Abstract
Individuals in groups are often thought to scan their surroundings for threats independently of one another. Models, however, suggest that foragers should monitor the vigilance level of their neighbours to prevent cheating, and to gather information about incipient predation risk. Evidence for monitoring of vigilance is scant. Here, I examined changes in vigilance levels in sleeping gulls (Larus sp.) surrounded by neighbours in various states of alertness. Controlling for group size and neighbour density, gulls interrupted sleep more often to scan their surroundings, and were therefore more vigilant, when their neighbours were alert rather than sleeping or preening. The results provide evidence for copying of vigilance within groups of birds, suggesting a complex flow of information about predation risk in groups.
Keywords: anti-predator vigilance, group size, gulls, Larus sp., sleep, visual monitoring
1. Introduction
Animals in groups often interrupt their foraging activities to scan their surroundings. Such vigilance may be aimed at detecting predators or to monitor the behaviour of their neighbours (Krause & Ruxton 2002). Anti-predator vigilance usually decreases with group size, as groups provide more opportunities to detect predators and dilute predation risk for each individual (Elgar 1989).
While early models of anti-predator vigilance assumed that individuals scan independently of one another (Pulliam 1973), recent models imply that individuals in a group monitor the vigilance behaviour of their neighbours. Such monitoring may be essential to ensure that others do their fair share of vigilance (Lima 1995), to increase feeding when others are more vigilant (Bahr & Bekoff 1999), or to copy the vigilance state of neighbours (Desportes et al. 1991; Sirot 2006). Copying vigilance could be advantageous for a group member to assess threats detected by vigilant neighbours sooner, or to avoid being left behind when companions flee.
Despite its key role, empirical evidence for monitoring the vigilance of neighbours is scant. In one study, neighbours failed to change their own vigilance level in response to an experimental reduction in the vigilance of one group member (Lima 1995). In another study, the presence of a barrier preventing individuals from seeing each other also failed to alter individual vigilance levels, despite the fact that monitoring of vigilance was prevented (Beauchamp 2002). Evidence for waves of collective vigilance (Lazarus 1979; Fernàndez-Juricic et al. 2004; Pays et al. 2007a,b), whereby many members in a group are vigilant at the same time, offers indirect support for the monitoring hypothesis. However, systematic changes in vigilance levels within a group may be a response to external stimuli to which many group members respond simultaneously, or indicative of systematic changes in fear perception or feeding motivation.
Here, I provide more direct evidence for monitoring of vigilance in sleeping gulls (Larus sp.). Gulls loaf in groups in which some individuals are alert, while others preen or sleep. While sleeping, gulls, as other animals, open their eyes to peek at their surroundings (Lendrem 1983; Gauthier-Clerc et al. 1998). I examined whether vigilance in sleeping gulls, as measured by peeking behaviour, varied as a function of the state of alertness of their nearest neighbours, which would be expected if monitoring of vigilance takes place between close neighbours. State of alertness was supposed to be the lowest when individuals are sleeping or preening, because these two activities impair visual scanning. Focusing on the nearest neighbours also makes sense, because the behavioural effects caused by companions have been shown to decrease with inter-individual distances (Blumstein et al. 2001; Fernandez-Juricic et al. 2007).
2. Material and methods
Observations were carried out from late July to early August in 2007 and 2008 in the Shepody area of the upper Bay of Fundy, New Brunswick, Canada (45.73° N, 64.65° W). Ring-billed (Larus delawarensis) and herring gulls (Larus argentatus), and occasionally greater black-backed gulls (Larus marinus), forage at low tide in the mudflats and gather at high tide on nearby beaches and agricultural fields.
Groups of gulls were located opportunistically at known loafing sites during the few hours preceding and following high tides. Gulls do not forage at loafing sites and spend their time in one of three main activities: (i) alert, in which individuals stand on their two feet and scan their surroundings, (ii) preening, and (iii) sleeping. Sleeping occurred while birds stood on one foot or in a crouched position but most often with bill tucked under scapulars. In these relaxed positions, gulls alternate between periods with eyes open and closed.
When a group was located, I counted the number of gulls present and gathered behavioural data on the available sleeping gulls. I aimed to watch a focal bird for two to three minutes, unless the bird woke up or group size changed. During each focal observation, I dictated on a portable tape recorder the sequence of eye openings and closings as events unfolded. At the beginning of the focal observation, I noted the distance of the two nearest neighbours in gull length units and the behavioural state of each of these two neighbours.
From the taped recordings, I obtained the percentage time spent sleeping for each focal observation, defined at the total duration of all sequences with eyes closed and divided by the total duration of the focal observation. I used a mixed linear model to analyse variation in percentage time spent sleeping including group identity as a random factor to account for a possible correlation in sleeping time between individuals of the same group (Littell et al. 1996). Independent variables included centred group size, squared centred group size to model nonlinear trends in the data, the average of the two nearest-neighbour distances, age of the focal species (classified as adult versus immature based on plumage coloration) and focal species identity. I also included the behavioural state of the two nearest neighbours as a categorical variable (alert, when the two nearest neighbours were alert; preen, when the two nearest neighbours were preening; sleep, when the two nearest neighbours were sleeping; and mixed, when the two nearest neighbours showed different behavioural states).
3. Results
The dataset consisted of 80 focal observations in 22 different groups ranging in size from 3 to 104. The two nearest neighbours of focal sleeping gulls also slept in 10 focal observations (12.5%). The two nearest neighbours were not generally sleeping and included at least one alert gull in 56 per cent of the observations. Observations were never interrupted by external causes and alert gulls were simply scanning their surroundings.
In the mixed linear model, age and focal species identity were not significant and dropped from further analyses (p>0.30). Group identity accounted for 12 per cent of the total variance in percentage time spent sleeping, which indicates a weak correlation in percentage time spent sleeping among birds of the same group.
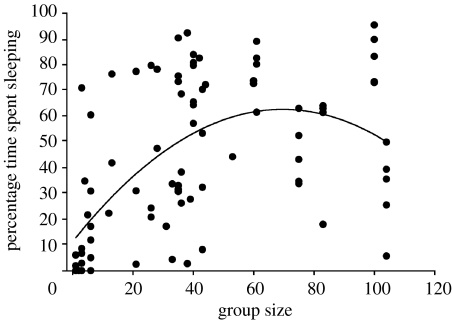
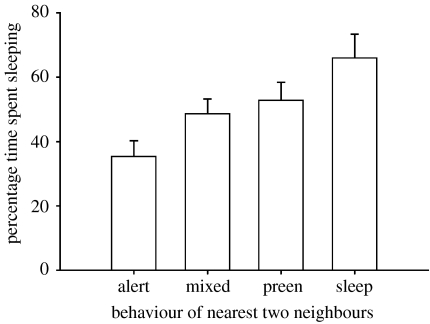
Percentage time spent sleeping increased with group size (β(s.e.)=0.39 (0.12), F1,50=10.3, p=0.002). However, a nonlinear trend was apparent and percentage time spent sleeping actually decreased in the larger groups (figure 1; β(s.e.)=−0.009 (0.003), F1,50=8.0, p=0.007). Percentage time spent sleeping decreased with increasing average neighbour distance (β(s.e.)=−1.47 (0.53), F1,50=7.7, p=0.008). The behavioural state of the two nearest neighbour influenced percentage time spent sleeping in focal gulls (figure 2; F3,50=4.8, p=0.005). Post hoc tests, with the Bonferroni sequential correction, indicated that mean percentage time spent sleeping in focal gulls was significantly lower when the two nearest neighbours were alert rather than either preening or sleeping, and marginally lower (p=0.04 before correction) than when the two neighbours showed non-synchronized behavioural states.
Figure 1.
Changes in the percentage time spent sleeping by focal gulls (Larus sp.) as a function of group size. The simple polynomial regression line is drawn to illustrate the nonlinear relationship between sleeping and group size.
Figure 2.
Changes in least square means of the percentage time spent sleeping by focal gulls (Larus sp.) as a function of the behaviour of the two nearest neighbours. The mixed category consists of cases where the two nearest neighbours showed different behavioural states. Sample sizes are indicated below each bar. Standard error bars are shown.
In loafing groups, not all gulls synchronized their activities, offering an opportunity to compare the behaviour of focal gulls within the same flock when their neighbours were alert to different extents. In groups with more than two focal observations, I matched a focal observation where one or two neighbours were alert to a focal observation where neither neighbours were alert. I then compared percentage time spent sleeping in these matched observations using a paired t-test. Results indicated that focal gulls with more alert neighbours spent on average 22 per cent (s.d.=30%, n=17) less time sleeping than focal gulls from the same flocks with less alert neighbours (t=3.0, d.f.=16, p=0.009).
4. Discussion
Results provide support for monitoring of vigilance in loafing groups of gulls. Gulls with more alert neighbours sleep less than those with less alert neighbours that are either sleeping or preening. The evidence is compelling because all focal gulls are engaged in the same activity and adjusted their vigilance levels according to the state of alertness of their neighbours controlling for group size and neighbour density. Other factors that are known to influence vigilance such as age, food density or food competition (Elgar 1989) did not play a role here, or are simply irrelevant since birds are sleeping. In addition, the results are not simply an indication that members of a group are either all alert or all sleeping, since it was very common to find sleeping gulls surrounded by individuals in various states of alertness. Within the same group, and therefore within minutes of one another, observations also revealed that focal gulls with more alert neighbours sleep less than those nearby with less alert neighbours. Finally, during my observations, alert gulls were simply monitoring their surroundings, a component of vigilance referred to as routine vigilance (Blanchard & Fritz 2007) ensuring that sleeping gulls were not simply responding to external cues detected by alert companions.
Results provide support for a positive correlation between the vigilance levels of the nearest neighbours, as opposed to the negative correlation predicted by Bahr & Bekoff (1999). Instances of matched vigilance among close neighbours are predicted by a model which assumes that information about predation threats is gleaned from the vigilance levels of neighbours (Sirot 2006). Waves of collective vigilance that have been observed in other studies as mentioned earlier could be explained by the pattern of vigilance matching described here.
While an increase in sleeping time with group size has been documented in other species (Lendrem 1984; Gauthier-Clerc et al. 1998), the decrease in percentage time spent sleeping in larger groups was unexpected and may indicate that disturbances caused by predators or neighbours are more common in these groups (Boukhriss et al. 2007). The decrease in sleeping time when neighbours are more distant is also consistent with the finding that nearby group members have more influence on feeding and vigilance (Blumstein et al. 2001; Fernandez-Juricic et al. 2007). Since focal gulls with distant neighbours often occurred at the edges of groups, the results are also consistent with the oft-repeated observation that individuals at the edges of groups are more vigilant (Hirsch 2007).
While the results indicate that monitoring of vigilance takes places, it is not clear how finely tuned such monitoring of vigilance can be. This could be assessed in future work by comparing the level of sleep in gulls surrounded by companions with varying levels of peeking frequency.
The results indicate that, at least in loafing gulls, vigilance is not independent among group members. Recent work in many fields indicates that choices made by others have a large impact on foraging and reproductive tactics (Danchin et al. 2004). It is perhaps time to consider that vigilance in groups is also a process where the behaviour of group members is a source of information about the environment.
References
- Bahr D.B., Bekoff M. Predicting flock vigilance from simple passerine interactions: modelling with cellular automata. Anim. Behav. 1999;58:831–839. doi: 10.1006/anbe.1999.1227. doi:10.1006/anbe.1999.1227 [DOI] [PubMed] [Google Scholar]
- Beauchamp G. Little evidence for visual monitoring of vigilance in zebra finches. Can. J. Zool. 2002;80:1634–1637. doi:10.1139/z02-156 [Google Scholar]
- Blanchard P., Fritz H. Induced or routine vigilance while foraging. Oikos. 2007;116:1603–1608. doi:10.1111/j.0030-1299.2007.15799.x [Google Scholar]
- Blumstein D.T., Daniel J.C., Evans C.S. Yellow-footed rock-wallaby group size effects reflect a trade-off. Ethology. 2001;107:655–664. doi:10.1046/j.1439-0310.2001.00699.x [Google Scholar]
- Boukhriss J., Selmi S., Nouira S. Time allocation and vigilance behaviour of greater flamingos (Phoenicopterus roseus) wintering in the Gulf of Gabes, Tunisia. Ostrich. 2007;78:459–461. doi:10.2989/OSTRICH.2007.78.2.54.134 [Google Scholar]
- Danchin E., Giraldeau L.A., Valone T.J., Wagner R.H. Public information: from nosy neighbors to cultural evolution. Science. 2004;305:487–491. doi: 10.1126/science.1098254. doi:10.1126/science.1098254 [DOI] [PubMed] [Google Scholar]
- Desportes J.-P., Cézilly F., Gallo A. Modelling and analysing vigilance behaviour. Acta Oecol. 1991;12:227–236. [Google Scholar]
- Elgar M.A. Predator vigilance and group size in mammals and birds. Biol. Rev. 1989;64:13–33. doi: 10.1111/j.1469-185x.1989.tb00636.x. doi:10.1111/j.1469-185X.1989.tb00636.x [DOI] [PubMed] [Google Scholar]
- Fernàndez-Juricic E., Siller S., Kacelnik A. Flock density, social foraging, and scanning: an experiment with starlings. Behav. Ecol. 2004;15:371–379. doi:10.1093/beheco/arh017 [Google Scholar]
- Fernàndez-Juricic E., Beauchamp G., Bastain B. Group-size and distance-to-neighbour effects on feeding and vigilance in brown-headed cowbirds. Anim. Behav. 2007;73:771–778. doi:10.1016/j.anbehav.2006.09.014 [Google Scholar]
- Gauthier-Clerc M., Tamisier A., Cézilly F. Sleep-vigilance trade-off in green-winged teals (Anas crecca crecca) Can. J. Zool. 1998;76:2214–2218. doi:10.1139/cjz-76-12-2214 [Google Scholar]
- Hirsch B.T. Costs and benefits of within-group spatial position: a feeding competition model. Q. Rev. Biol. 2007;82:9–27. doi: 10.1086/511657. doi:10.1086/511657 [DOI] [PubMed] [Google Scholar]
- Krause J., Ruxton G.D. Oxford University Press; Oxford, UK: 2002. Living in groups. [Google Scholar]
- Lazarus J. The early warning function of flocking in birds: an experimental study with captive quelea. Anim. Behav. 1979;27:855–865. doi:10.1016/0003-3472(79)90023-X [Google Scholar]
- Lendrem D.W. Sleeping and vigilance in birds. I. Field observations of the mallard (Anas platyrhynchos) Anim. Behav. 1983;31:532–538. doi:10.1016/S0003-3472(83)80076-1 [Google Scholar]
- Lendrem D.W. Sleeping and vigilance in birds. II. An experimental study of the Barbary dove (Streptopelia risoria) Anim. Behav. 1984;32:243–248. doi:10.1016/S0003-3472(84)80343-7 [Google Scholar]
- Lima S.L. Back to the basics of anti-predatory vigilance: the group-size effect. Anim. Behav. 1995;49:11–20. doi:10.1016/0003-3472(95)80149-9 [Google Scholar]
- Littell R.M., Milliken G.A., Stroup W.W., Wolfinger R.D. SAS Institute, Inc; Cary, NC: 1996. SAS system for mixed models. [Google Scholar]
- Pays O., Jarman P.J., Loisel P., Gerard J.-F. Coordination, independence or synchronization of individual vigilance in the eastern grey kangaroo? Anim. Behav. 2007a;73:595–604. doi:10.1016/j.anbehav.2006.06.007 [Google Scholar]
- Pays O., Renaud P., Loisel P., Petit M., Gerard J., Jarman P. Prey synchronize their vigilant behaviour with other group members. Proc. R. Soc. B. 2007b;274:1287–1291. doi: 10.1098/rspb.2006.0204. doi:10.1098/rspb.2006.0204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulliam H.R. On the advantages of flocking. J. Theor. Biol. 1973;38:419–422. doi: 10.1016/0022-5193(73)90184-7. doi:10.1016/0022-5193(73)90184-7 [DOI] [PubMed] [Google Scholar]
- Sirot E. Social information, antipredatory vigilance and flight in bird flocks. Anim. Behav. 2006;72:373–382. doi:10.1016/j.anbehav.2005.10.028 [Google Scholar]