Abstract
A large skull is disadvantageous to animals that move quickly in three-dimensional space, such as fishes and birds in water or air. A cerebral neocortex with a six-layered sheet has not evolved, most likely due to the limited cranial space. Instead of the laminar cortex, telencephalic nuclear masses seem to have evolved as the pallium in teleost fishes. We consider that the nuclear masses contain rather simple neural circuits sharing a skeleton of simple circuits in the mammalian cortex, which have been elaborated by additional circuits in mammals. Such basic similarities at the connectional level shared by nuclear and cortical pallium might underlie similar or equivalent functions.
Keywords: cortex, pallium, evolution, actinopterygian, teleost
1. Nuclear versus laminar telencephalic pallium
A distinctive feature of the mammalian telencephalon is the presence of a six-layered pallium or neocortex, which is considered to be responsible for ‘higher’ functions. Despite several ‘intelligent’ behaviours, however, the pallium of birds is composed mostly of nuclear masses with only a small laminar structure, the Wulst (Shimizu 2007). Laminar pallium, therefore, does not appear to be a requisite for such functions. Karten (1991) suggested that visual information processing in the bird telencephalon is comparable with that in the mammalian neocortex.
The pallium of teleosts also lacks six-layered cortex (or even layered structures), although behavioural studies show some higher cortical-like functions in teleosts including transitive inference (Demski 1983; Grosenick et al. 2007; Ito et al. 2007). In the present paper, we survey afferent, efferent and intrinsic connections of the teleost telencephalon in search of pallial circuitries comparable with mammalian neocortex.
2. Overview of teleost telencephalon
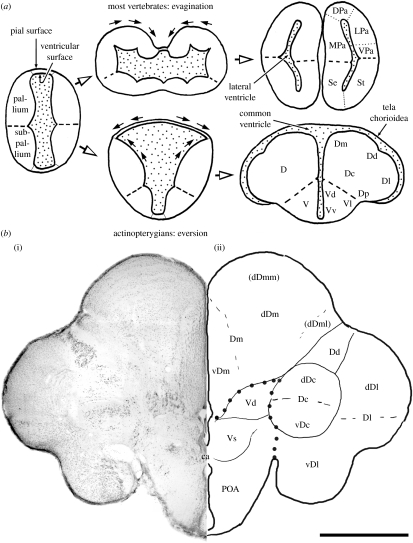
The developmental process of the telencephalon is unique in ray-finned fishes. The developing telencephalon does not show evagination being different from other vertebrates, but instead shows eversion. Accordingly, the telencephalon does not form paired tubular structures that contain the lateral ventricles, but forms two solid hemispheres composed of several nuclear masses (figure 1). The hemispheres are separated by a T-shaped common ventricle and covered with a thin tela chorioidea (figure 1). This unique developmental process might explain the formation of the non-laminar telencephalon in ray-finned fishes. Eversion significantly reduces the pial surface area on the lateral wall of the pallium (figure 1a), leaving little room for cortex formation. Recently, Striedter & Northcutt (2006) have proposed that the eversion evolved because embryos of ray-finned fishes are so small that the telencephalon cannot evaginate, but instead squeeze into a rostral narrow space of the cranium.
Figure 1.
(a) Schematic of telencephalic development of actinopterygians (teleost as an example) in comparison with other vertebrates and (b) (i) a frontal section of the telencephalon of tilapia Oreochromis niloticus stained by cresyl violet and (ii) line drawing indicating telencephalic structures. Figure 1a adapted from Yamamoto et al. (2007). Thick broken lines in (a) and large dots in (b) indicate the boundary between the areas dorsalis (D: presumed pallial homologue) and ventralis (V: presumed subpallial homologue). ca, commissura anterior; dDc, dorsal region of Dc; dDm, dorsal region of Dm; dDml, lateral portion of dDm; dDmm, medial portion of dDm; DPa, dorsal pallium; LPa, lateral pallium; MPa, medial pallium; POA, area preoptica; Se, septum; St, striatum; vDc, ventral region of Dc; vDm, ventral region of Dm; VPa, ventral pallium. See text for other abbreviations. Scale bar, 1 mm.
The areas dorsalis telencephali (D) and ventralis telencephali (V) named by Nieuwenhuys (1963) are considered to be homologous approximately to the pallium and subpallium of other vertebrates, respectively (Alunni et al. 2004; Kage et al. 2004; figure 1). The D can be subdivided into several parts: pars centralis (Dc); pars dorsalis (Dd); pars medialis (Dm); pars lateralis (Dl); and pars posterior (Dp). The D also includes the nucleus taenia (nT). These parts may be subdivided further. For example, the Dl is composed at least of two components: dorsal (dDl) and ventral (vDl) regions (Yamane et al. 1996). Similarly, the V is composed of pars dorsalis (Vd), pars ventralis (Vv), pars lateralis (Vl), pars supracommissuralis (Vs), pars postcommissuralis (Vp) and so on. In the present survey, we mainly focus on the area dorsalis, the presumed pallial homologue, which we hypothesize to contain functional equivalents to the mammalian neocortex.
3. Sensory pathways to the telencephalon
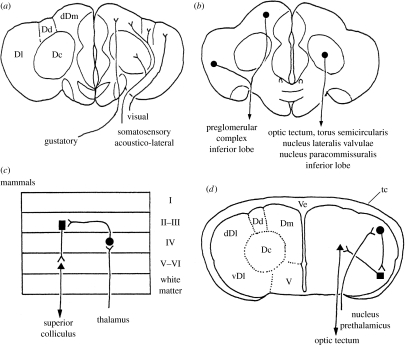
Although the telencephalon of fishes has been regarded purely as an olfactory centre for a long time (e.g. Herrick 1924), non-olfactory sensory pathways to the telencephalon have been reported in some teleosts (Ebbesson 1980; Finger 1980; Echteler & Saidel 1981). Based upon more detailed experiments and observations, it has been clarified that different sensory modalities reach different pallial targets (figure 2a; for review see, Yamamoto et al. 2007). Secondary olfactory fibres reach the Dp and nT (Yamamoto & Ito 2000). Visual information reaches mainly the dDl mediated by the nucleus prethalamicus (Ito & Vanegas 1983) or a presumed prethalamic homologue within the preglomerular complex, a collection of nuclei in the ventrolateral diencephalon (Northcutt 2006; Yamamoto & Ito 2008). Octavolateral as well as somatosensory information reaches the Dm, Dc and Dl mediated by the ventromedial thalamic nucleus (Ito et al. 1986) and the preglomerular complex (Murakami et al. 1986a,b; Striedter 1991; Yamamoto & Ito 2005a,b, 2008; Northcutt 2006). Gustatory information reaches mainly the Dm relayed by a portion of the preglomerular complex or its homologue (Kanwal et al. 1988; Yoshimoto et al. 1998), reaching a region presumably distinct from octavolateral terminal zones within the Dm (Yamamoto & Ito 2005b). Ascending pathways mediated by the preglomerular complex enumerated above exhibit a considerable degree of modality-specific organization (Yamamoto et al. 2007; Yamamoto & Ito 2008), similarly to mammalian thalamo-cortical pathways.
Figure 2.
Line drawings illustrating (a) ascending sensory pathways to and (b) descending pathways originating from the area dorsalis telencephali in teleosts, and an example of the input–output pathways involved in telencephalic visual processing that can be deduced from tract-tracing studies in (d) acanthopterygian teleosts (Ito et al. 1980; Ito & Vanegas 1983; Murakami et al. 1983; Yamane et al. 1996; Imura et al. 2003), in comparison with (c) the mammalian cortical circuit. Ve, common ventricle; tc, tela chorioidea. Other abbreviations are as in the text and those of figure 1.
It is a critical issue, whether or not the preglomerular complex corresponds to the dorsal thalamus. Northcutt and his co-workers regard the complex as a migrated part of the posterior tuberculum, a non-thalamic diencephalic basal plate derivative, based on developmental analyses with non-experimental materials (Braford & Northcutt 1983; Northcutt 2008). However, analyses of Pax6 expression during ontogeny suggest that preglomerular neurons are derived from diencephalic alar plate (Kage et al. 2004; Ishikawa et al. 2007). We therefore consider it likely that preglomerulo-pallial pathways correspond to dorsal thalamo-neocortical pathways in mammals.
4. Descending pathways from the telencephalon
Descending pathways originate mainly from the Dc (figure 2b), which are mostly directed to the optic tectum, torus semicircularis, nucleus lateralis valvulae, nucleus paracommissuralis and inferior lobes of the hypothalamus (Murakami et al. 1983; Imura et al. 2003; Yang et al. 2004, 2007). The Dl and Dm also give rise to descending fibres (figure 2b), which are targeted mainly to the inferior lobes of the hypothalamus and preglomerular complex (Folgueira et al. 2004; Yamamoto & Ito 2005b, 2008; Northcutt 2006; Yang et al. 2007). Thus, the Dc seems to give rise to longer descending pathways that reach down to the meso-rhombencephalic level than those originating from the Dl and Dm that mostly end in the diencephalon. In addition to neurons that project fibres directly out of the telencephalon, the Dl and Dm appear to contain different populations of neurons that send intra-telencephalic fibres to the Dc (Murakami et al. 1983; Yamane et al. 1996; Yamamoto & Ito 2005b, 2008; Northcutt 2006).
5. Non-laminar cortex
Neurons in the Dc resemble those in layer V of the mammalian neocortex, which give rise to long descending fibres. The Dl and Dm appear to contain neurons similar to those in layer VI in terms of originating rather short descending fibres to the diencephalon. The Dl and Dm also contain neuronal populations that participate in telencephalic intrinsic connections as neurons located in other cortical layers in mammals.
Although more detailed analysis of the fibre connections is required to test such a comparison, it appears quite possible that neuronal populations functionally equivalent to neocortical layers in mammals do not form laminar structures but aggregate to form nuclear masses in teleosts, similar to the situation proposed by Karten (1991) for birds. It requires further analyses to see whether the similar neuronal circuitries and their component neuronal populations are homologous.
Acknowledgments
All experimental procedures were performed under the official Japanese regulations for research on animals and those of the Animal Care Committee of our institutions.
Dr Yuji Ishikawa of National Institute of Radiological Sciences provided important data and helpful suggestions. This study was supported in part by a grant from Fujiwara Natural History Foundation to N.Y.
Footnotes
One contribution of 10 to a Special Feature on ‘Brain evolution’.
References
- Alunni A., Blin M., Deschet K., Bourrat F., Vernier P., Rétaux S. Cloning and developmental expression patterns of Dlx2, Lhx7 and Lhx9 in the medaka fish (Oryzias latipes) Mech. Dev. 2004;121:977–983. doi: 10.1016/j.mod.2004.03.023. doi:10.1016/j.mod.2004.03.023 [DOI] [PubMed] [Google Scholar]
- Braford Jr, M. R. & Northcutt, R. G. 1983 Organization of the diencephalon and pretectum of the ray-finned fishes. In Fish neurobiology, vol. 2 (eds R. E. Davis & R. G. Northcutt), pp. 117–163. Ann Arbor, MI: University of Michigan Press.
- Demski, L. 1983 Behavioral effects of electrical stimulation of the brain. In Fish neurobiology, vol. 2 (R. E. Davis & R. G. Northcutt), pp. 317–359. Ann Arbor, MI: University of Michigan Press
- Ebbesson S.O.E. A visual thalamo-telencephalic pathway in a teleost fish (Holocentrus rufus) Cell Tissue Res. 1980;213:505–508. doi: 10.1007/BF00237895. doi:10.1007/BF00237895 [DOI] [PubMed] [Google Scholar]
- Echteler S.M., Saidel W.M. Forebrain connections in the goldfish support telencephalic homologies with land vertebrates. Science. 1981;212:683–685. doi: 10.1126/science.6971493. doi:10.1126/science.6971493 [DOI] [PubMed] [Google Scholar]
- Finger T.E. Nonolfactory sensory pathway to the telencephalon in a teleost fish. Science. 1980;210:671–673. doi: 10.1126/science.7192013. doi:10.1126/science.7192013 [DOI] [PubMed] [Google Scholar]
- Folgueira M., Anadón R., Yáñez J. Experimental study of the connections of the telencephalon in the rainbow trout (Oncorhynchus mykiss). II: dorsal area and preoptic region. J. Comp. Neurol. 2004;480:204–233. doi: 10.1002/cne.20341. doi:10.1002/cne.20341 [DOI] [PubMed] [Google Scholar]
- Grosenick L., Clement T.S., Fernald R.D. Fish can infer social rank by observation alone. Nature. 2007;445:429–432. doi: 10.1038/nature05511. doi:10.1038/nature05511 [DOI] [PubMed] [Google Scholar]
- Herrick C.J. Hafner Publishing Company; New York, NY: 1924. 1962. Neurological foundations of animal behavior. (Reprinted) [Google Scholar]
- Imura K., Yamamoto N., Sawai N., Yoshimoto M., Yang C.-Y., Xue H.-G., Ito H. Topographical organization of an indirect telencephalo-cerebellar pathway through the nucleus paracommissuralis in a teleost, Oreochromis niloticus. Brain Behav. Evol. 2003;61:70–90. doi: 10.1159/000069353. doi:10.1159/000069353 [DOI] [PubMed] [Google Scholar]
- Ishikawa Y., Yamamoto N., Yoshimoto M., Ito H. Developmental origin of diencephalic sensory relay nuclei in teleosts. Brain Behav. Evol. 2007;69:87–95. doi: 10.1159/000095197. doi:10.1159/000095197 [DOI] [PubMed] [Google Scholar]
- Ito H., Vanegas H. Cytoarchitecture and ultrastructure of nucleus prethalamicus, with special reference to degenerating afferents from optic tectum and telencephalon, in a teleost (Holocentrus ascensionis) J. Comp. Neurol. 1983;221:401–415. doi: 10.1002/cne.902210404. doi:10.1002/cne.902210404 [DOI] [PubMed] [Google Scholar]
- Ito H., Morita Y., Sakamoto N., Ueda S. Possibility of telencephalic visual projections in a teleost, Holocentrus rufus. Brain Res. 1980;197:219–222. doi: 10.1016/0006-8993(80)90448-5. doi:10.1016/0006-8993(80)90448-5 [DOI] [PubMed] [Google Scholar]
- Ito H., Murakami T., Fukuoka T., Kishida R. Thalamic fiber connections in a teleost (Sebastiscus marmoratus): visual, somatosensory, octavolateral, and cerebellar relay region to the telencephalon. J. Comp. Neurol. 1986;250:215–227. doi: 10.1002/cne.902500208. doi:10.1002/cne.902500208 [DOI] [PubMed] [Google Scholar]
- Ito H., Ishikawa Y., Yoshimoto M., Yamamoto N. Diversity of brain morphology in teleosts: brain and ecological niche. Brain Behav. Evol. 2007;69:76–86. doi: 10.1159/000095196. doi:10.1159/000095196 [DOI] [PubMed] [Google Scholar]
- Kage T., et al. Morphogenesis and regionalization of the medaka embryonic brain. J. Comp. Neurol. 2004;476:219–239. doi: 10.1002/cne.20219. doi:10.1002/cne.20219 [DOI] [PubMed] [Google Scholar]
- Kanwal J.S., Finger T.E., Caprio J. Forebrain connections of the gustatory system in ictalurid catfishes. J. Comp. Neurol. 1988;278:353–376. doi: 10.1002/cne.902780306. doi:10.1002/cne.902780306 [DOI] [PubMed] [Google Scholar]
- Karten H.J. Homology and evolutionary origins of the ‘neocortex’. Brain Behav. Evol. 1991;38:264–272. doi: 10.1159/000114393. doi:10.1159/000114393 [DOI] [PubMed] [Google Scholar]
- Murakami T., Morita Y., Ito H. Extrinsic and intrinsic fiber connections of the telencephalon in a teleost, Sebastiscus marmoratus. J. Comp. Neurol. 1983;216:115–131. doi: 10.1002/cne.902160202. doi:10.1002/cne.902160202 [DOI] [PubMed] [Google Scholar]
- Murakami T., Fukuoka T., Ito H. Telencephalic ascending acousticolateral system in a teleost (Sebastiscus marmoratus), with special reference to fiber connections of the nucleus preglomerulosus. J. Comp. Neurol. 1986a;247:383–397. doi: 10.1002/cne.902470308. doi:10.1002/cne.902470308 [DOI] [PubMed] [Google Scholar]
- Murakami T., Ito H., Morita Y. Telencephalic afferent nuclei in the carp diencephalon, with special reference to fiber connections of the nucleus preglomerulosus pars lateralis. Brain Res. 1986b;382:97–103. doi: 10.1016/0006-8993(86)90115-0. doi:10.1016/0006-8993(86)90115-0 [DOI] [PubMed] [Google Scholar]
- Nieuwenhuys R. The comparative anatomy of the actinopterygian forebrain. J. Hirnforsch. 1963;6:171–192. [PubMed] [Google Scholar]
- Northcutt R.G. Connections of the lateral and medial divisions of the goldfish telencephalic pallium. J. Comp. Neurol. 2006;494:903–943. doi: 10.1002/cne.20853. doi:10.1002/cne.20853 [DOI] [PubMed] [Google Scholar]
- Northcutt R.G. Forebrain evolution in bony fishes. Brain Res. Bull. 2008;75:191–205. doi: 10.1016/j.brainresbull.2007.10.058. doi:10.1016/j.brainresbull.2007.10.058 [DOI] [PubMed] [Google Scholar]
- Shimizu, T. 2007 The avian brain revisited: anatomy and evolution of the telencephalon. In Integration of comparative neuroanatomy and cognition (eds S. Watanabe & M. H. Hofman), pp. 55–73. Tokyo, Japan: Keio University Publications.
- Striedter G.F. Auditory, electrosensory, and mechanosensory lateral line pathways through the forebrain in channel catfishes. J. Comp. Neurol. 1991;312:311–331. doi: 10.1002/cne.903120213. doi:10.1002/cne.903120213 [DOI] [PubMed] [Google Scholar]
- Striedter G.F., Northcutt R.G. Head size constrains forebrain development and evolution in ray-finned fishes. Evol. Dev. 2006;8:215–222. doi: 10.1111/j.1525-142X.2006.00091.x. doi:10.1111/j.1525-142X.2006.00091.x [DOI] [PubMed] [Google Scholar]
- Yamamoto N., Ito H. Afferent sources to the ganglion of the terminal nerve in teleosts. J. Comp. Neurol. 2000;428:355–375. doi: 10.1002/1096-9861(20001211)428:2<355::aid-cne12>3.0.co;2-w. doi:10.1002/1096-9861(20001211)428:2<355::AID-CNE12>3.0.CO;2-W [DOI] [PubMed] [Google Scholar]
- Yamamoto N., Ito H. Fiber connections of the central nucleus of semicircular torus in cyprinids. J. Comp. Neurol. 2005a;491:186–211. doi: 10.1002/cne.20683. doi:10.1002/cne.20683 [DOI] [PubMed] [Google Scholar]
- Yamamoto N., Ito H. Fiber connections of the anterior preglomerular nucleus in cyprinids with notes on telencephalic connections of the preglomerular complex. J. Comp. Neurol. 2005b;491:212–233. doi: 10.1002/cne.20681. doi:10.1002/cne.20681 [DOI] [PubMed] [Google Scholar]
- Yamamoto N., Ito H. Visual, lateral line, and auditory ascending pathways to the dorsal telencephalic area through the rostrolateral region of lateral preglomerular nucleus in cyprinids. J. Comp. Neurol. 2008;508:615–647. doi: 10.1002/cne.21717. doi:10.1002/cne.21717 [DOI] [PubMed] [Google Scholar]
- Yamamoto N., Ishikawa Y., Yoshimoto M., Xue H.-G., Bahaxar N., Sawai N., Yang C.-Y., Ozawa H., Ito H. A new interpretation on the homology of the teleostean telencephalon based on hodology and a new eversion model. Brain Behav. Evol. 2007;69:96–104. doi: 10.1159/000095198. doi:10.1159/000095198 [DOI] [PubMed] [Google Scholar]
- Yamane Y., Yoshimoto M., Ito H. Area dorsalis pars lateralis of the telencephalon in a teleost (Sebastiscus marmoratus) can be divided into dorsal and ventral regions. Brain Behav. Evol. 1996;48:338–349. doi: 10.1159/000113212. doi:10.1159/000113212 [DOI] [PubMed] [Google Scholar]
- Yang C.-Y., Yoshimoto M., Xue H.-G., Yamamoto N., Imura K., Sawai N., Ishikawa Y., Ito H. Fiber connections of the lateral valvular nucleus in a percomorph teleost, tilapia (Oreochromis niloticus) J. Comp. Neurol. 2004;474:209–226. doi: 10.1002/cne.20150. doi:10.1002/cne.20150 [DOI] [PubMed] [Google Scholar]
- Yang C.-Y., Xue H.-G., Yoshimoto M., Ito H., Yamamoto N., Ozawa H. Fiber connections of the corpus glomerulosum pars rotunda, with special reference to efferent projection pattern to the inferior lobe in a percomorph teleost, tilapia (Oreochromis niloticus) J. Comp. Neurol. 2007;501:582–607. doi: 10.1002/cne.21261. doi:10.1002/cne.21261 [DOI] [PubMed] [Google Scholar]
- Yoshimoto M., Albert J.S., Sawai N., Shimizu M., Yamamoto N., Ito H. Telencephalic ascending gustatory system in a cichlid fish, Oreochromis (Tilapia) niloticus. J. Comp. Neurol. 1998;392:209–226. doi:10.1002/(SICI)1096-9861(19980309)392:2<209::AID-CNE5>3.0.CO;2-6 [PubMed] [Google Scholar]