Abstract
Recent research has documented a variety of ovulatory cues in humans, and in many nonhuman species, the vocal channel provides cues of reproductive state. We collected two sets of vocal samples from 69 normally ovulating women: one set during the follicular (high-fertility) phase of the cycle and one set during the luteal (low-fertility) phase, with ovulation confirmed by luteinizing hormone tests. In these samples we measured fundamental frequency (pitch), formant dispersion, jitter, shimmer, harmonics-to-noise ratio and speech rate. When speaking a simple introductory sentence, women's pitch increased during high- as compared with low-fertility, and this difference was the greatest for women whose voices were recorded on the two highest fertility days within the fertile window (the 2 days just before ovulation). This pattern did not occur when the same women produced vowels. The high- versus low-fertility difference in pitch was associated with the approach of ovulation and not menstrual onset, thus representing, to our knowledge, the first research to show a specific cyclic fertility cue in the human voice. We interpret this finding as evidence of a fertility-related enhancement of femininity consistent with other research documenting attractiveness-related changes associated with ovulation.
Keywords: menstrual cycle, ovulation, vocal cues, sexual communication
1. Introduction
Recent research has documented several detectable ovulatory cues in humans, including midcycle increases in body scent attractiveness, flirtation, and attention to style of dress (see Haselton et al. 2007). Mated women report that their male partners are more attentive and jealous near ovulation as compared with other cycle phases (Gangestad et al. 2002), and one study showed that exotic dancers earned the most tips when nearest to ovulation (Miller et al. 2007). These results call into question the traditional assumption that humans differ from many other primates in the lack of any obvious cues of fertility. Clearly, there exist many possible channels for detection of ovulation beyond those described thus far.
Most documented cues of reproductive state in mammals are either visual or olfactory (Dixson 1998), but recent studies have shown that vocalizations reveal fertility in dairy cattle (Schon et al. 2007), elephants (Leong et al. 2003) and two primate species (Semple et al. 2002). Several studies have shown vocal changes associated with the human menstrual cycle, typically in connection with the use of contraceptive pills or vocal hoarseness associated with menstruation (e.g. Higgins & Saxman 1989; Abitbol et al. 1999; Amir & Biron-Shental 2003; Amir & Kishon-Rabin 2004; Whiteside et al. 2004). In summary, existing acoustical research suggests that circulating hormones affect vocal characteristics, but no study has yet documented changes across the human cycle that could be cues of the approach of ovulation, rather than differences precipitated by menstrual onset alone. A recent study showed that women's voices were judged more attractive on mid-cycle days as compared with other days (Pipitone & Gallup 2008). This study included just 17 naturally cycling women, did not hormonally confirm ovulation, and did not perform acoustical analyses. Thus, it is suggestive of an ovulatory cue, although it did not identify one.
Known ovulatory cues are tied to femininity and female attractiveness (Haselton et al. 2007); thus we predicted that sexually dimorphic vocal cues associated with female attractiveness would shift with fertility across the cycle. Fundamental frequency (F0), the acoustic correlate of perceived pitch, is sexually dimorphic due to differential vocal fold development during puberty. Formant dispersion (Df) is the averaged distance between adjacent resonating frequencies (formants) and is also sexually dimorphic. We expected that both vocal characteristics would shift towards more feminine values on fertile days of the cycle, although we considered a change in Df less likely given the relatively greater constraints on manipulating this vocal dimension. We also examined changes in other speech parameters across the cycle, including those investigated previously.
2. Material and methods
We followed existing cycle-tracking methods (Gangestad et al. 2002; Haselton et al. 2007). Subjects were 69 women from the University of California, Los Angeles (UCLA) campus (mean age=20.30; range=18–39). All the women reported regular menstrual cycles between 21 and 33 days in length and none were using hormonal contraceptives.
After initial screening, the women were scheduled for their next possible session (low- or high-fertility) given their current cycle day. Low-fertility sessions were scheduled 4–10 days prior to the next estimated menstrual onset. On average, low-fertility sessions took place 6.36 days prior to actual menstrual onset (s.d.=3.08). High-fertility sessions were scheduled 15–17 days prior to the next estimated menstrual onset. Using an unmarked urine test (Clearblue), all the women were judged to have a luteinizing hormone (LH) surge between 3 days after and 2 days before their high-fertility session. An LH surge typically precedes ovulation by 24–48 hours (Lynch et al. 2006); thus all the women were near onset of ovulation during their high-fertility session. Within this window, conception probability peaks just before ovulation (Lynch et al. 2006). Therefore, we estimated days-to-ovulation by adding 2 to days-to-LH surge (mean=1.49, s.d.=1.48) and included this in analyses.
These 69 women were a subset of 114 recruits. Ineligible women showed no LH surge (n=15), completed low-fertility sessions outside of the luteal phase (as confirmed by next menstrual onset, n=4), were scheduled incorrectly (n=2), missed their menstrual onset date by more than 30 days (n=2) or failed to complete all sessions (n=20). Vocal recordings were not obtained for two women due to experimenter error. At the end of the study, the women completed biographical questionnaires. Of women completing the entire study (n=90), women included in analyses and ineligible women did not differ across pertinent biographical variables, including age, relationship status and self-rated attractiveness (p>0.20). Retention rates in the study are comparable to previous work using similar methods (e.g. Gangestad et al. 2002).
Voices were recorded on a digital (16 bit, 44.1 kHz) recorder (Marantz PMD-660 or M-Audio MicroTrack 24/96) with a cardioid condenser microphone (AKG C535 EB) in a quiet room 15–20 cm from the microphone. The women were instructed before recording what speech to produce exactly. Corrections were requested until the participants produced utterances adequately. Female experimenters ran all sessions. Target speech was extracted using Cool Edit Pro software (v. 2.1); sound files were re-sampled to 11.025 kHz with a low pass anti-aliasing filter.
Participants produced (i) the sentence, ‘Hi, I'm a student at UCLA,’ (ii) five monopthong vowels (‘eh’ as in bet, ‘ee’ as in beet, ‘ah’ as in bought, ‘oh’ as in boat and ‘oo’ as in boot and (iii) the prolonged (∼5 s) monopthong vowel ‘ah’.
Samples were analysed using Praat, v. 4.6.03 (www.praat.org). F0 was measured using Praat's autocorrelation algorithm with a search setting of 100–600 Hz. In the sentence, mean F0, F0 s.d. and speech rate (mean syllabic duration) were measured across the entire utterance. For the five monopthongs, F0 and Df were calculated. Df was calculated as the following: (F4−F3)+(F3−F2)+(F2−F1)/3 with calculations of all formants for each vowel, and values averaged for the final Df value. Overall F0 was calculated by averaging F0 values across the five vowels. For the single monopthong vowel, frequency and amplitude perturbation were measured by averaging three jitter (local, relative average perturbation, and 5-point period perturbation quotient) and three shimmer (local, 5-point perturbation quotient and 11-point perturbation quotient) values. Harmonics-to-noise ratio (HNR) was calculated. HNR indexes the degree of acoustic periodicity in dB as the periodic energy divided by total energy. F0 was measured on the single vowel.
3. Results
Statistical analyses were run using SPSS-PC 15.0 generalized linear model repeated measures. Fertility (high versus low session) was a repeated factor, order (high- versus low-fertility session first) was a between-groups factor, and days-to-ovulation in the high-fertility session and days-to-menstrual onset in the low-fertility session were covariates. Inclusion of the covariates allowed us to assess whether high- versus low-fertility differences were driven by proximity to ovulation in the high-fertility session or proximity to menstrual onset in the low-fertility session. This analysis was repeated across all vocal measures.
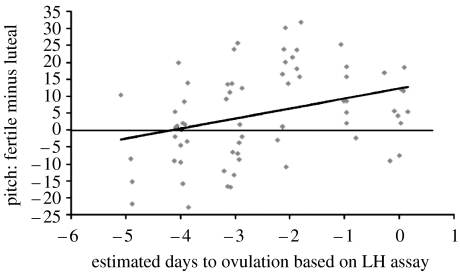
For the spoken sentence only, high-fertility recordings were significantly higher in pitch than low-fertility recordings, F1,65=5.63, p=0.02. This effect was moderated by days-to-ovulation, F1,65=7.95, p=0.006, indicating that differences between low- and high-fertility recordings were greater for women whose high-fertility sessions fell closer to the day of ovulation (rpartial=0.330, see figure 1). The highest probability of conception is on the 2 days preceding ovulation (which include the day of LH surge and the following day); thus, if pitch tracks fertility, we expected it to change most dramatically on these 2 days. As expected, estimated marginal means of the pitch increase on these days was 15.6 Hz (day-of-LH-surge) and 10.0 Hz (the next day). There were no significant effects of order or days-to-menstrual-onset.
Figure 1.
Correlation of high- versus low-fertility differences in voice pitch and proximity to ovulation in the high-fertility recording session, rpartial=0.33, p=0.006, controlling for proximity to menstrual onset in the low-fertility session and session order. This plot shows that fertile phase increases in pitch were greater for women whose voices were recorded closer to the day of ovulation within the fertile window.
With two exceptions, there were no main effects or interactions involving fertility for any other vocal parameters. Table 1 presents means of all vocal measurements in low- and high-fertility sessions. The exceptions were mundane fertility-by-order interactions involving duration, F1,65=4.07, p=0.048, and jitter, F1,65=5.44, p=0.023. In each, the pattern showed that both high- and low-fertility recordings taken during first sessions were characterized by larger values than recordings taken during second sessions.
Table 1.
Means of all vocal measurements in low- and high-fertility sessions. (s.d. in parentheses; analyses controlled for days-to-menstrual-onset, estimated days-to-ovulation, and session order. *p=0.02.)
| sessions | |||
|---|---|---|---|
| recording | acoustic dimensions | low fertility | high fertility |
| spoken sentence | F0 (Hz) | 206 (26.0) | 211 (28.0)* |
| F0 s.d. (Hz) | 27.0 (12.7) | 28.1 (13.0) | |
| mean syllabic duration (ms) | 169 (29.0) | 171 (24.1) | |
| sustained vowel | avg. jitter (%) | 0.49 (0.40) | 0.52 (0.62) |
| avg. shimmer (%) | 3.70 (2.45) | 3.90 (2.58) | |
| harmonic-to-noise ratio (dB) | 18.09 (4.5) | 17.74 (4.2) | |
| F0 (Hz) | 209 (26.5) | 206 (26.5) | |
| mono vowels | Df (Hz) | 1138 (48.9) | 1134 (50.4) |
| F0 (Hz) | 212 (21.6) | 213 (23.7) | |
To confirm that the pitch changes were perceptible, we presented the pairs (in random order with fertility counterbalanced) to participants (n=15) and asked which sample had higher pitch. For pairs in which the high-fertility recording was higher pitched (43 of 69, mean F0 difference=13 Hz), participants were correct at above-chance levels (55% of the time, t(42)=2.25, p=0.03).
4. Discussion
We expected that pitch and, to a lesser extent, Df would shift across the cycle to become more feminine at high fertility. We confirmed this expectation for pitch. When speaking a simple introductory sentence, women's pitch was greater at high- as compared to low-fertility, and this increase varied as a function of proximity to ovulation: women closer to ovulation within the fertile window showed greater differences between their high- and low-fertility vocal pitch. Further, this difference was the greatest on the 2 days preceding ovulation, when fertility within the cycle is the highest. We found no effects of proximity to menstrual onset within the low-fertility window. Together, these results indicate that high- versus low-fertility pitch difference is driven by changes associated with the approach of ovulation and not the approach of menstrual onset. Changes in pitch, therefore, appear to track cycling fertility.
These results are, to our knowledge, the first showing a specific cyclic fertility cue in the human voice. Although the pitch shift at peak fertility is the crucial measurement, even the average shift across high- and low-fertility windows exceeded the threshold of pitch discriminability (Moore 2008). When pitch increased in the high-fertility session (the only pairs where the cue is present), the pitch difference was well beyond the known perceptual threshold, which we confirmed with a simple perception task.
We did not find that Df differed across the cycle. We also did not find other changes in women's voices across the cycle, unlike some previous studies (Higgins & Saxman 1989; Abitbol et al. 1999). These null results are probably due to methodological differences between studies. Whereas our study compared follicular with luteal days, other research compared menstrual days with other phases of the cycle (Higgins & Saxman 1989; Abitbol et al. 1999; Whiteside et al. 2004). Ours is, to our knowledge, the first study to use a hormone assay to confirm cycle phase and to compare high- and low-fertility days apart from menstrual days.
An important question remains about whether this ovulatory cue could have social effects. Men prefer higher pitch relative to lower pitch in the same women (Feinberg et al. 2005, 2008), and these judgments are affected by cues of social interest in the speech (Jones et al. 2008). In the current study, pitch increases only occurred in speech with semantic meaning, and not vowel production. It is feasible that these changes in vocal femininity occur primarily or exclusively during social communicative tasks, raising the intriguing possibility that cues of ovulation appear more during social interactions and could serve a communicative function. These results are consistent with other findings revealing women's tendency during high fertility to accentuate sexually differentiated traits such as wearing fashionable clothing (Haselton et al. 2007) and preferring male masculinity (Gangestad et al. 2005). Future research should explore contextual effects on vocal production in association with the ovulatory cycle. Manipulations involving the presence of attractive others, speech content, and other stimuli before and during recording sessions might reveal systematic communicative signals, which in turn should be detectable and found attractive by judges.
Acknowledgments
We thank Unipath Diagnostics for donating hormone tests, Phil Ender for statistical consulting, Elizabeth Pillsworth for experiment oversight, Andreas Wilke for experimental programming, and Ashleigh Denny, Cari Goetz, Jennifer Huang, Vanessa Hurless, Brianne Latthitham, Proud Usahacharoenporn and Andrea Yocum for research assistance.
References
- Abitbol J., Abitbol P., Abitbol B. Sex hormones and the female voice. J. Voice. 1999;13:424–446. doi: 10.1016/s0892-1997(99)80048-4. doi:10.1016/S0892-1997(99)80048-4 [DOI] [PubMed] [Google Scholar]
- Amir O., Biron-Shental T. The impact of hormonal fluctuations on female vocal folds. Curr. Opin. Otolaryngol. Head Neck Surg. 2003;12:180–184. doi: 10.1097/01.moo.0000120304.58882.94. [DOI] [PubMed] [Google Scholar]
- Amir O., Kishon-Rabin L. Association between birth control pills and voice quality. Laryngoscope. 2004;114:1021–1026. doi: 10.1097/00005537-200406000-00012. doi:10.1097/00005537-200406000-00012 [DOI] [PubMed] [Google Scholar]
- Dixson A. Oxford University Press; New York, NY: 1998. Primate sexuality: comparative studies of the prosimians, monkeys, apes, and human beings. [Google Scholar]
- Feinberg D.R., et al. The voice and face of woman: one ornament that signals quality? Evol. Hum. Behav. 2005;26:398–408. doi:10.1016/j.evolhumbehav.2005.04.001 [Google Scholar]
- Feinberg D.M., DeBruine L.M., Jones B.C., Perrett D.I. The relative role of femininity and averageness in aesthetic judgments of women's voices. Perception. 2008;37:615–623. doi: 10.1068/p5514. doi:10.1068/p5514 [DOI] [PubMed] [Google Scholar]
- Gangestad S.W., Thornhill R., Garver C.E. Changes in women's sexual interests and their partners' mate-retention tactics across the menstrual cycle: evidence for shifting conflicts of interest. Proc. R. Soc. B. 2002;269:975–982. doi: 10.1098/rspb.2001.1952. doi:10.1098/rspb.2001.1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangestad S.W., Thornhill R., Garver-Apgar C.E. Adaptations to ovulation. In: Buss D.M., editor. The handbook of evolutionary psychology. Wiley; Hoboken, NJ: 2005. pp. 344–371. [Google Scholar]
- Haselton M.G., Mortezaie M., Pillsworth E.G., Bleske-Rechek A., Frederick D.A. Ovulatory shifts in human female ornamentation: near ovulation, women dress to impress. Horm. Behav. 2007;51:40–45. doi: 10.1016/j.yhbeh.2006.07.007. doi:10.1016/j.yhbeh.2006.07.007 [DOI] [PubMed] [Google Scholar]
- Higgins M.B., Saxman J.H. Variations in vocal frequency perturbation across the menstrual cycle. J. Voice. 1989;3:233–243. doi:10.1016/S0892-1997(89)80005-0 [Google Scholar]
- Jones B.C., Feinberg D.R., DeBruine L.M., Little A.C., Vukovic J. Integrating cues of social interest and voice pitch in men's preferences for women's voices. Biol. Lett. 2008;4:192–194. doi: 10.1098/rsbl.2007.0626. doi:10.1098/rsbl.2007.0626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong K.M., Ortolani A., Graham L.H., Savage A. The use of low-frequency vocalizations in African elephant (Loxodonta africana) reproductive strategies. Horm. Behav. 2003;43:433–443. doi: 10.1016/s0018-506x(03)00025-4. doi:10.1016/S0018-506X(03)00025-4 [DOI] [PubMed] [Google Scholar]
- Lynch C.D., Jackson L.W., Buck Louis G.M. Estimation of the day-specific probabilities of conception: current state of the knowledge and the relevance for epidemiological research. Paediatr. Perinat. Epidemiol. 2006;20:3–12. doi: 10.1111/j.1365-3016.2006.00765.x. doi:10.1111/j.1365-3016.2006.00765.x [DOI] [PubMed] [Google Scholar]
- Miller G.F., Tybur J., Jordan B. Ovulatory cycle effects on tip earnings by lap-dancers: economic evidence for human estrus? Evol. Hum. Behav. 2007;6:375–381. doi:10.1016/j.evolhumbehav.2007.06.002 [Google Scholar]
- Moore B.C.J. Basic auditory processes involved in the analysis of speech sounds. Phil. Trans. R. Soc. B. 2008;363:947–963. doi: 10.1098/rstb.2007.2152. doi:10.1098/rstb.2007.2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipitone R.N., Gallup G.G. Women's voice attractiveness varies across the menstrual cycle. Evol. Hum. Behav. 2008;29:268–274. doi:10.1016/j.evolhumbehav.2008.02.001 [Google Scholar]
- Schon P.C., Hamel K., Puppe B., Tuchscherer A., Kanitz A., Manteuffel G. Altered vocalization rate during the estrous cycle in dairy cattle. J. Dairy Sci. 2007;90:202–206. doi: 10.3168/jds.S0022-0302(07)72621-8. [DOI] [PubMed] [Google Scholar]
- Semple S., McComb K., Alberts S., Altmann J. Information content of female copulation calls in yellow baboons. Am. J. Primatol. 2002;56:43–56. doi: 10.1002/ajp.1062. doi:10.1002/ajp.1062 [DOI] [PubMed] [Google Scholar]
- Whiteside S.P., Hanson A., Cowell P.E. Hormones and temporal components of speech: sex differences and effects of menstrual cyclicity on speech. Neurosci. Lett. 2004;367:44–47. doi: 10.1016/j.neulet.2004.05.076. doi:10.1016/j.neulet.2004.05.076 [DOI] [PubMed] [Google Scholar]