent study (Smith et al., 2006) indicates that THP withdrawal in the female mouse reverses the effect of THP from anxiolytic to anxiogenic (Fig. 6), an effect which may be relevant for PMS (Schmidt et al., 1998).
Fig. 6.
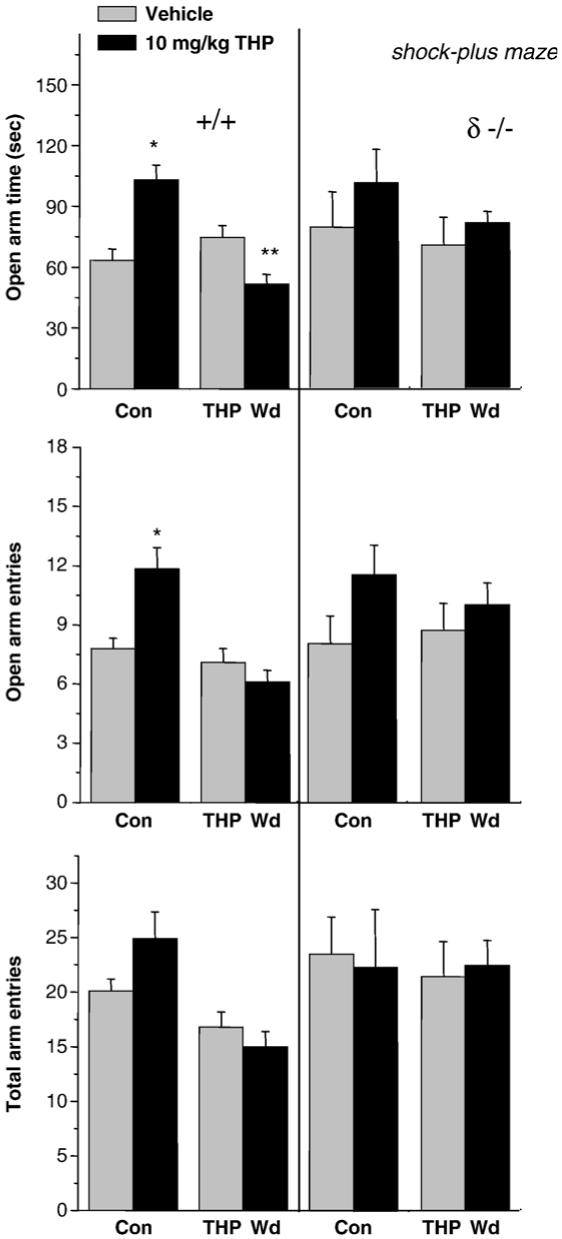
THP increases anxiety in a shock-paired plus maze paradigm following THP withdrawal in mice. For this study, mice were subjected to withdrawal from their naturally high levels of endogenous THP with a 5α-reductase blocker (finasteride, 50 mg/kg, i.p., 3 days). When tested in the elevated plus maze following a brief shock (left panel), THP decreased open arm time following THP Wd, in contrast to its effect in control mice, where it increased this parameter. (*P<0.05 vs. vehicle, n = 17-26). The total number of arm entries (lower panel) was not altered by any experimental treatment. This anxiogenic effect of THP (right panel) was not observed after THP Wd in δ knock-out mice, implicating δ-GABAR in this paradoxical effect of the steroid.
4.8. Steroid withdrawal and hypothalamic neurons
In the hypothalamus, the effects of hormone withdrawal may have very different implications. During pregnancy, activity in oxytocin-containing neurons is depressed due to extensive GABAergic innervation of these neurons and the influence of high-ambient THP levels on synaptic current (sIPSC), which results in prolongation of the decay time (Brussaard et al., 1997). Additionally, THP prevents suppression of sIPSCs by oxytocin, an effect mediated by protein kinase C (PKC)-induced phosphorylation, thus, further ensuring that premature labor does not occur (Brussaard et al., 2000). At the time of parturition, however, a number of changes in GABAergic inhibition occur which tip the balance of neuronal excitability in the positive direction. GABAR subunit composition in oxytocin neurons within the supraoptic nuclei is altered during pregnancy, with an increase in α2:α1 subunit ratio occurring at the time of partrurition as well as a more modest increase in α4 levels (Brussaard et al., 1997, 1999). Consistent with previous reports of GABAR subunit effects on the kinetics of GABA-gated current (Lavoie et al., 1997), the increase in α2-containing GABAR was associated with a marked increase in τ for GABAergic monoquantal sIPSCs. In fact, this subunit switch was the mediating factor in promoting the increase in τ, as antisense-induced suppression of α2 expression prevented the change in τ (Brussaard et al., 1997). However, oxytocin-induced activation of PKC produced a relative neurosteroid insensitivity which was independent of receptor isoform (Koksma et al., 2003). During partrurition and lactation, this steroid insensitivity rendered oxytocin neurons with a net acceleration in decay time, as compared with this value during pregnancy, a time of elevated THP levels. Initial observations during early pregnancy also demonstrated an increase in the frequency of these monoquantal events, a finding consistent with other reports of increases in GABAergic innervation of these neurons during pregnancy. In order to determine the net GABAergic inhibition of this circuit, the authors calculated the synaptic current densities by integrating synaptic current in the presence of THP across time and express this value with respect to the cell capacitance, a quantitative measure of the cell membrane surface area and thus cell size. Because swelling of oxytocin cell bodies occurs during pregnancy and lactation (Hatton, 1997), cell capacitance measurements also increase across the period of lactation. Assessed in this way, the total synaptic inhibition was markedly decreased, beginning in late pregnancy and continuing throughout the lactation period, compared with that determined for virgin control animals. Such a change would permit increases in excitability of the oxytocin neurons whose bursting discharge would release oxytocin to promote uterine contractility necessary for the birth process and subsequent lactation period.
5. The estrous cycle and GABAA receptor expression
5.1. GABAA receptor δ subunit
Several studies have correlated changes in GABAR subunit expression with the estrous cycle. In one landmark study (Maguire et al., 2005), increases in δ subunit expression on diestrus-1 were correlated with increased amplitude of the tonic current recorded from the dentate gyrus granule cells. Diestrus-1 is associated with a secondary peak of THP, and the δ-containing GABAR are the most sensitive targets for this steroid (Wohlfarth et al., 2002). Indeed, increased expression of δ was associated with a decrease in the γ2 subunit but no change in the α4 subunit, suggesting that α4 changed its coexpression to form α4βδ GABAR. This increase in tonic inhibitory current decreased susceptibility to seizure activity as well as decreasing anxiety, consistent with the increase in GABAergic tone at this time (Maguire et al., 2005). This effect of the estrous cycle on δ expression was prevented with finasteride and mimicked with exogenous administration of progesterone to male or ovariectomized female mice, suggesting that increases in δ expression are produced by THP (Maguire & Mody, 2007). These results are important in validating that normally occurring fluctuations in endogenous steroids can not only alter inhibition through direct effects on the GABAR, but also by increasing receptor populations that are the most sensitive target for the steroid.
5.2. Stress
Stress is known to increase circulating levels of both THP and THDOC (Purdy et al., 1991). One recent study (Maguire & Mody, 2007) demonstrated that acute stress could upregulate δ subunit expression in dentate gyrus within 30 min. This effect was also demonstrated in an in vitro slice preparation, where the stress steroid THDOC also increased δ expression within 30 min. Thus, these findings suggest that a relatively short period of steroid exposure can increase δ subunit expression.
5.3. Dentate gyrus
The dentate gyrus is the initial component of the limbic system which receives and integrates afferent input from related cortical areas. Because it is one of the CNS areas with the highest expression levels of α4 and δ subunits (Wisden et al., 1992), it is one of the most sensitive targets for steroids, such as THP and THDOC. Indeed α4βδ GABAR here underlie a tonic current (Stell & Mody, 2002), which is highly sensitive to physiologically relevant 10 nM concentrations of the steroid THDOC (Stell et al., 2003) and THP (Mtchedlishvili & Kapur, 2006). Acute application of this steroid doubled the tonic GABAergic current, resulting in decreased neuronal excitability. In contrast, the synaptic current was unaffected by the steroid, suggesting that the tonic current is the target for these endogenous steroids. As predicted by this finding, increases in tonic current on diestrus-1 would be further increased by THP to result in high levels of inhibitory tone (Maguire et al., 2005). In fact, mice tested on the day of diestrus-1 exhibit a higher threshold for seizure induction and a lower level of anxiety when tested on the elevated plus maze.
Steroid effects in dentate gyrus, however, may be developmentally regulated due to the emergence of steroid metabolizing enzymes. In young rats, the presence of the membrane-bound form of 3α-HSD (Mellon & Vaudry, 2001) favors the reverse metabolism of THP back to 5α-DHP, which is inactive as a GABA-modulatory steroid. Therefore, physiological levels of THP are ineffective in modulatory GABAergic inhibition under these conditions in the rat. In a recent study (Belelli & Herd, 2003), the metabolically inert ganaxolone was shown to potentiate GABAergic IPSCs at concentrations where THP was ineffective, while another synthetic steroid, Provera, blocked the actions of 3α-HSD to allow steroid potentiation of GABAergic synaptic current. Thus, the net effect of the steroid is dependent not only on the prevalence of the target GABAR postsynaptically but also on the relative prevalence of cytosolic versus membrane-bound forms of 3α-HSD.
5.4. Periaqueductal gray
In contrast to the dentate gyrus, the midbrain periaqueductal gray (PAG) is an area with a direct role in mediating the panic response (Lovick, 2000), and the activity of the output neurons in this area is under the control of GABAergic inhibition. Recent studies (Lovick et al., 2005) have demonstrated that fluctuations in endogenous steroids across the estrous cycle alter the expression of GABAR subunit combinations in this region: α4, β1, and δ subunit expression was increased on PAG interneurons on the day of late diestrus. This stage of the cycle follows the secondary peak in progesterone and THP, which occurs on early diestrus, and could thus be considered a time of THP “withdrawal.” In fact, the authors have a second study demonstrating similar increases in α4, β1, and δ GABAR subunit expression following withdrawal from administered progesterone (Griffiths & Lovick, 2005). Because this increase in GABAR expression is on the inhibitory interneurons, it would effectively “disinhibit” the output neurons, as has recently been shown. In this case, both application of bicuculline as well as the panicogenic cholecystokinin ligand pentagastrin increased PAG neuronal activity to a greater extent on estrus and late diestrus (Brack & Lovick, 2007), suggesting a reduced GABAergic tone on the output neurons on these 2 days of THP “withdrawal” across the estrous cycle. Activation of these PAG output neurons has been shown to elicit a panic reaction in both humans and rodents (Lovick, 2000), because it is part of an afferent pathway to brainstem respiratory neurons that control the rate and amplitude of respiratory signals (Voituron et al., 2005). These findings have important clinical significance as well because many women with panic disorder exhibit premenstrual exacerbation during the decline in progesterone at the end of the luteal phase (Yonkers, 1997) (i.e., a THP “withdrawal” state).
6. Puberty
It is well-known that puberty is associated with mood swings and aversive responses to stress (Buchanan et al., 1992; Hayward & Sanborn, 2002; Modesti et al., 2004). Because puberty is also associated with fluctuations in circulating levels of THP (Fadalti et al., 1999), we examined the expression levels of α4 and δ subunits in CA1 hippocampus immediately before and after the onset of puberty. In fact the onset of puberty resulted in markedly increased expression of α4 and δ subunits along the dendrites of CA1 pyramidal cells from nearly undetectable levels before puberty (Shen et al., 2007). Tonic current recorded from these cells was increased at puberty onset, compared to levels before puberty, and was pharmacologically consistent with increased α4βδ GABAR expression, i.e., showing an increased response to THIP and blockade by La3+. Surprisingly, acute application of THP reduced the tonic current recorded from CA1 hippocampal pyramidal cells at puberty (Shen et al., 2007), recorded with perforated patch recording techniques to maintain physiological Cl- gradients (outward current) and intracellular messengers. In contrast, in slices from pre-pubertal mice, tonic current was not affected by THP, as has been reported in the adult (Stell et al., 2003). Tonic current was similarly not affected by THP in slices from pubertal δ knock-out mice, implicating α4βδ GABAR. The decrease in tonic inhibition from these dendritic GABAR increased the input resistance of the neuron, reducing the current necessary to trigger an action potential. This paradoxical excitatory effect of THP was also reflected behaviorally: THP increased the anxiety response in pubertal mice (Shen et al., 2007), in contrast to its well-known anxiolytic action before puberty and in the adult (Bitran et al., 1999). Increases in anxiety were also triggered by restraint stress in pubertal animals, a stressful stimulus known to markedly increase brain THP levels compensatorily (Purdy et al., 1991). Again, this is in contrast to the effect of restraint stress before puberty, which decreased anxiety (Shen et al., 2007).
Because this was a paradoxical effect of THP presumably mediated by α4βδ GABAR, we investigated effects of the steroid on recombinant α4β2δ GABAR. Here, THP decreased outward current at these receptors, an effect dependent upon arginine 353 in the intracellular loop of α4, a putative Cl- modulatory site (Shen et al., 2007), and was mediated by an increase in the rate of desensitization, consistent with previous reports (Bianchi et al., 2002). In contrast, inward current was potentiated by THP, consistent with other reports of steroid effects at δ-GABAR (Belelli et al., 2002; Wohlfarth et al., 2002; Bianchi et al., 2002). These findings suggest that the effect of this neurosteroid on α4βδ GABAR is dependent upon the direction of Cl- current in the brain, This, in turn, will determine its ultimate behavioral effect. That is, in CA1 hippocampus, where Cl- current is outward (Staley & Proctor, 1999), THP reduces inhibition, but in dentate gyrus and cortex, areas where Cl- current is inward (Staley & Mody, 1992; Gulledge & Stuart, 2003) in association with relatively high levels of α4βδ GABAR expression (Wisden et al., 1992), neurosteroids would enhance inhibition, as has been reported (Stell et al., 2003).
7. Comparison with other GABA-modulatory drugs
7.1. Benzodiazepines
The neurosteroid-induced increase in α4 subunit expression may be linked to sustained increases in GABA-mediated inhibition, because α4 expression is regulated by other positive modulators of the GABAR. Indeed, chronic 7- or 14-day treatment with the BDZ diazepam or the α1-selective zolpidem increases α4 mRNA expression in rat cortex (Holt et al., 1996), in association with decreases in α1 expression. Other studies have indicated that withdrawal from chronic treatment with either diazepam or zolpidem also increases α4 mRNA expression in cultured cerebellar neurons (Holt et al., 1997; Follesa et al., 2001, 2002).
7.2. Ethanol
Similarly, chronic intake of ethanol or its withdrawal results in increased α4 expression. In the chronic intermittent ethanol (CIE) model of ethanol dependence in the rat, multiple withdrawal cycles of ethanol result in a kindling effect to increase seizure susceptibility, increase anxiety and produce insomnia, as well as promote self-administration (Olsen et al., 2005). This hyperexcitability state is a result of decreased hippocampal circuit excitability as evidenced by decreases in paired pulse inhibition (Kang et al., 1996). Increased α4 expression was found in the CIE model across multiple regions of the hippocampal formation, including dentate gyrus, CA3 and CA1 (Mahmoudi et al., 1997). More recent studies suggest that α4 here coexpresses with the γ2 subunit, because δ subunit expression was decreased in the CIE rat, while the pharmacology of CA1 hippocampal neurons was consistent with α4βγ2 expression (Cagetti et al., 2003), that is, insensitive to a BDZ agonist, but increased responsiveness to the BDZ partial inverse agonist RO15-4513.
As shown after chronic THP treatment, increased α4 expression in the hippocampus of the CIE rat was also accompanied by faster kinetics of the synaptic current. Specifically, mIPSCs recorded from CA1 hippocampal pyramidal cells exhibited an acceleration in the fast component of decay (Cagetti et al., 2003). This acceleration in deactivation would reduce the total charge transfer and would thus reduce the level of inhibition. Thus, these findings may explain the hyperexcitability state seen after ethanol withdrawal.
Studies from other laboratories have also noted increases in α4 expression after chronic ethanol treatment (Devaud et al., 1997), which were associated with decreases in α1 expression (Kumar et al., 2003), suggesting that this represents a subunit switch. In fact, this may be related directly to effects of phosphorylation (Kumar et al., 2002). A recent study has also demonstrated that ethanol withdrawal increases expression of α4 mRNA after a 5-day administration to cultured hippocampal neurons (Sanna et al., 2003).
8. Mechanisms of α4 expression
The mechanism for the 48-hr steroid-induced upregulation of α4 subunit expression has not been established, but it requires low background levels of steroid, and is most effectively observed after neuronal differentiation by nerve growth factor in a neuroblastoma cell line (Zhou & Smith, 2007). THP is not the only steroid which is associated with higher levels of α4-containing GABAR. Another steroid hormone, 17β-estradiol, increases α4 levels when administered across a 48-hr or 7-day exposure (Pierson et al., 2005; Zhou & Smith, 2007), most likely through a different mechanism. Recent studies have identified the α4 promoter (Ma et al., 2004; Roberts et al., 2005), which is activated by PKC and early growth factor 3 to increase α4 expression. More recent studies have shown that brain derived neurotrophic factor is the endogenous signal which initiates these events via the tyrosine kinase receptor (Roberts et al., 2006). In addition to GABAR ligands, seizure activity also increases α4 expression, suggesting that altered neuronal excitability states may underlie the expression of the receptor (Roberts et al., 2005). However, multiple start boxes have also been identified on the α4 promoter, suggesting that multiple mechanisms may exist for triggering its expression (Ma et al., 2004).
9. Relevance for clinical issues
9.1. Puberty
Puberty is well-known as a time when mood swings occur (Hayward & Sanborn, 2002), and responses to stressful events become exacerbated (Modesti et al., 2004). Although an array of hormonally mediated events occur at this time, the onset of puberty is associated with a decline in circulating levels of THP (Palumbo et al., 1995; Fadalti et al., 1999). The onset of puberty also increases the likelihood of developing anxiety disorders, including panic disorder, twice as likely in girls compared to boys (Hayward & Sanborn, 2002). Altered GABAR subunit composition (i.e., increased expression of α4βδ GABAR), as we have shown (see above), is one likely mechanism for this change in behavior.
9.2. Premenstrual syndrome
PMS is a disorder of mood that occurs in a cyclic manner across the menstrual cycle (Endicott et al., 1999). The psychological symptoms which are most commonly reported include anxiety, depression and irritability, and some reports suggest that up to 70% of the population can be affected (Endicott et al., 1999; Angst et al., 2001; Rapkin, 2003; Pearlstein et al., 2005). Although the onset of this disorder is variable, symptoms are most likely to occur during the late luteal phase when progesterone and THP levels are declining, forming arelative “THP withdrawal” state (Rapkin et al., 1997). Thus, the increase in anxiety observed during THP withdrawal in rodent models may be relevant for this condition.
A more severe form of PMS is PMDD. This occurs in 3-5% of the population as described in the DSM-IV (Endicott et al., 1999; Backstrom et al., 2003). The most common symptom reported is emotional reactivity or irritability (Angst et al., 2001), but anxiety and depression also typify the condition (Yonkers, 1997). Unlike PMS, PMDD symptoms can begin early to midluteal phase when THP levels are rising. Although GABAR subunit changes cannot be evaluated directly, women with PMDD have been shown to be relatively insensitive to BDZs (Sundstrom et al., 1997), assessed both with objective tests such as saccade velocity, as well as with subjective self-reports. Thus, these findings are consistent with the possibility that sustained elevations in THP levels increase expression of the BDZ-insensitive α4 subunit.
One recent study has employed transcranial stimulation to investigate CNS excitability in women with PMDD (Smith et al., 2003). Similar to the paired-pulse stimulation routinely used to assess hippocampal excitability, this protocol employs paired stimulation to assess the level of inhibitory tone in cortex. Similar to the results obtained after THP withdrawal in the rodent (Hsu & Smith, 2003), women with PMDD exhibit paired-pulse facilitation during the luteal phase, in contrast to control subjects who typically exhibit paired-pulse inhibition (Smith et al., 1999). Another recent study (Epperson et al., 2007) has shown that the acoustic startle response is increased in women with PMDD during the luteal phase, compared with the follicular phase of the menstrual cycle. This outcome is also similar to findings in rodents in a model of PMS (Gulinello et al., 2003b) where progesterone withdrawal triggered an increase in the acoustic startle response. These findings, coupled with the relative BDZ insensitivity observed in PMDD (Sundstrom et al., 1997), are consistent with upregulation of the α4 subunit in rodent models.
Recent reports also suggest that women with PMDD have abnormal responses to ovarian steroids. In one landmark study (Schmidt et al., 1998), PMDD symptoms were suppressed when ovarian cyclicity was halted pharmacologically. However, symptoms were triggered by exogenous administration of ovarian steroids, including progesterone, suggesting a paradoxical effect. This finding was supported by other reports showing that symptom improvement in PMDD was well-correlated with decreases, rather than increases in serum THP levels (Freeman et al., 2002).
One possibility for this apparent paradoxical effect of THP is via reduction in tonic inhibition mediated by outward current generated by α4β2δ GABAR, as we have shown (Smith et al., 2006; Shen et al., 2007) after THP withdrawal, when it increases anxiety (Fig. 6, Smith et al., 2006). Another possibility for this finding is that the steroids were acting preferentially at interneurons, where fluctuations in steroids increase α4 and δ subunit expression in PAG (Lovick et al., 2005).
A second hallmark of PMDD is an increase in the panic response to administration of the BDZ anagonist flumazenil (Le Melledo et al., 2000), similar to individuals with panic disorder. This pharmacological finding may be explained by the increased expression of α4 subunits on interneurons of the midbrain PAG, which is reported in rodents after progesterone withdrawal (Griffiths & Lovick, 2005) and in late diestrus (Lovick et al., 2005), the rodent equivalent of the “late luteal phase.” When coexpressed with γ2, α4βγ2 yields receptors which are potentiated by flumazenil (Wafford et al., 1996). Thus, flumazenil would effectively disinhibit the PAG output neurons; this increase in PAG output would thus trigger a panic reaction.
9.3. Catamenial epilepsy
Exacerbation of seizure activity across the menstrual cycle has been reported clinically, where seizure likelihood increases mid-cycle and in the late luteal phase (Backstrom, 1976; Herzog et al., 1997; Herzog & Friedman, 2001). Most likely the increase in seizures mid-cycle is triggered by estradiol which can enhance effects at excitatory synapses, while the increase in the late luteal phase may be related to THP withdrawal (2003). A number of groups have established rodent models where progesterone or THP withdrawal increased seizure susceptibility and seizure severity (Frye & Bayon, 1998; Smith et al., 1998a; Reddy & Rogawski, 2001; Rogawski, 2003). That this may be a result of increased α4 expression was suggested by the finding that suppression of α4 expression in hippocampus prevented the increase in seizure susceptibility after steroid withdrawal (Smith et al., 1998a). In fact, progesterone administration is effective therapeutically in women with this disorder, suggesting a role for the steroid in reducing CNS excitability. In addition, barbiturate sensitivity is increased in catamenial epilepsy (Reddy et al., 2001), consistent with increased expression of α4βγ2 GABAR (Wafford et al., 1996).
9.4. Postpartum and postmenopausal dysphoria
Two additional periods of steroid declines include the postpartum and postmenopausal periods. Following the increase in THP during pregnancy when levels are increased several-fold beyond their levels during the cycle, the decline in THP at partrurition represents the most significant steroid withdrawal state. Emotional reactivity is very common in the early postpartum period, when anxiety and irritability are also reported (Kendell et al., 1981). In the pseudopregnancy rodent model, direct withdrawal from THP (Smith et al., 1998b) in the presence of high circulating levels of progesterone increased the α4 subunit in association with increases in anxiety. Postpartum blues, however, may be related to changes other than GABAR-related alterations (Kendell et al., 1981).
Several studies suggest that the postmenopausal period can also result in increased emotional reactivity, with the emergence of anxiety or panic disorders (Claudia et al., 2004). The effectiveness of progesterone and thus THP in alleviating these symptoms is dose-dependent with midluteal circulating concentrations of THP producing aversive symptoms, but either low or high levels of THP effective at reducing anxiety (Andreen et al., 2004). Thus, although steroid withdrawal is associated with anxiety states clinically, the effect of the steroid is variable, and may depend upon receptor targets as well as on the circulating level of the steroid.
10. Conclusions
Taken together, the results from these varied basic science and clinical studies suggest that beyond the acute effects of neurosteroids, chronic exposure to steroids such as THP and THDOC produce compensatory changes in target receptor populations. Notable among the subunits which are altered include the α4 and δ subunits. Increases in α4 expression would produce BDZ insensitivity, one common factor in rodent models and clinical examples of PMDD. Depending on the direction of Cl- current in target populations, responses to acute increases in steroids would either increase or decrease tonic inhibition, with behavioral outcomes.
In contrast, increased expression of synaptic α4βγ2 GABAR during THP withdrawal (i.e., the late luteal phase) would increase neuronal excitability due to the faster deactivation kinetics of this receptor, while increases in α4βδ-generated tonic current on diestrus-1 or early in the luteal phase would enhance inhibition and suppress anxiety. However, the direction of the Cl- gradient would ultimately determine whether current gated by α4β2δ GABAR is increased or decreased by THP, with implications for paradoxical anxiety-producing effects of the steroid mediated via reduction in outward current.
Both chronic exposure and withdrawal from exogenous neurosteroids increase α4 expression to result in states of CNS hyperexcitability, similar to other GABA-modulatory compounds. Thus, these results not only have important implications for hormonal cycle-associated changes in neuronal activation and behavior, but also suggest a common underlying mechanism for the role of inhibition in triggering compensatory changes in neuronal excitability states due to the plasticity of GABAR populations with different pharmacological and biophysical properties.
Abbreviations
- 3α,5α-THP
3α-OH-5α-pregnan-20-one (allopregnanolone)
- 3α,5β-THP
3α-OH-5β-pregnan-20-one (pregnanolone)
- 3α-HSD
3α-hydroxysteroid-oxidoreductase
- BDZ
benzodiazepine
- CIE
chronic intermittent ethanol
- CNS
central nervous system
- EPSP
excitatory postsynaptic potential
- GABA
gamma-aminobutyric acid
- GABAR
GABAA receptor
- gaboxadol
4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridine-3-ol (THIP)
- HEK
human embryonic kidney
- mIPSC
miniature inhibitory postsynaptic current
- mRNA
messenger ribonucleic acid
- PAG
periaqueductal gray
- PKC
protein kinase C
- PMDD
premenstrual dysphoric disorder
- PMS
premenstrual syndrome
- sIPSC
spontaneous inhibitory postsynaptic current
- Tau, τ
time constant of decay
- THDOC
3α,21-dihydroxy-5α-pregnan-20-one
- THP
3α-OH-5α[β]-pregnan-20-one
References
- Agis-Balboa RC, Pinna G, Zhubi A, Maloku E, Veldic M, Costa E, et al. Characterization of brain neurons that express enzymes mediating neurosteroid biosynthesis. Proc Natl Acad Sci. 2006;103:14602–14607. doi: 10.1073/pnas.0606544103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akk G, Bracamontes J, Steinbach JH. Activation of GABAA receptors containing the alpha4 subunit by GABA and pentobarbital. J Phys. 2004;556:387–399. doi: 10.1113/jphysiol.2003.058230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreen L, Sundstrom-Poromaa I, Bixo M, Andersson A, Nyberg S, Backstrom T. Relationship between allopregnanolone and negative mood in postmenopausal women taking sequential hormone replacement therapy with vaginal progesterone. Psychoneuroendocrinology. 2004;30:212–224. doi: 10.1016/j.psyneuen.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Angst J, Sellaro R, Merikangas KR, Endicott J. The epidemiology of premenstrual psychological symptoms. Acta Psychiatr Scand. 2001;104:110–116. doi: 10.1034/j.1600-0447.2001.00412.x. [DOI] [PubMed] [Google Scholar]
- Backstrom T. Epileptic seizures in women related to plasma estrogen and progesterone during the menstrual cycle. Acta Neurol Scand. 1976;54:321–347. doi: 10.1111/j.1600-0404.1976.tb04363.x. [DOI] [PubMed] [Google Scholar]
- Backstrom T, Andreen L, Birzniece V, Bjorn I, Johannson IM, Nordenstam-Haghjo M, et al. The role of hormones and hormonal treatments in premenstrual syndrome. CNS Drugs. 2003;17:325–342. doi: 10.2165/00023210-200317050-00003. [DOI] [PubMed] [Google Scholar]
- Barbaccia ML, Roscetti G, Trabucchi M, Mostallino MC, Concas A, Purdy RH, et al. Time-dependent changes in rat brain neuroactive steroid concentrations and GABAA receptor function after acute stress. Neuroendocrinology. 1996;63:166–172. doi: 10.1159/000126953. [DOI] [PubMed] [Google Scholar]
- Barbaccia ML, Serra M, Purdy RH, Biggio G. Stress and neuroactive steroids. Int Rev Neurobiol. 2001;46:243–272. doi: 10.1016/s0074-7742(01)46065-x. [DOI] [PubMed] [Google Scholar]
- Belelli D, Herd MB. The contraceptive agent Provera enhances GABAA receptor-mediated inhibitory neurotransmission in the rat hippocampus: evidence for endogenous neurosteroids? J Neurosci. 2003;23:10013–10020. doi: 10.1523/JNEUROSCI.23-31-10013.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Casula A, Ling A, Lambert JJ. The influence of subunit composition on the interaction of neurosteroids with GABAA receptors. Neuropharmacology. 2002;43:651–661. doi: 10.1016/s0028-3908(02)00172-7. [DOI] [PubMed] [Google Scholar]
- Belelli D, Peden DR, Rosahl TW, Wafford K, Lambert JJ. Extrasynaptic GABA-A receptors for thalamocortical neurons: a molecular target for hypnotics. J Neurosci. 2005;25:11513–11520. doi: 10.1523/JNEUROSCI.2679-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benke D, Michel C, Mohler H. GABAA receptors containing the α4-subunit: prevalence, distribution, pharmacology, and subunit architecture in situ. J Neurochem. 1997;69:806–814. doi: 10.1046/j.1471-4159.1997.69020806.x. [DOI] [PubMed] [Google Scholar]
- Bianchi MT, Macdonald RL. Neurosteroids shift partial agonist activation of GABA (A) receptor channels from low-to high-efficacy gating patterns. J Neurosci. 2003;23:10934–10943. doi: 10.1523/JNEUROSCI.23-34-10934.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi MT, Haas KF, Macdonald RL. Alpha1 and alpha6 subunits specify distinct desensitization, deactivation and neurosteroid modulation of GABA(A) receptors containing the δ subunit. Neuropharmacology. 2002;43:492–502. doi: 10.1016/s0028-3908(02)00163-6. [DOI] [PubMed] [Google Scholar]
- Binkley P, Ticku MK. Histidine residue is crucial for the binding of ligands to the benzodiazepine site except RO15-4513. Eur J Pharmacol. 1991;208:269–270. doi: 10.1016/0922-4106(91)90106-r. [DOI] [PubMed] [Google Scholar]
- Bitran D, Shiekh M, McLeod M. Anxiolytic effect of progesterone is mediated by the neurosteroid allopregnanolone at brain GABAA receptors. J Neuroendocrinol. 1995;7:171–177. doi: 10.1111/j.1365-2826.1995.tb00744.x. [DOI] [PubMed] [Google Scholar]
- Bitran D, Dugan M, Renda P, Ellis R, Foley M. Anxiolytic effects of the neuroactive steroid pregnanolone (3α-OH-5β-pregnan-20-one) after microinjection in the dorsal hippocampus and lateral septum. Brain Res. 1999;850:217–224. doi: 10.1016/s0006-8993(99)02150-2. [DOI] [PubMed] [Google Scholar]
- Bixo M, Andersson A, Winblad B, Purdy RH, Backstrom T. Progesterone, 5alpha-pregnane-3, 20-dione and 3alpha-OH-5alpha-pregnane-20-one in specific regions of the human female brain in different endocrine states. Brain Res. 1997;764:173–178. doi: 10.1016/s0006-8993(97)00455-1. [DOI] [PubMed] [Google Scholar]
- Borghese CM, et al. The δ subunit of gamma-aminobutyric acid type A receptors does not confer sensitivity to low concentrations of ethanol. J Pharmacol Exp Ther. 2006;316:1360–1368. doi: 10.1124/jpet.105.092452. [DOI] [PubMed] [Google Scholar]
- Brack KE, Lovick TA. Neuronal excitability in the periaqueductal grey matter during the estrous cycle in female Wistar rats. Neuroscience. 2007;144:325–335. doi: 10.1016/j.neuroscience.2006.08.058. [DOI] [PubMed] [Google Scholar]
- Brickley SG, Revilla V, Cull-Candy SG, Wisden W, Farrant M. Adaptive regulation of neuronal excitability by a voltage-independent potassium conductance. Nature. 2001;409:88–92. doi: 10.1038/35051086. [DOI] [PubMed] [Google Scholar]
- Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA. Pharmacological characterization of a novel cell line expressing human alpha (4)beta (3)delta GABA (A) receptors. Br J Pharmacol. 2002;136:965–974. doi: 10.1038/sj.bjp.0704795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brussaard AB, Kits KS, Baker RE, Willems WP, Leyting-Vermuelen JW, Voom P, et al. Plasticity in fast synaptic inhibition of adult oxytocin neurons by switch in GABA (A) receptor subunit expression. Neuron. 1997;19:1103–1114. doi: 10.1016/s0896-6273(00)80401-8. [DOI] [PubMed] [Google Scholar]
- Brussaard AB, Devay P, Leyting-Vermuelen JW, Kits KS. Changes in properties and neurosteroid regulation of GABAergic synapses in the supraoptic nucleus during the mammalian female reproductive cycle. J Physiol. 1999;516:513–524. doi: 10.1111/j.1469-7793.1999.0513v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brussaard AB, Wossink J, Lodder JC, Kits KS. Progesterone-metabolite prevents protein kinase C-dependent modulation of γ-aminobutyric acid type A receptors in oxytocin neurons. PNAS. 2000;97:3625–3630. doi: 10.1073/pnas.050424697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan CM, Eccles JS, Becker JB. Are adolescents the victims of raging hormones: evidence for activational effects of hormones on moods and behavior at adolescence. Psychol Bull. 1992;111:62–107. doi: 10.1037/0033-2909.111.1.62. [DOI] [PubMed] [Google Scholar]
- Burgard EC. Properties of recombinant gamma-aminobutyric acid A receptor isoforms containing the alpha 5 subunit subtype. Mol Pharmacol. 1996;50(1):119–127. [PubMed] [Google Scholar]
- Cagetti E, Liang J, Spigelman I, Olsen RW. Withdrawal from chronic intermittent ethanol treatment changes subunit composition, reduces synaptic function, and decreases behavioral responses to positive allosteric modulators of GABA-A receptors. Mol Pharmacol. 2003;63:53–64. doi: 10.1124/mol.63.1.53. [DOI] [PubMed] [Google Scholar]
- Callachan H, Cottrell GA, Hather NY, Lambert JJ, Nooney JM, Peters JA. Modulation of the GABAA receptor by progesterone metabolites. Proc R Soc Lond B Biol Sci. 1987;231:359–369. doi: 10.1098/rspb.1987.0049. [DOI] [PubMed] [Google Scholar]
- Caraiscos VB, Elliott EM, You-Ten KE, Cheng VY, Belelli D, Newell JG, et al. Tonic inhibition in mouse hippocampal CA1 pyramidal neurons is mediated by alpha 5 subunit-containing gamma-aminobutyric acid type A receptors. Proc Natl Acad Sci. 2004;101:3662–3667. doi: 10.1073/pnas.0307231101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra D, Jia F, Liang J, Peng Z, Suryanarayanan A, Werner DF, et al. GABA-A receptor alpha-4 subunits mediate extrasynaptic inhibition in thalamus and dentate gyrus and the action of gaboxadol. Proc Natl Acad Sci. 2006;103:15230–15235. doi: 10.1073/pnas.0604304103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y, Wang R, Barot S, Weiss DS. Stoichiometry of a recombinant GABA-A receptor. J Neurosci. 1990;16:534–541. doi: 10.1523/JNEUROSCI.16-17-05415.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claudia P, Andrea C, Chiara C, Stefano L, Giuseppe M, Vincenzo de L, et al. Panic disorder in menopause: a case control study. Maturitas. 2004;48:147–154. doi: 10.1016/j.maturitas.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Compagnone NA, Mellon SH. Neurosteroids: biosynthesis and function of these novel neuromodulators. Front Neuroendocrinol. 2000;21:1–56. doi: 10.1006/frne.1999.0188. [DOI] [PubMed] [Google Scholar]
- Concas A, Follesa P, Barbaccia ML, Purdy RH, Biggio G. Physiological modulation of GABA (A) receptor plasticity by progesterone metabolites. Eur J Pharmacol. 1999;375:225–235. doi: 10.1016/s0014-2999(99)00232-0. [DOI] [PubMed] [Google Scholar]
- Cope DW, Hughes SW, Crunelli V. GABA-A receptor-mediated tonic inhibition in thalamic neurons. J Neurosci. 2005;25:11553–11563. doi: 10.1523/JNEUROSCI.3362-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpechot C, Collins B, Carey M, Tsouros A, Robel P, Fry J. Brain neurosteroids during the mouse oestrous cycle. Brain Res. 1997;766:276–280. doi: 10.1016/s0006-8993(97)00749-x. [DOI] [PubMed] [Google Scholar]
- Davis M, Gendelman DS, Tischler MD, Gendelman PM. A primary acoustic startle circuit: lesion and stimulation studies. J Neurosci. 1982;2:791–805. doi: 10.1523/JNEUROSCI.02-06-00791.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaud LL, Fritschy J-M, Sieghar W, Morrow AL. Bidirectional alterations of GABAA receptor subunit peptide levels in rat cortex during chronic ethanol consumption and withdrawal. J Neurochem. 1997;69:126–130. doi: 10.1046/j.1471-4159.1997.69010126.x. [DOI] [PubMed] [Google Scholar]
- Dong E, Matsumoto K, Uzunova V, Sugaya I, Takahata H, Nomura H, et al. Brain 5alpha-dihydroprogesterone and allopregnanolone synthesis in a mouse model of protracted social isolation. Proc Natl Acad Sci. 2001;98:2849–2854. doi: 10.1073/pnas.051628598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droogleever Fortuyn HA, van Broekhoven F, Span PN, Backstrom T, Zitman FG, Verkes RJ. Effects of PhD examination stress on allopregnanolone and cortisol plasma levels and peripheral benzodiazepine receptors density. Psychoneuroendocrinology. 2004;29:1341–1344. doi: 10.1016/j.psyneuen.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Endicott J, Amsterdam J, Eriksson E, Frank E, Freeman E, Hirschfeld R, et al. Is premenstrual dysphoric disorder a distinct clinical entity? J Women’s Health Gend Based Med. 1999;8:663–679. doi: 10.1089/jwh.1.1999.8.663. [DOI] [PubMed] [Google Scholar]
- Epperson CN, Pittman B, Czarkowski KA, Stiklus S, Krystal JH, Grillon C.Luteal-phase accentuation of acoustic startle response in women with premenstrual dysphoric disorder Neuropsychopharmacology 21Feb2007(Electronic Publication, PMID17314917) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadalti M, et al. Changes of serum allopregnanolone levels in the first two years of life and during pubertal development. Pediatr Res. 1999;46:323–327. doi: 10.1203/00006450-199909000-00013. [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme:phasic and tonic activation of GABA (A) receptors. Nat Rev Neurosci. 2005;6(3):215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Feng H-J, Kang J-Q, Song L, Dibbens L, Mulley J, Macdonald RL. Delta subunit susceptibility variants E177A and R220H associated with complex epilepsy alter channel gating and surface expression of alpha4-beta2-delta GABA-A receptors. J Neurosci. 2006;26:1499–1506. doi: 10.1523/JNEUROSCI.2913-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follesa P, Serra M, Cagetti E, Pisu MG, Porta S, Floris S, et al. Allopregnanolone synthesis in cerebellar granule cells: roles in regulation of GABA(A) receptor expression and function during progesterone treatment and withdrawal. Mol Phamacol. 2000;57:1262–1270. [PubMed] [Google Scholar]
- Follesa P, Cagetti E, Mancuso L, Biggio F, Manca A, Maciocco E, et al. Increase in expression of the GABA (A) receptor alpha (4) subunit gene induced by withdrawal of, but not by long-term treatment with, benzodiazepine full or partial agonists. Brain Res Mol Brain Res. 2001;92:138–148. doi: 10.1016/s0169-328x(01)00164-4. [DOI] [PubMed] [Google Scholar]
- Follesa P, Mancuso L, Biggio F, Cagetti E, Franco M, Trapani G, et al. Changes in GABA (A) receptor gene expression induced by withdrawal of, but not by long-term exposure to, zaleplon or zolpidem. Neuropharmacology. 2002;42:191–198. doi: 10.1016/s0028-3908(01)00167-8. [DOI] [PubMed] [Google Scholar]
- Freeman EW, Frye CA, Rickels K, Martin PA, Smith SS. Allopregnanolone levels and symptom improvement in severe premenstrual syndrome. J Clin Psychopharmacol. 2002;22:516–520. doi: 10.1097/00004714-200210000-00013. [DOI] [PubMed] [Google Scholar]
- Friedman LK, Gibbs TT, Farb DH. Gamma-aminobutyric acidA receptor regulation: heterologous uncoupling of modulatory site interactions induced by chronic steroid, barbiturate, benzodiazepine, or GABA treatment in culture. Brain Res. 1996;707:100–109. doi: 10.1016/0006-8993(95)01226-5. [DOI] [PubMed] [Google Scholar]
- Frye CA, Bayon LE. Seizure activity is increased in endocrine states characterized by decline in endogenous levels of the neurosteroid 3 alpha,5 alpha-THP. Neuroendocrinology. 1998;68:272–280. doi: 10.1159/000054375. [DOI] [PubMed] [Google Scholar]
- Frye CA, Walf AA. Changes in progesterone metabolites in the hippocampus can modulate open field and forced swim test behavior of proestrous rats. Horm Behav. 2002;41:306–315. doi: 10.1006/hbeh.2002.1763. [DOI] [PubMed] [Google Scholar]
- Frye CA, Petralia SM, Rhodes ME. Estrous cycle and sex differences in performance on anxiety tasks coincide with increases in hippocampal progesterone and 3alpha,5alpha-THP. Pharmacol Biochem Behav. 2000;67:587–596. doi: 10.1016/s0091-3057(00)00392-0. [DOI] [PubMed] [Google Scholar]
- Fujii E, Mellon SH. Regulation of uterine gamma amino-butyric acid (A) receptor subunit expression throughout pregnancy. Endocrinology. 2001;142:1770–1777. doi: 10.1210/endo.142.5.8153. [DOI] [PubMed] [Google Scholar]
- Gallo MA, Smith SS. Progesterone withdrawal decreases latency to and increases duration of electrified prod burial: a possible rat model of PMS anxiety. Pharmacol Biochem Behav. 1993;46:897–904. doi: 10.1016/0091-3057(93)90219-j. [DOI] [PubMed] [Google Scholar]
- Gee KW, Chang W-C, Brinton RE, McEwen BS. GABA-dependent modulation of the Cl-ionophore by steroids in rat brain. Eur J Pharmacol. 1987;136:419–423. doi: 10.1016/0014-2999(87)90317-7. [DOI] [PubMed] [Google Scholar]
- Gilbert Evans SE, Ross LE, Sellers EM, Purdy RH, Romach MK. 3 alpha-reduced neuroactive steroids and their precursors during pregnancy and the postpartum period. Gynecol Endocrinol. 2005;21:268–279. doi: 10.1080/09513590500361747. [DOI] [PubMed] [Google Scholar]
- Gingrich KL, Roberts WA, Kass RS. Dependence of the GABAA receptor gating kinetics on the alpha subunit isoform: implications for structure-function relations and synaptic transmission. J Physiol. 1995;489:529–543. doi: 10.1113/jphysiol.1995.sp021070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girdler SS, Beth Mechlin M, Light KC, Morrow AL. Ethnic differences in allopregnanolone concentrations in women during rest and following mental stress. Psychophysiology. 2006;43:331–336. doi: 10.1111/j.1469-8986.2006.00410.x. [DOI] [PubMed] [Google Scholar]
- Gravielle MC, Faris R, Russek SJ, Farb DH. GABA induces activity dependent delayed-onset uncoupling of GABA/benzodiazepine site interactions in neocortical neurons. J Biol Chem. 2005;280:20954–20960. doi: 10.1074/jbc.M500131200. [DOI] [PubMed] [Google Scholar]
- Griffiths J, Lovick T. Withdrawal from progesterone increases expression of alpha4, beta1 and delta GABA (A) receptor subunits in neurons in the periaqueductal gray matter in female Wistar rats. J Comp Neurol. 2005;486:89–97. doi: 10.1002/cne.20540. [DOI] [PubMed] [Google Scholar]
- Grobin AC, Morrow AL. 3alpha-hydroxy-5alpha-pregnan-20-one exposure reduces GABA (A) receptor alpha4 subunit mRNA levels. Eur J Pharmacol. 2000;409:R1–R2. doi: 10.1016/s0014-2999(00)00797-4. [DOI] [PubMed] [Google Scholar]
- Grobin AC, VanDoren MJ, Porrino LJ. Cortical 3alpha-OH-5alpha-pregnan-20-one levels after acute administration of delta 9-tetracannabinol, cocaine and morphine. Psychopharmacology. 2005;179:544–550. doi: 10.1007/s00213-004-2084-3. [DOI] [PubMed] [Google Scholar]
- Gulinello M, Smith SS. Anxiogenic effects of neurosteroid exposure: sex differences and altered GABA-A receptor pharmacology in adult rats. J Pharmacol Exp Ther. 2003;305:541–548. doi: 10.1124/jpet.102.045120. [DOI] [PubMed] [Google Scholar]
- Gulinello M, Gong QH, Li X, Smith SS. Short-term exposure to a neuroactive steroid increases α4 GABAA receptor subunit levels in association with increased anxiety. Brain Res. 2001;910:55–66. doi: 10.1016/s0006-8993(01)02565-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulinello M, Gong QH, Smith SS. Progesterone withdrawal increases the anxiolytic actions of gaboxadol: role of alpha4betadelta GABA (A) receptors. Neuroreport. 2003;14:43–46. doi: 10.1097/01.wnr.0000050303.92401.9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulinello M, Orman R, Smith SS. Sex differences in anxiety, sensorimotor gating and expression of the alpha-4 subunit of the GABA-A receptor in the amygdala after progesterone withdrawal. Eur J Neurosci. 2003;17:1–8. doi: 10.1046/j.1460-9568.2003.02479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulledge AT, Stuart GJ. Excitatory actions of GABA in the cortex. Neuron. 2003;37:299–309. doi: 10.1016/s0896-6273(02)01146-7. [DOI] [PubMed] [Google Scholar]
- Hatton GI. Function-related plasticity in hypothalamus. Annu Rev Neurosci. 1997;20:375–397. doi: 10.1146/annurev.neuro.20.1.375. [DOI] [PubMed] [Google Scholar]
- Hayward C, Sanborn K. Puberty and the emergence of gender differences in psychopathology. J Adolesc Health. 2002;30S:49–58. doi: 10.1016/s1054-139x(02)00336-1. [DOI] [PubMed] [Google Scholar]
- Herzog A, Friedman M. Menstrual cycle interval and ovulation in women with localization-related epilepsy. Neurology. 2001;57:2133–2135. doi: 10.1212/wnl.57.11.2133. [DOI] [PubMed] [Google Scholar]
- Herzog AG, Klein P, Ransil BJ. Three patterns of catamenial epilepsy. Epilepsia. 1997;38:1082–1088. doi: 10.1111/j.1528-1157.1997.tb01197.x. [DOI] [PubMed] [Google Scholar]
- Hevers W, Luddens H. The diversity of GABAA receptors. Pharmacological and electrophysiological properties of GABAA channel subtypes. Mol Neurobiol. 1998;18:35–86. doi: 10.1007/BF02741459. [DOI] [PubMed] [Google Scholar]
- Higashi T, Takido N, Shimada K. Studies on neurosteroids XVII. Analysis of stress-induced changes in neurosteroid levels in rat brains using liquid chromatography-electron capture atmosperic pressure chemical ionization-mass spectrometry. Steroids. 2005;70:1–11. doi: 10.1016/j.steroids.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Holt RA, Bateson AN, Martin IL. Chronic treatment with diazepam or abecarnil differently affects the expression of GABAA receptor subunit mRNAs in the rat cortex. Neuropharmacology. 1996;35:1457–1463. doi: 10.1016/s0028-3908(96)00064-0. [DOI] [PubMed] [Google Scholar]
- Holt RA, Bateson AN, Martin IL. Chronic zolpidem treatment alters GABA (A) receptor mRNA levels in the rat cortex. Eur J Pharmacol. 1997;329:129–132. doi: 10.1016/s0014-2999(97)00168-4. [DOI] [PubMed] [Google Scholar]
- Hsu F-C, Smith SS. Progesterone withdrawal reduces paired-pulse inhibition in rat hippocampus: dependence on GABA-A receptor alpha-4 upregulation. J Neurophysiol. 2003;89:186–198. doi: 10.1152/jn.00195.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu F-C, Waldeck R, Faber DS, Smith SS. Neurosteroid effects on GABAergic synaptic plasticity in hippocampus. J Neurophysiol. 2003;89:1929–1940. doi: 10.1152/jn.00780.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia F, Pignataro L, Schofield C, Yue M, Harrison NL, Goldstein PA. An extrasynaptic GABA-A receptor mediates tonic inhibition in thalamic VB neurons. J Neurophysiol. 2005;94:4491–4501. doi: 10.1152/jn.00421.2005. [DOI] [PubMed] [Google Scholar]
- Kang M, Spigelman I, Sapp DW, Olsen RW. Persistent reduction of GABA (A) receptor-mediated inhibition in rat hippocampus after chronic intermittent ethanol treatment. Brain Res. 1996;709:221–228. doi: 10.1016/0006-8993(95)01274-5. [DOI] [PubMed] [Google Scholar]
- Kendell RE, McGuire RE, Connor Y, Cox JL. Mood changes in the first three weeks after childbirth. J Affect Disord. 1981;3:i317–i326. doi: 10.1016/0165-0327(81)90001-x. [DOI] [PubMed] [Google Scholar]
- Koksma JJ, van Kesteren RE, Rosahl TW, Zwart R, Smit AB, Luddens H, et al. Oxytocin regulates neurosteroid modulation of GABA (A) receptors in supraoptic nucleus around parturition. J Neurosci. 2003;23:788–797. doi: 10.1523/JNEUROSCI.23-03-00788.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Sieghart W, Morrow AL. Association of protein kinase C with GABA (A) receptors containing alpha1 and alpha4 subunits in the cerebral cortex: selective effects of chronic ethanol consumption. J Neurochem. 2002;82:110–117. doi: 10.1046/j.1471-4159.2002.00943.x. [DOI] [PubMed] [Google Scholar]
- Kumar S, Kralic JE, O’Buckley TK, Grobin AC, Morrow AL. Chronic ethanol consumption enhances internalization of alpha1 subunit-containing GABAA receptors in cerebral cortex. J Neurochem. 2003;86:700–708. doi: 10.1046/j.1471-4159.2003.01894.x. [DOI] [PubMed] [Google Scholar]
- Lavoie AM, Tingey JJ, Harrison NL, Pritchett DB, Twyman RE. Activation and deactivation rates of recombinant GABAA receptor channels Are dependent on α-subunit isoform. Biophys J. 1997;73:1–9. doi: 10.1016/S0006-3495(97)78280-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Melledo JM, Van Driel M, Coupland NJ, Lott P, Jhangri GS. Response to flumazenil in women with premenstrual dysphoric disorder. Am J Psychiatry. 2000;157:821–823. doi: 10.1176/appi.ajp.157.5.821. [DOI] [PubMed] [Google Scholar]
- Liang J, Cagetti E, Olsen RW, Spigelman I. Altered pharmacology of synaptic and extrasynaptic GABAA receptors on CA1 hippocampal neurons is consistent with subunit changes in a model of alcohol withdrwal and dependence. J Pharmacol Exp Ther. 2004;310:1234–1245. doi: 10.1124/jpet.104.067983. [DOI] [PubMed] [Google Scholar]
- Liang J, Zhang N, Cagetti E, Houser CR, Olsen RW, Spigelman I. Chronic intermittent ethanol-induced switch of ethanol actions from extrasynaptic to synaptic hippocampal GABAA receptors. J Neurosci. 2006;26:1749–1758. doi: 10.1523/JNEUROSCI.4702-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lofgren M, Johansson IM, Meyerson B, Lundgren P. Progesterone withdrawal effects in the open field test can be predicted by elevated plus maze performance. Horm Behav. 2006;50:208–215. doi: 10.1016/j.yhbeh.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Lovick T. Panic disorder: a malfunction of multiple transmitter control systems within the midbrain periaqueductal gray matter? Neuroscientist. 2000;6:48–59. [Google Scholar]
- Lovick TA, Griffiths JL, Dunn SM, Martin IL. Changes in GABA (A) receptor subunit expression in the midbrain during the oestrous cycle in Wistar rats. Neuroscience. 2005;131:397–405. doi: 10.1016/j.neuroscience.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Lyons HR, Land MB, Gibbs TT, Frab DH. Distinct signal transduction pathways for GABA-induced GABA(A) receptor down-regulation and uncoupling in neuronal culture: a role for voltage-gated calcium channels. J Neurochem. 2001;78:1114–1126. doi: 10.1046/j.1471-4159.2001.00501.x. [DOI] [PubMed] [Google Scholar]
- Ma L, Song L, Radoi GE, Harrison NL. Transcriptional regulation of the mouse gene encoding the alpha[4] subunit of the GABA-A receptor. J Biol Chem. 2004;279:40451–40461. doi: 10.1074/jbc.M406827200. [DOI] [PubMed] [Google Scholar]
- Maguire J, Mody I. Neurosteroid synthesis-mediated regulation of GABA(A) receptors: relevance to the ovarian cycle and stress. J Neurosci. 2007;27:2155–2162. doi: 10.1523/JNEUROSCI.4945-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in GABA (A) receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci. 2005;8:797–804. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- Mahmoudi M, Kang MH, Tillakaratne N, Tobin AJ, Olsen RW. Chronic intermittent ethanol treatment in rats increases GABAA receptor α4 subunit expression- possible relevance to alcohol dependence. J Neurochem. 1997;68:2485–2492. doi: 10.1046/j.1471-4159.1997.68062485.x. [DOI] [PubMed] [Google Scholar]
- Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232:1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- Mannan MA, O’Shaughnessy PJ. Ovarian steroid metabolism during post-natal development in the normal mouse and in the adult hypogonadal (hpg) mouse. J Reprod Fertil. 1988;82:727–734. doi: 10.1530/jrf.0.0820727. [DOI] [PubMed] [Google Scholar]
- Mellon SH, Vaudry H. Biosynthesis of neurosteroids and regulation of their synthesis. Int Rev Neurobiol. 2001;46:33–78. doi: 10.1016/s0074-7742(01)46058-2. [DOI] [PubMed] [Google Scholar]
- Mihalek RM, et al. Attenuated sensitivity to neuroactive steroids in gamma-aminobutyrate type A receptor delta subunit knockout mice. Proc Natl Acad Sci. 1999;96:12905–12910. doi: 10.1073/pnas.96.22.12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modesti PA, et al. Changes in blood pressure reactivity and 24-hourblood pressure profileoccuring at puberty. Angiology. 1994;45:443–450. doi: 10.1177/000331979404500605. [DOI] [PubMed] [Google Scholar]
- Mody I. Aspects of the homeostatic plasticity of GABA-A receptor-mediated inhibition. J Physiol. 2005;562.1:37–46. doi: 10.1113/jphysiol.2004.077362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostallino MC, Mura ML, Maciocco E, Murru L, Sanna E, Biggio G. Changes in expression of the delta subunit of the GABA (A) receptor and in receptor function induced by progesterone exposure and withdrawal. J Neurochem. 2006;99:321–332. doi: 10.1111/j.1471-4159.2006.04055.x. [DOI] [PubMed] [Google Scholar]
- Mtchedlishvili Z, Kapur J. High-affinity, slowly desensitizing GABA-A receptors mediate tonic inhibition in hippocampal dentate granule cells. Mol Pharmacol. 2006;69:564–575. doi: 10.1124/mol.105.016683. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Benke D, Fritschy J-M, Somogyi P. Differential synaptic localization of two major γ-aminobutyric acid type A receptor α subunits on hippocampal pyramidal cells. PNAS. 1996;93:11939–11944. doi: 10.1073/pnas.93.21.11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Somogyi P. Segregation of differing GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J Neurosci. 1998;18:1693–1703. doi: 10.1523/JNEUROSCI.18-05-01693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RW, Liang J, Cagetti E, Spigelman I. Plasticity of GABA-A receptors in brains of rats treated with chronic intermittent ethanol. Neuroschem Res. 2005;30:1579–1588. doi: 10.1007/s11064-005-8836-6. [DOI] [PubMed] [Google Scholar]
- Palumbo MA, Salvestroni C, Gallo R, Guo AL, Genazzani AD, Artini PG, et al. Allopregnanolone concentration in hippocampus of prepubertal rats and female rats throughout estrous cycle. J Endocrinol Invest. 1995;18:853–856. doi: 10.1007/BF03349832. [DOI] [PubMed] [Google Scholar]
- Paoletti AM, Romagnino S, Contu R, Orru MM, Marotto MF, Zedda P, et al. Observational study on the stability of the psychological status during normal pregnancy and increased blood levels of neuroactiver steroids wtih GABA-A receptor agonist activity. Psychoneuroendocrinology. 2006;31:485–492. doi: 10.1016/j.psyneuen.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Pearlstein T, Yonkers KA, Fayyad R, Gillespie JA. Pretreatment pattern of symptom expression in premenstrual dysphoric disorder. J Affect Disord. 2005;85:275–282. doi: 10.1016/j.jad.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Pierson RC, Lyons AM, Greenfield LJ., Jr. Gonadal steroids regulate GABA-A receptor subunit mRNA expression in NT2-N cells. Brain Res Mol Brain Res. 2005;138:105–115. doi: 10.1016/j.molbrainres.2004.10.047. [DOI] [PubMed] [Google Scholar]
- Plappert CF, Rodenbucher AM, Pilz PK. Effects of sex and estrous cycle on modulation of the acoustic startle response in mice. Physiol Behav. 2005;84:585–594. doi: 10.1016/j.physbeh.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Purdy RH, Morrow AL, Moore PH, Jr., Paul SM. Stress-induced elevations of gamma-aminobutyric acid type A receptor-active steroids in the rat. PNAS. 1991;88:4553–4557. doi: 10.1073/pnas.88.10.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapkin A. A review of treatment of premenstrual syndrome and premenstrual dysphoric disorder. Psychoneuroendocrinology. 2003;28:39–53. doi: 10.1016/s0306-4530(03)00096-9. [DOI] [PubMed] [Google Scholar]
- Rapkin A, Morgan M, Goldman L, Brann D, Simone D, Mahesh V.Progesterone metabolite allopregnanolone in women with premenstrual syndrome Obstet Gynecol 199790709–714. (Ref Type: Generic) [DOI] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Enhanced anticonvulsant activity of neuroactive steroids in a rat model of catamenial epilepsy. Epilepsia. 2001;42:337–344. doi: 10.1046/j.1528-1157.2001.10200.x. [DOI] [PubMed] [Google Scholar]
- Reddy D, Kim H, Rogawski M. Neurosteroid withdrawal model of perimenstrual catamenial epilepsy. Epilepsia. 2001;42:328–336. doi: 10.1046/j.1528-1157.2001.10100.x. [DOI] [PubMed] [Google Scholar]
- Roberts DS, Raol YH, Bandyopadhyay S, Lund IV, Budreck EC, Passini MA, et al. Egr3 stimulation of GABARA4 promoter activity as a mechanism for seizure-induced up-regulation of GABA (A) receptor alpha4 subunit expression. Proc Natl Acad Sci. 2005;102:11894–11899. doi: 10.1073/pnas.0501434102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DS, Hu Y, Lund IV, Brooks-Kayal AR, Russek SJ. Brain-derived neurotrophic factor (BDNF)-induced synthesis of early growth response factor 3 (Egr3) controls the levels of type A GABA receptor alpha4 subunits in hippocampal neurons. J Biol Chem. 2006;281:29431–29435. doi: 10.1074/jbc.C600167200. [DOI] [PubMed] [Google Scholar]
- Rogawski MA. Progesterone, neurosteroids, and the hormonal basis of catamenial epilepsy. Ann Neurol. 2003;53:288–291. doi: 10.1002/ana.10534. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Crestani F, Benke D, Brünig I, Benson JA, Fritschy J-M, et al. Benzodiazepine actions mediated by specific γ-aminobutyric acidA receptor subtypes. Nature. 1999;401:796–800. doi: 10.1038/44579. [DOI] [PubMed] [Google Scholar]
- Sanna E, Mostallino MC, Busonero F, Talani G, Tranquilli S, Mameli M, et al. Changes in GABA-A receptor gene expression associated with selective alterations in receptor function and pharmacology after ethanol withdrawal. J Neurosci. 2003;23:11711–11724. doi: 10.1523/JNEUROSCI.23-37-11711.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt P, Purdy RH, Moore PH, Jr., Paul S, Rubinow D. Circulating levels of anxiolytic steroids in the luteal phase in women with premenstrual syndrome and in control subjects. J Clin Endocrinol Metab. 1994;79:1256–1260. doi: 10.1210/jcem.79.5.7962316. [DOI] [PubMed] [Google Scholar]
- Schmidt P, Nieman L, Danaceau M, Adams L, Rubinow D. Differential behavioral effects of gonadal steroids in women with premenstrual syndrome. New Engl J Med. 1998;338:209–216. doi: 10.1056/NEJM199801223380401. [DOI] [PubMed] [Google Scholar]
- Shen H, Gong QH, Yuan M, Smith SS. Short-term steroid treatment increases delta GABA-A receptor subunit expression in rat CA1 hippocampus: pharmacological and behavioral effects. Neuropharmacology. 2005;49:573–586. doi: 10.1016/j.neuropharm.2005.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Gong QH, Aoki C, Yuan M, Ruderman Y, Dattilo M, et al. Reversal of neurosteroid effects at α4β2δ GABAA receptors triggers anxiety at puberty. Nat Neurosci. 2007;10:469–477. doi: 10.1038/nn1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS. Neurosteroid Effects in the Central Nervous System: The role of the GABAA receptor. CRC Press; Boca Raton, Florida: 2003. Withdrawal effects of a neuroactive steroid as a model of PMS: synaptic physiology to behavior; pp. 110–130. [Google Scholar]
- Smith SS, Chapin JK. The estrous cycle and the olivo-cerebellar circuit: II. Enhanced selective sensory gating of responses from the rostral dorsal accessory olive. Exp Brain Res. 1996;111:371–384. doi: 10.1007/BF00228727. [DOI] [PubMed] [Google Scholar]
- Smith SS, Gong QH. Neurosteroid administration and withdrawal alter GABA-A receptor kinetics in CA1 hippocampus of female rats. J Physiol. 2005;564:421–436. doi: 10.1113/jphysiol.2004.077297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS, Waterhouse BD, Woodward DJ. Progesterone alters GABA and glutamate responsiveness: a possible mechanism for its anxiolytic action. Brain Res. 1987;400:353–359. doi: 10.1016/0006-8993(87)90634-2. [DOI] [PubMed] [Google Scholar]
- Smith SS, Gong QH, Hsu FC, Markowitz RS, ffrench-Mullen JMH, Li X. GABAA receptor α4 subunit suppression prevents withdrawal properties of an endogenous steroid. Nature. 1998;392:926–929. doi: 10.1038/31948. [DOI] [PubMed] [Google Scholar]
- Smith SS, Gong QH, Li X, Moran MH, Bitran D, Frye CA, et al. Withdrawal from 3α-OH-5α-pregnan-20-one withdrawal using a pseudopregnancy model alters the kinetics of hippocampal GABAA-gated current and increases the GABAA receptor α4 subunit in association with increased anxiety. J Neurosci. 1998;18:5275–5284. doi: 10.1523/JNEUROSCI.18-14-05275.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M, Keel J, Greeberg B, Adams L, Schmidt P, Rubinow D, et al. Menstrual cycle effects on cortical excitability. Neurology. 1999;53:2069–2072. doi: 10.1212/wnl.53.9.2069. [DOI] [PubMed] [Google Scholar]
- Smith MJ, Adams LF, Schmidt PJ, Rubinow DR, Wasserman EJ. Abnormal luteal phase excitability of the motor cortex in women with premenstrual syndrome. Biol Psychol. 2003;54:757–762. doi: 10.1016/s0006-3223(02)01924-8. [DOI] [PubMed] [Google Scholar]
- Smith SS, Ruderman Y, Frye CA, Homanics GE, Yuan M. Steroid withdrawal in the mouse results in anxiogenic effects of 3,5-THP: a possible model of premenstrual dysphoric disorder. Psychopharmacology. 2006;186:323–333. doi: 10.1007/s00213-005-0168-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spigelman I, Li Z, Liang J, Cagetti E, Samzadeh S, Mihalek RM, et al. Reduced inhibition and sensitivity to neurosteroids in hippocampus of mice lacking the GABA(A) receptor delta subunit. J Neurophysiol. 2003;90:903–910. doi: 10.1152/jn.01022.2002. [DOI] [PubMed] [Google Scholar]
- Staley KJ, Mody I. Shunting of excitatory input to dentate gyrus granule cells by a depolarizing GABA-A receptor-mediated postsynaptic cinductance. J Neurophysiol. 1992;68:197–212. doi: 10.1152/jn.1992.68.1.197. [DOI] [PubMed] [Google Scholar]
- Staley KJ, Proctor WR. Modulation of mammalian dendritic GABA (A) receptor function by the kinetics of Cl- and HCO3-transport. J Physiol. 1999;519:693–712. doi: 10.1111/j.1469-7793.1999.0693n.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stell BM, Mody I. Receptors with different affinities mediate phasic and tonic GABA (A) conductances in hippocampal neurons. J Neurosci. 2002;22:RC223. doi: 10.1523/JNEUROSCI.22-10-j0003.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by delta subunit-containing GABA-A receptors. Proc Natl Acad Sci. 2003;100:14439–14444. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundstrom I, Nyberg S, Backstrom T. Patients with premenstrual syndrome have reduced snsitivity to midazolam compared to control subjects. Neuropsychopharmacology. 1997;17:370–381. doi: 10.1016/S0893-133X(97)00086-9. [DOI] [PubMed] [Google Scholar]
- Sundstrom-Poromaa I, Smith DH, Gong Q, Sabado TN, Li X, Light A, et al. Hormonally regulated α4β2δ GABAA receptors are a target for alcohol. Nat Neurosci. 2002;5:721–722. doi: 10.1038/nn888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sur C, Farrar S, Kerby J, Whiting PJ, Atack JR, McKernan RM. Preferential coassembly of alpha-4 and delta subunits of the GABA-A receptor in rat thalamus. Mol Pharmacol. 1999;56:110–115. doi: 10.1124/mol.56.1.110. [DOI] [PubMed] [Google Scholar]
- Turner DM, Ransom RW, Yang JS, Olsen RW. Steroid anesthetics and naturally occurring analogs modulate the gamma-aminobutyric acid receptor complex at a site distinct from barbiturates. J Pharmacol Exp Ther. 1989;248:960–966. [PubMed] [Google Scholar]
- Twyman RE, Macdonald RL. Neurosteroid regulation of GABAA receptor-single channel kinetic properities of mouse spinal cord neurons in culture. J Physiol. 1992;456:215–245. doi: 10.1113/jphysiol.1992.sp019334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanDoren MJ, Matthews DB, Janis GC, Grobin AC, Devaud LL, Morrow AL. Neuroactive steroid 3alpha-hydroxy-5alpha-pregnan-20-one modulates electrophysiological and behavioral actions of ethanol. J Neurosci. 2000;20:1982–1989. doi: 10.1523/JNEUROSCI.20-05-01982.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voituron N, Frugiere A, Gros F, Macron JM, Bodineau L. Diencephalic and mesencephalic influences on ponto-medullary respiratory control in normoxic and hypoxic conditions: an in vitro study on central nervous system preparations from newborn rat. Neuroscience. 2005;132:843–854. doi: 10.1016/j.neuroscience.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Wafford KA, Thompson SA, Sikela J, Wilcox AS, Whiting PJ. Functional characterization of human GABA receptors containing the α4 subunit. Mol Pharmacol. 1996;50:670–678. [PubMed] [Google Scholar]
- Wallner M, Hanchar HJ, Olsen RW. Ethanol enhances α4β3δ and α6β3δ gamma-aminobutyric acid type A receptors at low concentrations known to affect humans. Proc Natl Acad Sci. 2003;101:2–3. doi: 10.1073/pnas.2435171100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Zhang N, Peng Z, Houser CR, Mody I. Perisynaptic localization of delta subunit-containing GABA (A) receptors and their activation by GABA spillover in the mouse dentate gyrus. J Neurosci. 2003;23:10650–10661. doi: 10.1523/JNEUROSCI.23-33-10650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland NG, Orchinik M. Specific subunit mRNAs of the GABAA receptor are regulated by progesterone in subfields of the hippocampus. Brain Res Mol Brain Res. 1995;32:271–278. doi: 10.1016/0169-328x(95)00087-9. [DOI] [PubMed] [Google Scholar]
- Wieland HA, Luddens H, Seeburg PH. A single histidine in GABA-A receptors is essential for benzodiazepine agonist binding. J Biol Chem. 1992;267:1426–1429. [PubMed] [Google Scholar]
- Wisden W, Laurie DJ, Monyer H, Seeburg P. Cloning, pharmacological characteristis and expression pattern of the rat GABAA receptor α4 subunit. FEBS Lett. 1991;289:227–230. doi: 10.1016/0014-5793(91)81076-k. [DOI] [PubMed] [Google Scholar]
- Wisden W, Laurie DJ, Monyer H, Seeburg P. The distribution of 13 GABA-A receptor subunit mRNAs in the rat brain: I. Telencephalon, diencephalon, mesencephalon. J Neurosci. 1992;12:1040–1062. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlfarth KM, Bianchi MT, Macdonald RL. Enhanced neurosteroid potentiation of ternary GABA (A) receptors containing the delta subunit. J Neurosci. 2002;22:1541–1549. doi: 10.1523/JNEUROSCI.22-05-01541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Wang W, Richerson G. Vigabatrin induces tonic inhibition via GABA transporter reversal without increasing vesicular GABA release. J Neurophysiol. 2003;89:2021–2034. doi: 10.1152/jn.00856.2002. [DOI] [PubMed] [Google Scholar]
- Yonkers KA. Anxiety symptoms and anxiety disorders: how are they related to premenstrual disorders? J Clin Psychiatry. 1997;58:62–69. [PubMed] [Google Scholar]
- Yu R, Ticku MK. Chronic neurosteroid treatment decreases the efficacy of benzodiazepine ligands and neurosteroids at the GABAA receptor complex in mammalian cortical neurons. JPET. 1995;275:784–789. [PubMed] [Google Scholar]
- Yu R, Ticku MK. Chronic neurosteroid treatment produces functional heterologous uncoupling at the gamma-aminobutyric acid type A/benzodiazepine receptor complex in mammalian cortical neurons. Mol Pharmacol. 1995;47:603–610. [PubMed] [Google Scholar]
- Yu R, Follesa P, Ticku MK. Down-regulation of the GABA receptor subunit mRNA levels in mammalian cultured cortical neurons following chronic neurosteroid treatment. Brain Res Mol Brain Res. 1996;41:163–168. doi: 10.1016/0169-328x(96)00087-3. [DOI] [PubMed] [Google Scholar]
- Yu R, Hay M, Ticku MK. Chronic neurosteroid treatment attenuates single cell GABAA response and its potentiation by modulators in cortical neurons. Brain Res. 1996;706:160–162. doi: 10.1016/0006-8993(95)01247-8. [DOI] [PubMed] [Google Scholar]
- Zhou X, Smith SS. Steroid requirements for regulation of the alpha-4 subunit of the GABA-A receptor in an in vitro model. Neurosci Lett. 2007;411:61–66. doi: 10.1016/j.neulet.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuluaga MJ, Agrati D, Pereira M, Uriarte N, Fernandez-Guasti A, Ferreira A. Experimental anxiety in the black and white model in cycling, pregnant and lactating rats. Physiol Behav. 2005;84:279–286. doi: 10.1016/j.physbeh.2004.12.004. [DOI] [PubMed] [Google Scholar]


