Abstract
Some mammals and birds independently evolved an enlarged telencephalon. They appear to have done so, at least in part, by developing a thick telencephalic subventricular zone (SVZ). We suggest that this correlation between telencephalic enlargement and SVZ expansion is due to a mechanical constraint acting on the proliferative ventricular zone (VZ). Essentially, we argue that rapid proliferation in the VZ after post-mitotic cells in the overlying mantle zone have begun to form limits the VZ's tangential expandability and forces some proliferating cells to emigrate from the VZ and expand the pool of proliferating cells that comprise the SVZ.
Keywords: development, neocortex, birds
Most of the dividing cells in embryonic vertebrate brains are located in the so-called ventricular zone (VZ), which surrounds the cerebral ventricles. The nuclei of cells in this VZ regularly move towards the ventricular surface, divide there and then move away from the ventricle again. This process is called interkinetic nuclear migration. The mammalian neocortex and striatum contain an additional proliferative zone that lies superficial to the VZ and is called the subventricular zone (SVZ). Cells in this SVZ do not exhibit interkinetic nuclear migration and undergo mitosis at scattered locations throughout the SVZ.
The thickness of the SVZ differs across species, especially in the developing neocortex and its non-mammalian homologues (Striedter 1997). It is rudimentary, at best, in turtles, probably absent in marsupials, relatively thin in rats, thicker in ferrets and thickest in primates (Smart et al. 2002; Martínez-Cerdeño et al. 2006; Abdel-Mannan et al. 2008). By contrast, VZ thickness varies much less. These observations have been used to argue that (i) the invention of a proper SVZ caused the adult mammalian neocortex to become thicker than its reptilian homologue (Molnar et al. 2005; Martínez-Cerdeño et al. 2006) and (ii) the phylogenetic expansion of this SVZ caused the adult neocortex to be thicker in primates than in rats (Smart et al. 2002; Kriegstein et al. 2006). These gains in thickness are thought to involve primarily the upper cortical layers, which develop largely from the SVZ.
An SVZ has also been described in the telencephalon of birds (Striedter & Keefer 2000). This avian SVZ is thicker in embryonic parrots than in age-matched quail (Striedter & Charvet 2008), an observation that correlates with a difference in adult telencephalon volume, because adult parrots have a proportionately larger telencephalon than adult quail or other fowl (Boire & Baron 1994). The telencephalic expansion in adult parrots involves the striatum as well as several pallial regions. This correlates with our finding that the parrot's SVZ is thickened in both the striatum and the pallium (Striedter & Charvet 2008). Collectively, these observations suggest that the SVZ expanded convergently in parrots and mammals.
Instances of convergent evolution often indicate the existence of underlying causal principles that can account for the evolved similarities. Of course, some convergences may simply be due to chance. However, the argument for chance becomes less likely if the convergent similarities show up in diverse lineages. It is useful, therefore, to ask whether the SVZ is expanded also in other species that have enlarged their telencephalon.
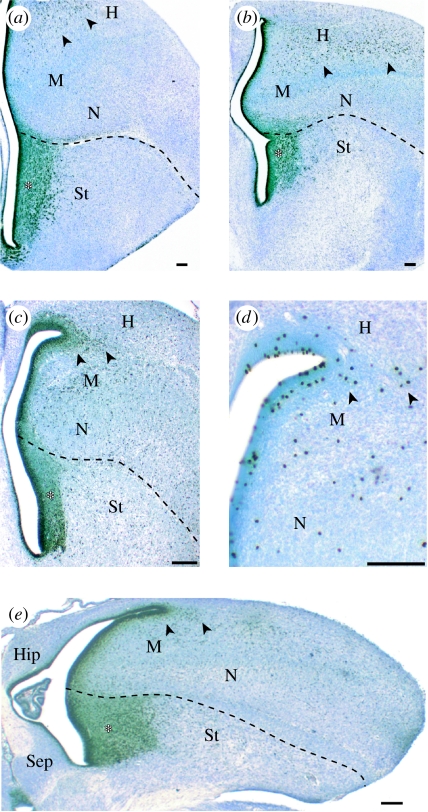
An obvious taxon to examine in this context are songbirds, as they rival parrots in their degree of telencephalic enlargement (Boire & Baron 1994). Previous studies described only a thin SVZ in the subpallium and lateral pallium of a juvenile songbird, the zebra finch, 30 days after hatching (Dewulf & Bottjer 2002). However, we find that the telencephalon of newly hatched zebra finches contains a large SVZ that is similar in thickness and extent to that of parrots (figure 1). Therefore, songbirds also appear to have enlarged their telencephalon, at least in part, by expanding their SVZ. Whether the SVZ expanded independently in songbirds and parrots remains unclear because songbirds may be closely related to parrots (Hackett et al. 2008). However, the fact that songbird and parrot brains differ in numerous major respects (Stingelin 1958) strongly suggests that these two taxonomic groups enlarged their telencephalons independently of one another.
Figure 1.
Sections through the telencephalon of (a, b) parakeets and (c–e) zebra finches labelled with antibodies against (a–c, e) proliferating cells (anti-PCNA) or (d) mitotic cells (anti-phosphorylated histone H3). The thick SVZ in the striatum (St) is marked with an asterisk. Mitotic cells in the hyperpallium, which is probably homologous to at least part of the mammalian neocortex, are indicated with arrowheads. H, hyperpallium; M, mesopallium; N, nidopallium; Hip, hippocampus; Sep, septum. Scale bar, 100 μm.
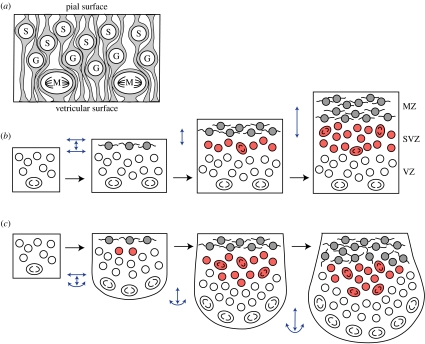
To discover causal principles underlying the convergent evolution of expanded SVZs in mammals and birds, we begin with Smart's (1972) insight that the organization of the VZ, with its interkinetic nuclear migration and ventricular mitoses, imposes significant constraints (figure 2). One constraint is that rapid proliferation within the VZ requires, and generally co-occurs with, tangential VZ expansion. This tangential expansion accounts for the ballooning of the telencephalic vesicle early in embryogenesis. However, if tangential expansion cannot occur, then the VZ must slow its proliferation rate or allow some mitoses to occur away from the ventricle in an SVZ (Smart 1972). Thus, the SVZ allows rapid proliferation to occur in regions of the telencephalon that are limited in their degree of tangential expansion. The most obvious such region is the ganglionic eminence, which bulges into the telencephalic ventricle, gives rise to parts of the basal ganglia and contains a thick SVZ (Smart & Sturrock 1979).
Figure 2.
(a) In the VZ, most mitoses (M) occur at the ventricular surface; G- and S-phase cells are located away from the ventricle. (b) Early in embryogenesis, the VZ expands mainly tangentially. After the MZ has formed, tangential VZ expansion is limited by the processes of post-mitotic MZ cells. This, in turn, increases congestion within the VZ to the point where some proliferating cells emigrate from the VZ and form the SVZ. (c) If the mechanical coupling between the VZ and the MZ is low, then a rapidly proliferating VZ may continue to expand tangentially when the MZ does not. In such cases, an intraventricular ridge with a large SVZ is formed. Blue arrows indicate radial and tangential expansion along the pial and ventricular surfaces.
Thickening the telencephalic walls by means of an expanded SVZ is one effective strategy for packing many cells into a small space. An alternative strategy is to expand the telencephalon tangentially, causing the telencephalic vesicle to balloon more, and then to bend the tissue into complex folds, as happens with the cerebral cortex in large-brained mammals (Prothero & Sundsten 1984). Thus, one can view the expanded SVZ in parrots and songbirds as convergent uses of the same radial expansion strategy for enlarging the telencephalon. Primates, by contrast, employ a hybrid strategy that relies on tangential expansion to enlarge the cortical surface and on an expanded SVZ to thicken the neocortex.
Expanding an SVZ may be a good strategy, but what is its proximate cause? All SVZs initially develop as a thin layer just superficial to the VZ (Kriegstein et al. 2006; Charvet & Striedter 2008), suggesting that the first SVZ cells are descendents of VZ cells that emigrated from the VZ (Fish et al. 2008). The subsequent expansion of the SVZ is probably due to continued emigration from the VZ and to the proliferation of SVZ cells in situ. SVZ cells differ from VZ cells not only in their migratory behaviour but also in their pattern of gene expression. For example, they express Tbr-2, which is not expressed in VZ cells (Englund et al. 2005). Unfortunately, these molecular differences do not explain why some cells leave the VZ to become SVZ cells, while others remain behind. Perhaps the VZ contains a molecularly distinct subpopulation of cells that is fated to form the SVZ. Some evidence for such molecular heterogeneity has been described (Englund et al. 2005; Pinto et al. 2008).
An alternative possibility is that some VZ cells are mechanically forced out of the VZ. Once they have lost their attachment to the ventricle, these budding SVZ cells may adopt a unique pattern of gene expression and start behaving differently from VZ cells. Analogous mechanical effects on gene expression have been described in other proliferating cells (Huang & Ingber 1999). We hypothesize that the force pushing the cells out of the VZ results when the VZ is proliferating rapidly but its tangential expansion is constrained. This portion of our argument is based on Smart's (1972) insights. We further suggest that what constrains the VZ is the presence of post-mitotic cells just superficial to the emerging SVZ in the young mantle zone (MZ; figure 2). These post-mitotic cells tend to have long tangential processes that may limit the MZ's tangential expandability, which then constrains the VZ, to which the MZ is linked by radial glia.
This set of interlinked hypotheses was originally derived from the observation that the SVZ in both mammals and birds consistently begins to form just after the MZ appears. More direct support comes from a recent study of transgenic mice that express a constitutively active form of β-catenin in the neocortical VZ (Wrobel et al. 2007). Because activated β-catenin promotes cell cycle re-entry over cell cycle exit (Chenn & Walsh 2002), these transgenic animals exhibit a significant delay in neurogenesis onset, which means that MZ cells form abnormally late. As our hypothesis predicts, in these transgenic mice, SVZ formation is delayed. The study's authors did not discuss the possibility of a link between delaying neurogenesis and delaying SVZ formation, but their findings are consistent with our proposal.
The observation that turtles thicken their lateral telencephalic wall but exhibit, at best, a rudimentary SVZ (Molnar et al. 2005; Martínez-Cerdeño et al. 2006) implies that telencephalic walls can be thickened without a proper SVZ. Such SVZ-independent thickening might occur when VZ cells proliferate faster than the MZ can be stretched, but slowly enough to prevent proliferating cells from losing their attachment to the ventricular surface. Slow proliferation might also explain the somewhat surprising absence of an SVZ in opossums. Another complication for our proposal is that brains do expand tangentially long after the SVZ has formed. This late tangential expansion differs from the earlier expansion, however, in being part of a more uniform expansion due to the growth of post-mitotic neurons and the late addition of glial cells.
The most potent means of enlarging a brain region is to prolong its period of precursor proliferation (Smart 1972; Finlay & Darlington 1995). The downside of prolonging proliferation is that it delays maturation, as mature neurons do not divide. One way to minimize this problem is to delay neurogenesis offset without delaying neurogenesis onset (Striedter & Charvet 2008), thereby allowing at least some neurons to differentiate early. According to our hypothesis, this strategy creates a thick SVZ and a thick adult structure, which is what we observe in the telencephalon of parrots and songbirds. Of course, a delay in telencephalic neurogenesis offset causes most telencephalic neurons to mature relatively late. This is not a problem for parrots or songbirds, as these altricial birds feed their babies. However, precocial species might well adopt a different strategy for enlarging their telencephalon. Testing of this hypothesis is ongoing.
Acknowledgments
We thank Edwin Monuki for his valuable feedback and the NSF for grant support (IOS-0744332).
Footnotes
One contribution of 10 to a Special Feature on ‘Brain evolution’.
References
- Abdel-Mannan O., Cheung A.F., Molnár Z. Evolution of cortical neurogenesis. Brain Res. Bull. 2008;75:398–404. doi: 10.1016/j.brainresbull.2007.10.047. doi:10.1016/j.brainresbull.2007.10.047 [DOI] [PubMed] [Google Scholar]
- Boire D., Baron G. Allometric comparison of brain and main brain subdivisions in birds. J. Hirnforsch. 1994;35:49–66. [PubMed] [Google Scholar]
- Charvet C.J., Striedter G.F. Developmental species differences in brain cell cycle rates between Northern bobwhite quail (Colinus virginianus) and parakeets (Melopsittacus undulatus): implications for mosaic brain evolution. Brain Behav. Evol. 2008;72:295–306. doi: 10.1159/000184744. doi:10.1159/000184744 [DOI] [PubMed] [Google Scholar]
- Chenn A., Walsh C.A. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science. 2002;297:365–369. doi: 10.1126/science.1074192. doi:10.1126/science.1074192 [DOI] [PubMed] [Google Scholar]
- Dewulf V., Bottjer S. Age and sex differences in mitotic activity within the zebra finch telencephalon. J. Neurosci. 2002;22:4080–4094. doi: 10.1523/JNEUROSCI.22-10-04080.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund C., Fink A., Lau C., Pham D., Daza R.A., Bulfone A., Kowalczyk T., Hevner R.F. Pax6, Tbr2, and Tbr1 are expressed sequentially by radial glia, intermediate progenitor cells, and postmitotic neurons in developing neocortex. J. Neurosci. 2005;25:247–251. doi: 10.1523/JNEUROSCI.2899-04.2005. doi:10.1523/JNEUROSCI.2899-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B.L., Darlington R.B. Linked regularities in the development and evolution of mammalian brains. Science. 1995;268:1578–1584. doi: 10.1126/science.7777856. doi:10.1126/science.7777856 [DOI] [PubMed] [Google Scholar]
- Fish J.L., Dehay C., Kennedy H., Huttner W.B. Making bigger brains:the evolution of neural–progenitor-cell division. J. Cell Sci. 2008;121:2783–2793. doi: 10.1242/jcs.023465. doi:10.1242/jcs.023465 [DOI] [PubMed] [Google Scholar]
- Hackett S.J., et al. A phylogenomic study of birds reveals their evolutionary history. Science. 2008;320:1763–1768. doi: 10.1126/science.1157704. doi:10.1126/science.1157704 [DOI] [PubMed] [Google Scholar]
- Huang S., Ingber D.E. The structural and mechanical complexity of cell-growth control. Nat. Cell Biol. 1999;1:E131–E138. doi: 10.1038/13043. doi:10.1038/13043 [DOI] [PubMed] [Google Scholar]
- Kriegstein A., Noctor S., Martínez-Cerdeño V. Patterns of neural stem and progenitor cell division may underlie evolutionary cortical expansion. Nat. Rev. Neurosci. 2006;7:883–890. doi: 10.1038/nrn2008. doi:10.1038/nrn2008 [DOI] [PubMed] [Google Scholar]
- Martínez-Cerdeño V., Noctor S.C., Kriegstein A.R. The role of intermediate progenitor cells in the evolutionary expansion of the cerebral cortex. Cereb. Cortex. 2006;16(Suppl. 1):i152–i161. doi: 10.1093/cercor/bhk017. doi:10.1093/cercor/bhk017 [DOI] [PubMed] [Google Scholar]
- Molnar Z., Tavare A., Cheung A.F.P. The origin of neocortex: lessons from comparative embryology. In: Striedter G.F., Rubenstein J.L., editors. Evolution of nervous systems. Elsevier; Amsterdam: 2005. pp. 13–26. [Google Scholar]
- Pinto L., et al. Prospective isolation of functionally distinct radial glial subtypes-lineage and transcriptome analysis. Mol. Cell Neurosci. 2008;38:15–42. doi: 10.1016/j.mcn.2008.01.012. doi:10.1016/j.mcn.2008.01.012 [DOI] [PubMed] [Google Scholar]
- Prothero J.W., Sundsten J.W. Folding of the cerebral cortex in mammals. Brain Behav. Evol. 1984;24:152–167. doi: 10.1159/000121313. doi:10.1159/000121313 [DOI] [PubMed] [Google Scholar]
- Smart I. Proliferative characteristics of the ependymal layer during the early development of the spinal cord in the mouse. J. Anat. 1972;111:365. [PMC free article] [PubMed] [Google Scholar]
- Smart I.H.M., Sturrock R.R. Ontogeny of the neostriatum. In: Divac I., Öberg R.G.E., editors. The neostriatum. Oxford University Press; New York, NY: 1979. pp. 127–146. [Google Scholar]
- Smart I.H., Dehay C., Giroud P., Berland M., Kennedy H. Unique morphological features of the proliferative zones and postmitotic compartments of the neural epithelium giving rise to striate and extrastriate cortex in the monkey. Cereb. Cortex. 2002;12:37–53. doi: 10.1093/cercor/12.1.37. doi:10.1093/cercor/12.1.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stingelin W. Helbing & Lichtenhahn; Basel: 1958. Vergleichend morphologische Untersuchungen am Vorderhirn der Vögel ouf cytologischer und cytoarchitektonischer Grundlage. [Google Scholar]
- Striedter G.F. The telencephalon of tetrapods in evolution. Brain Behav. Evol. 1997;49:179–213. doi: 10.1159/000112991. doi:10.1159/000112991 [DOI] [PubMed] [Google Scholar]
- Striedter G.F., Charvet C.J. Developmental origins of species differences in telencephalon and tectum size: morphometric comparisons between a parakeet (Melopsittacus undulatus) and a quail (Colinus virgianus) J. Comp. Neurol. 2008;507:1663–1675. doi: 10.1002/cne.21640. doi:10.1002/cne.21640 [DOI] [PubMed] [Google Scholar]
- Striedter G.F., Keefer B.B. Cell migration and aggregation in the developing telencephalon: pulse-labeling chick embryos with bromodeoxyuridine. J. Neurosci. 2000;20:8021–8030. doi: 10.1523/JNEUROSCI.20-21-08021.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrobel C.N., Mutch C.A., Swaminathan S., Taketo M.M., Chenn A. Persistent expression of stabilized beta-catenin delays maturation of radial glial cells into intermediate progenitors. Dev. Biol. 2007;309:285–297. doi: 10.1016/j.ydbio.2007.07.013. doi:10.1016/j.ydbio.2007.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]