Abstract
Oxidative stress is suggested as a contributor to the ageing process. Knowledge of the relationship between age and energy expenditure may contribute to our understanding of ageing patterns, due to the link between oxygen consumption and free radical production. However, studies on basal metabolic rate (BMR) and age have generally been cross-sectional, which may confound estimates of the age effect due to disproportionate mortality (also known as ‘selective disappearance’). We therefore performed a longitudinal study of BMR using captive zebra finches (Taeniopygia guttata) up to 5 years of age. BMR declined with age in individuals of both sexes when body mass was controlled for. Males gained mass with age while females did not. There was no evidence for disproportionate mortality with respect to BMR in either sex. To our knowledge, this is the first longitudinal study of avian BMR over such a long proportion of the lifespan of the study species.
Keywords: ageing, basal metabolic rate, senescence, zebra finches, Taeniopygia guttata, energy metabolism
1. Introduction
Ageing (or senescence) is a decline in physiological functioning with age accompanied by a decrease in reproductive performance and an increase in mortality rate (Rose 1991). Energy metabolism has been a key issue for understanding the enigma of how we age, ever since the ‘rate of living’ theory was proposed (Pearl 1928). This theory proposed that the rate of ageing and life expectancy were determined by the rate of energy metabolism. This proposition had got a mechanistic explanation by the ‘free radical/oxidative stress’ theory (Harman 1956; Beckman & Ames 1998), which proposes that ageing results from the accumulation of damage caused by reactive oxygen species (ROS) and other free radicals. The free radical/oxidative stress theory receives much support (Beckman & Ames 1998; Finkel & Holbrook 2000). However, the relationship between energy metabolism and ageing is more complex than previously thought (Speakman 2005; Selman et al. 2008), depending, for example, on the relationship between ROS production and energy turnover (e.g. Speakman et al. 2004) and the counteractive effects of antioxidant mechanisms (Beckman & Ames 1998).
Energy metabolism itself may also be subject to age-related changes (Navarro & Boveris 2007), but little is known about this. Basal metabolic rate (BMR) or resting metabolic rate declines with age in humans (Roberts & Rosenberg 2006) as well as in rats (e.g. Even et al. 2001), dogs (Speakman et al. 2003) and some species of birds (Broggi et al. 2007). However, other studies on birds, naked mole rats and mice did not report any age-related decline in BMR (see references in Moe et al. 2007). Most of these studies are cross-sectional, which confounds estimates of within-individual changes when there is disproportionate mortality (also known as ‘selective disappearance’). Disproportionate mortality occurs, for example, when BMR is related to mortality as recently reported for humans (Ruggiero et al. 2008). We therefore performed a longitudinal study of captive zebra finches (Taeniopygia guttata), repeatedly measuring BMR of individual birds up to 5 years of age. Zebra finches can live up to 5 years in the wild (Zann 1996), and the oldest individuals in our captive population are 8 years old (C. Bech, B. Rønning and B. Moe 2008, unpublished data). Thus, the study covered a substantial part of their maximum lifespan. We applied a recently introduced statistical model (van de Pol & Verhulst 2006) to investigate whether BMR changed with age within individuals and whether it was related to mortality (Ruggiero et al. 2008).
2. Material and methods
We studied captive zebra finches T. guttata Vieillot (n=46) hatched in 2001. Housing conditions are described in Rønning et al. (2005), as this study used the same subjects. In September–December 2002 and October–December 2005, the birds were paired and allowed to breed in small cages.
BMR was measured as O2 consumption rate using open flow-through respirometry in metabolic chambers of 1.5 l (flow rate: 400 ml min−1). Measurements were made during the night in the dark at 35°C, within the thermoneutral zone (Calder 1964; see Rønning et al. (2005) for details). BMR was measured when the birds were 1, 3 and 5 years of age, always at least four months after breeding. The BMR measurements at age 1 and 3 are part of the data published in Rønning et al. (2005), but in the present study we only used the first measurement at each age. Hence, the analysis includes only one measurement per individual for each age. Sample sizes declined with age due to mortality, and were 46 (age 1), 37 (age 3) and 25 (age 5) birds.
Data were analysed using mixed linear models using JMP v. 7.0.1 (SAS Institute), following van de Pol & Verhulst (2006). In short, individual bird was included as a random effect, and age was represented by two terms: age and age at last measurement. The latter term estimates the level of disproportionate mortality and was retained in all models to yield an unbiased estimate of the within-individual effects. BMR and body mass were log transformed prior to analyses to ensure linearity.
3. Results
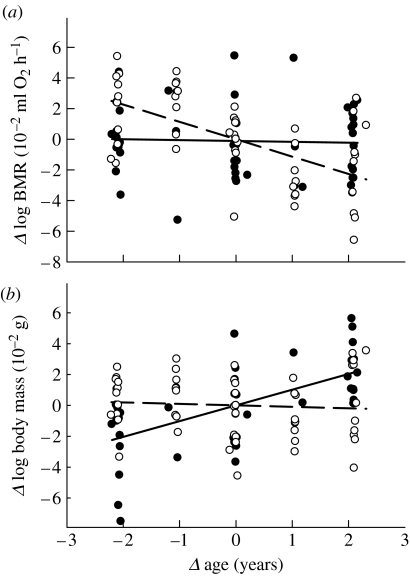
BMR not controlled for body mass decreased with age and was higher in females (table 1a). Age at last measurement was not significant, indicating that there was no disproportionate mortality with respect to BMR. The age effect on BMR differed significantly between the sexes (sex×age interaction added to model in table 1a: F1,60=9.95, p=0.0025; figure 1a). Age and BMR correlated in females (slope: −0.011±0.003; F1,29=17.22, p<0.001), but not in males (slope: −0.00004±0.003; F1,30<0.01, p=0.99), and thus females caused the overall effect when body mass was not controlled for (figure 1a).
Table 1.
Log BMR in relation to age. (a) Sexes combined (R2=0.52), (b) males only (R2=0.60) and (c) females only (R2=0.60). (Sex is a dummy variable (1=male, 2=female), ALM is age at last measurement (in years) and individual is included as random effect.)
| parameter | coefficient (s.e.) | F (d.f.) | p |
|---|---|---|---|
| (a) sexes combined | |||
| constant | 1.563 (0.022) | ||
| sex | 0.031 (0.010) | 9.45 (1, 61) | <0.004 |
| age | −0.005 (0.002) | 7.26 (1, 61) | <0.01 |
| ALM | −0.0004 (0.004) | 0.02 (1, 61) | 0.9 |
| (b) males | |||
| constant | 0.917 (0.094) | ||
| log mass | 0.583 (0.082) | 51.3 (1, 29) | <0.001 |
| age | −0.006 (0.023) | 6.95 (1, 29) | 0.01 |
| ALM | 0.001 (0.003) | 0.05 (1, 29) | 0.8 |
| (c) females | |||
| constant | 0.994 (0.121) | ||
| log mass | 0.563 (0.105) | 28.9 (1, 28) | <0.001 |
| age | −0.011 (0.003) | 15.4 (1, 28) | <0.001 |
| ALM | −0.003 (0.004) | 0.53 (1, 28) | 0.9 |
Figure 1.
Deviations (Δ) in (a) whole animal (log) BMR (ml O2 h−1) and (b) (log) body mass (g) as a function of deviations (Δ) in age (years). Deviations are calculated from each individual's mean (observed value minus the mean value). Males, filled symbols and solid regression lines; females, open symbols and broken regression lines.
Changes in metabolic rate can be due to changes in body mass. Male mass increased with age (F1,30=19.4, p<0.001; figure 1b), and when controlling for the effect of mass, male BMR declined significantly with age (table 1b). The age effect on female BMR was independent of mass (table 1c), since female mass was independent of age (F1,29=0.15, p=0.7; figure 1b). In neither sex was there a significant interaction between age and mass (p>0.25) with respect to BMR.
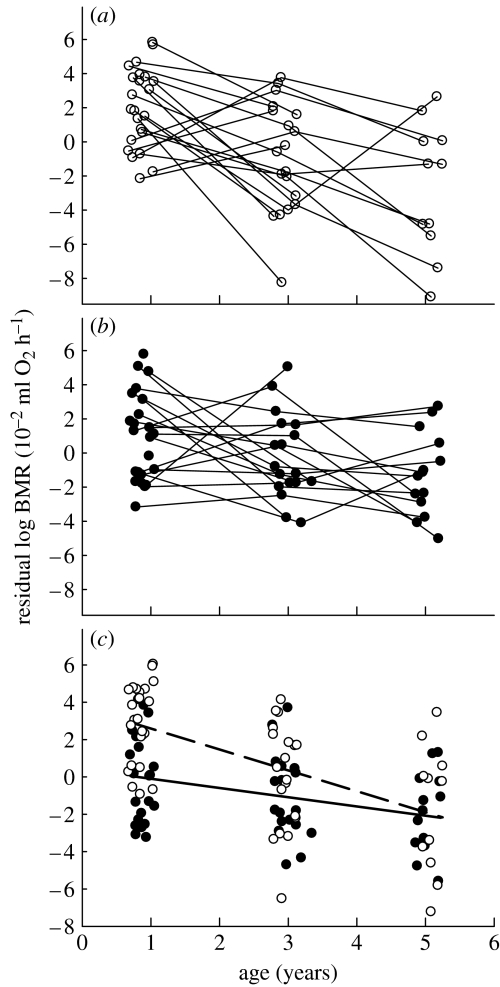
We pooled the data of the two sexes to verify whether the age effect differed between the sexes when mass was controlled for. When added to a model containing mass, sex and age, the interaction between age and sex did not quite reach significance (F1,59=3.19, p<0.08; figure 2c). When omitting the interaction, the effect of sex remained significant, with BMR being on average 0.027 (s.e.=0.006) log units higher in females (F1,60=18.2, p<0.001).
Figure 2.
Residual (log) BMR (ml O2 h−1) of zebra finches as a function of age (years). (a) Females, (b) males and (c) both sexes. (a,b) Lines connect subsequent measurements of individuals. (c) Males, filled symbols and solid line; females, open symbols and dashed line. Residuals were extracted from mixed linear models including (log) body mass and sex in (a,b), and only (log) body mass in (c).
4. Discussion
When studying age effects, longitudinal data make it possible to avoid biases caused by disproportionate mortality. Longitudinal studies of BMR over multiple years have been carried out in humans (e.g. Keys et al. 1973; Ruggiero et al. 2008) and rhesus monkeys (Raman et al. 2007). Using longitudinal data covering a large proportion of the animals' lives, we show for the first time that BMR decreased with age in individual birds. BMR declined with age in both sexes, but in males this was masked by an increase in body mass with age. There was no evidence for disproportionate mortality of individuals with high or low BMR. This contrasts to a recent study, which shows that high BMR in humans is related to higher mortality (Ruggiero et al. 2008). However, not all birds have died yet in our study, and it cannot be ruled out that there is an effect that we could not yet detect with the available sample size.
Metabolic ageing, an age-related decline in the ability of an organism to sustain a homeostatic metabolic rate, has now been demonstrated in several species, but it is not yet clear why or how metabolic ageing occurs. On the mechanistic level, it is suggested that BMR declines with age because the mass of metabolically active tissue as well as their mass-specific metabolic activity declines with age (e.g. Even et al. 2001; Roberts & Rosenberg 2006). The latter is also supported by the finding that tissue of aged rats shows a decreased capacity to produce ATP by oxidative phosphorylation (Navarro & Boveris 2007). The present study highlights the benefits of using longitudinal data, but we acknowledge that cross-sectional studies with invasive tissue sampling are still needed for the mechanistic understanding of energy metabolism and the ageing process. On another level, metabolic ageing may be an adjustment to reduced ability to withstand oxidative stress. Old individuals could theoretically benefit from lowering ROS production by lowering their BMR. However, ROS production can be modulated independent of energy metabolism (Barja 2007), so it is highly questionable whether low BMR is a strategy for low ROS production in old individuals.
Many bird species are long lived for their body sizes and are assumed to be relatively resistant to oxidative stress (Holmes & Ottinger 2003; Moe et al. 2007). The zebra finch, however, has a fast life history with short generation time and lifespan. The metabolic ageing we observed contrasts to the lack of age effect on BMR in long-lived snow petrels (Moe et al. 2007). The latter study was cross-sectional and that could potentially explain some of the difference. However, we think that the contrasted results are in line with the idea that the rate of ageing correlates with the ranking on the fast–slow life-history continuum (Jones et al. 2008). It is also in line with the ‘disposable soma theory’. Short-lived species with a fast life history and high extrinsic mortality (e.g. from predation, contagious disease, starvation, weather-related stress) are expected to invest less in mechanisms for somatic maintenance and repair, compared with long-lived species (Kirkwood & Rose 1991; Moe et al. 2007). The ageing process is therefore expected to be faster in these species. In this perspective, the BMR–age relationship may reflect the degree of investment in somatic maintenance and repair (Speakman et al. 2003; Moe et al. 2007). The comparative evolutionary explanations for metabolic ageing, as well as the mechanistic explanations, clearly deserve further study.
Acknowledgments
The laboratory animal unit is approved by the National Animal Research Authority and the methods in this study are in compliance with the Norwegian Regulations on Animal Experimentation.
We thank O. A. Indset and B. Simensen for their assistance, the Norwegian Science Research Council for financial support (nos. 138698/410 and 159584/V40), NTNU for a grant to B.M. and NWO for a Vici grant to S.V.
References
- Barja G. Mitochondrial oxygen consumption and reactive oxygen species production are independently modulated: implications for aging studies. Rejuv. Res. 2007;10:215–223. doi: 10.1089/rej.2006.0516. doi:10.1089/rej.2006.0516 [DOI] [PubMed] [Google Scholar]
- Beckman K.B., Ames B.N. The free radical theory of aging matures. Physiol. Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- Broggi J., Hohtola E., Koivula K., Orell M., Thomson R.L., Nilsson J.-Å. Sources of variation in winter metabolic rate in the great tit Parus major. Funct. Ecol. 2007;21:528–533. doi:10.1111/j.1365-2435.2007.01255.x [Google Scholar]
- Calder W.A. Gaseous metabolism and water relations of the zebra finch, Taeniopygia castanotis. Physiol. Zool. 1964;37:400–413. [Google Scholar]
- Even P.C., Rolland V., Roseau S., Bouthegourd J.-C., Tomé D. Prediction of basal metabolism from organ size in the rat: relationship to strain, feeding, age, and obesity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;280:R1887–R1896. doi: 10.1152/ajpregu.2001.280.6.R1887. [DOI] [PubMed] [Google Scholar]
- Finkel T., Holbrook N.J. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. doi:10.1038/35041687 [DOI] [PubMed] [Google Scholar]
- Harman D. Aging: a theory based on free radical and radiation biology. J. Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Holmes D.J., Ottinger M.A. Birds as long-lived models for the study of aging. Exp. Gerontol. 2003;38:1365–1375. doi: 10.1016/j.exger.2003.10.018. doi:10.1016/j.exger.2003.10.018 [DOI] [PubMed] [Google Scholar]
- Jones O.R., et al. Senescence rates are determined by the ranking on the fast–slow life-history continuum. Ecol. Lett. 2008;11:664–673. doi: 10.1111/j.1461-0248.2008.01187.x. doi:10.1111/j.1461-0248.2008.01187.x [DOI] [PubMed] [Google Scholar]
- Keys A., Taylor H.L., Grande F. Basal metabolism and age of adult man. Metabolism. 1973;22:579–587. doi: 10.1016/0026-0495(73)90071-1. doi:10.1016/0026-0495(73)90071-1 [DOI] [PubMed] [Google Scholar]
- Kirkwood T.B.L., Rose M.R. Evolution of senescence–late survival sacrificed for reproduction. Phil. Trans. R. Soc. B. 1991;332:15–24. doi: 10.1098/rstb.1991.0028. doi:10.1098/rstb.1991.0028 [DOI] [PubMed] [Google Scholar]
- Moe B., Angelier F., Bech C., Chastel O. Is basal metabolic rate influenced by age in a long-lived seabird, the snow petrel? J. Exp. Biol. 2007;210:3407–3414. doi: 10.1242/jeb.005090. doi:10.1242/jeb.005090 [DOI] [PubMed] [Google Scholar]
- Navarro A., Boveris A. The mitochondrial energy transduction system and the aging process. Am. J. Physiol. Cell Physiol. 2007;292:C670–C686. doi: 10.1152/ajpcell.00213.2006. doi:10.1152/ajpcell.00213.2006 [DOI] [PubMed] [Google Scholar]
- Pearl R. University of London Press; London, UK: 1928. The rate of living. [Google Scholar]
- Raman A., Ramsey J.J., Kemnitz J.W., Baum S.T., Newton W., Colman R.J., Weindruch R., Beasley M.T., Schoeller D.A. Influences of calorie restriction and age on energy expenditure in the rhesus monkey. Am. J. Physiol. Endocrinol. Metab. 2008;292:E101–E106. doi: 10.1152/ajpendo.00127.2006. doi:10.1152/ajpendo.00127.2006 [DOI] [PubMed] [Google Scholar]
- Roberts S.B., Rosenberg I. Nutrition and aging: changes in the regulation of energy metabolism with aging. Physiol. Rev. 2006;86:651–667. doi: 10.1152/physrev.00019.2005. doi:10.1152/physrev.00019.2005 [DOI] [PubMed] [Google Scholar]
- Rønning B., Moe B., Bech C. Long-term repeatability makes basal metabolic rate a likely heritable trait in the zebra finch Taeniopygia guttata. J. Exp. Biol. 2005;208:4663–4669. doi: 10.1242/jeb.01941. doi:10.1242/jeb.01941 [DOI] [PubMed] [Google Scholar]
- Rose M.R. Oxford University Press; New York, NY: 1991. Evolutionary biology of aging. [Google Scholar]
- Ruggiero C., Metter J.E., Melenovsky V., Cherubini A., Najjar S.S., Ble A., Senin U., Longo D.L., Ferrucci L. High basal metabolic rate is a risk factor for mortality: the Baltimore longitudinal study of aging. J. Gerontol. A. 2008;63:698–706. doi: 10.1093/gerona/63.7.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selman C., McLaren J.S., Collins A.R., Duthie G.G., Speakman J.R. The impact of experimentally elevated energy expenditure on oxidative stress and lifespan in the short-tailed field vole Microtus agrestis. Proc. R. Soc. B. 2008;275:1907–1916. doi: 10.1098/rspb.2008.0355. doi:10.1098/rspb.2008.0355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speakman J.R. Body size, energy metabolism and lifespan. J. Exp. Biol. 2005;208:1717–1730. doi: 10.1242/jeb.01556. doi:10.1242/jeb.01556 [DOI] [PubMed] [Google Scholar]
- Speakman J.R., van Acker A., Harper E.J. Age-related changes in the metabolism and body composition of three dog breeds and their relationship to life expectancy. Aging Cell. 2003;2:265–275. doi: 10.1046/j.1474-9728.2003.00061.x. doi:10.1046/j.1474-9728.2003.00061.x [DOI] [PubMed] [Google Scholar]
- Speakman J.R., et al. Uncoupled and surviving: individual mice with high metabolism have greater mitochondrial uncoupling and live longer. Aging Cell. 2004;3:87–95. doi: 10.1111/j.1474-9728.2004.00097.x. doi:10.1111/j.1474-9728.2004.00097.x [DOI] [PubMed] [Google Scholar]
- van de Pol M., Verhulst S. Age-dependent traits: a new statistical model to separate within- and between-individual effects. Am. Nat. 2006;167:766–773. doi: 10.1086/503331. doi:10.1086/503331 [DOI] [PubMed] [Google Scholar]
- Zann R.A. Oxford University Press; Oxford, UK: 1996. The zebra finch: a synthesis of field and laboratory studies. [Google Scholar]