Abstract
The immune response affects learning and memory in insects. Given this and the known fitness costs of both the immune system and learning, does an evolutionary trade-off exist between these two systems? We tested this by measuring the learning ability of 12 bumble-bee (Bombus terrestris) colonies in a free-flying paradigm. We then tested their immune response using the zone of inhibition assay. We found a positive relationship between colony learning performance and immune response, that is, fast-learning colonies also show high levels of antimicrobial activity. We conclude that there is no a priori reason to demand an evolutionary relationship between two traits that are linked physiologically.
Keywords: psychoneuroimmunology, crosstalk, social insects, learning speed
1. Introduction
There is extensive communication between the nervous system and immune system in mammals (Dantzer 2004). Many responses to parasites, such as fever, increased slow-wave sleep, reduced activity, exploration and sexual behaviour in mammals are orchestrated by immune products (proinflammatory cytokines) released in response to the detection of antigens (Maier & Watkins 1998). Links between nervous and immune systems are not unique to vertebrates. We have shown that both honeybees Apis mellifera (Mallon et al. 2003a) and bumble-bees Bombus terrestris (Riddell & Mallon 2006; Alghamdi et al. 2008) perform poorly in learning assays when their immune systems have been challenged by lipopolysaccaride (LPS). LPS is a component of gram-negative bacterial cell walls, which is a non-pathogenic elicitor of the immune response (Moret & Schmid-Hempel 2000). That is, we found that learning and memory are impaired by the immune response directly with no parasite present.
Given this physiological link between learning and immunity, and that learning and immunity have demonstrated fitness costs in insects (Kraaijeveld & Godfray 1997; Mery & Kawecki 2003; Raine & Chittka 2008), it seems reasonable to hypothesize an evolutionary trade-off between learning and immunity. An evolutionary trade-off is where the evolution of an increase in a given trait leads to a reduction in a different trait. This could be due to the pleiotropic effects of the genes involved, linkage disequilibrium with deleterious mutations or resource allocation during development (Schmid-Hempel 2005). While a recent paper showed no evidence of a trade-off between immunity and learning in different artificially selected Drosophila lines in the laboratory (Kolss et al. 2006), it might be more ecological relevant to examine natural levels of variation in these traits.
Bumble-bees are an obvious candidate for this approach as colonies show natural variation in learning performance (Raine et al. 2006b) and the physiological relationship between learning and immunity has been demonstrated in B. terrestris (Riddell & Mallon 2006; Alghamdi et al. 2008). Also there are ecological reasons to believe that learning and immunity could be more costly in bumble-bees than Drosophila, potentially leading to a higher likelihood of a trade-off.
The demands of foraging from many different flower species, which can vary dramatically in the quantity and quality of rewards they offer, and the need to find the nest after each foraging bout, mean that bees have highly developed cognitive abilities. Bumble-bees also learn from conspecifics, so-called social learning (Leadbeater & Chittka 2007). Furthermore, we would also expect that immunity would be a more important trait in social species that have high-contact rates with genetically close individuals leading to a greater chance of infection (Cremer et al. 2007).
In this study, we used a free-flying floral choice assay to test the learning abilities of bumble-bee colonies. We took workers from these colonies and tested their immune response using the antibacterial zone of inhibition (ZOI) assay. This allowed us to identify any evolutionary relationship between these two traits.
2. Material and methods
We obtained 12 bumble-bee (Bombus terrestris dalmatinus) colonies from Koppert Biological Systems (Berkel en Rodenrijs, The Netherlands). All workers were uniquely marked with Opalith tags (Christian Graze KG, Germany).
(a) Learning assay
Results from this associative learning assay are reported in a previous paper (Raine & Chittka 2008). Bees were pre-trained to forage from 20 bicoloured, blue and yellow, artificial flowers in a flight arena. During pre-training all flowers were rewarded with 50 per cent (w/w) sucrose solution providing previously colour-naive bees with an equal chance to associate both colours with reward (Raine et al. 2006b). Bees completing at least five consecutive foraging bouts were selected for training. These foragers were trained individually, in a flight arena containing 10 blue (Perspex Blue 727) and 10 yellow (Perspex Yellow 260) artificial flowers (each 24×24 mm). Yellow flowers were rewarding (each contained 15 μl of 50% (w/w) sucrose solution), while blue flowers were empty (unrewarding). Bees were regarded as choosing a flower when they either approached (inspected) or landed on it. Choosing a yellow flower was regarded as ‘correct’, while choosing a blue flower was deemed to be an ‘error’. We recorded the choice sequence made by each bee from the time it first entered the flight arena. Recording flower choices ceased once a bee made 99 flower choices after the first time it probed a rewarding (yellow) flower (Raine et al. 2006b). Flowers were changed and their positions re-randomized between foraging bouts to prevent bees using scent marks or previous flower positions as predictors of reward. Flower colours were selected so that bees had to overcome their strong, unlearned preference for blue, before associating one of their innately least favoured colours (yellow) with reward (Chittka et al. 2004; Raine et al. 2006a).
(b) Learning curves
The starting point for each bee's learning curve was the proportion of errors made (blue flowers chosen) before the bee first probed a rewarding (yellow) flower. Flower choices made by each bee after (and including) the first time it probed a rewarding (yellow) flower were evaluated as the number of errors (blue flowers chosen) in each group of 10 choices. Learning curves (exponential decay functions: y=y0+Ae−x/t) were fitted to these 11 data points (i.e. the start point and subsequent 10 groups of 10 flower choices) for each individual bee, using Microcal Origin (Chittka et al. 2004; Raine et al. 2006b). Here, x is the number of flower choices the bee made, starting with the first time it probed a yellow flower, and y is the number of errors. The saturation performance level (y0) is the number of errors made by a bee after finishing the learning process, i.e. when reaching a performance plateau. The decay constant (t) is a measure of learning speed: with lower t-values corresponding to faster learning speeds. A is the curve amplitude: the maximum displacement (height) of the curve above y0. Both amplitude (A) and saturation performance (y0) were constrained between 0 and 10 for curve fitting.
(c) Zone of inhibition assay
This assay measures antibacterial activity: it is based on the ability of immune proteins to inhibit bacterial growth when placed onto an agar plate seeded with bacteria (Arthrobacteur globiformis 105 bacteria per ml of agar). Workers from all 12 colonies were sacrificed after the learning assay and stored at −20°C for later analysis. Each thorax was homogenized in 300 μl of sodium cacodylate solution. Two microlitres of the supernatant from the centrifuged solution (1300g for 10 min at 4°C) were pippetted into a hole on the agar plate. This was incubated overnight (28°C). The resultant ZOI (mm) were measured as the mean of its longest and shortest axis (ZOI value).
3. Results
As reported in Raine & Chittka (2008), there was significant variation among colonies in learning speed (t-value: one way ANOVA: F11,160=1.900, p=0.043).
We tested the immune response of 55 bees from 12 colonies (mean number per colony (±s.d.)=4.58±0.67) using the ZOI assay. There was a significant difference between colonies in their immune response (colony F11,33=2.51, p=0.020), which could not be attributed to the effect of body size (head width F10,33=2.2, p=0.072).
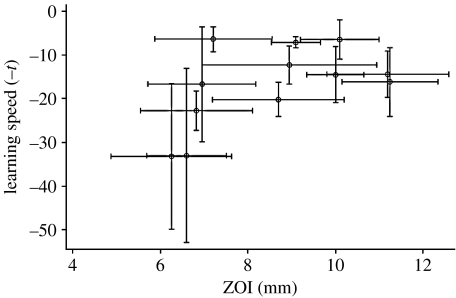
There was a significant negative correlation between the median t-value of a colony and its mean ZOI value (Spearman's rank: r=−0.608, n=12, p=0.036; figure 1). As high t-values correspond to slower learning speeds, this is a positive relationship between the ability of a colony to learn and the strength of its immune response.
Figure 1.
The relationship between median learning speed (negative t-values) and mean ZOI response of the 12 colonies. As high t-values correspond to slower learning speeds, we have plotted negative t-values to make clear the positive relationship between the colony learning ability and the strength of its immune response. Each point represents a colony. Vertical error bars represent median absolute deviation. Horizontal error bars represent standard error.
4. Discussion
We found a positive correlation between the ability of a colony's workers to learn and the strength of their immune response. Our initial hypothesis that learning ability and immune response would be in an evolutionary trade-off was not supported. Our result is in broad agreement with that of Kolss et al.'s (2006) artificial selection experiment, and expands our understanding of this potential learning and immunity trade-off by examining natural variation in both traits in unmanipulated organisms.
Foraging activity has been shown to decrease the immune response of bumble-bee workers (König & Schmid-Hempel 1995). All workers tested in the learning assay and subsequently used for ZOI assays had similar levels of foraging experience in the laboratory flight arena. Hence, as all our bees were foragers this could not explain variation in immune response. Potential exposure to pathogens which could induce stimulation of the immune system was identical for all 12 colonies that came directly from the bee breeder and were not exposed to field foraging conditions before this experiment.
Phenotypic correlations are generally seen as weak evidence for evolutionary trade-offs (Reznick et al. 2000). However, along with Kolss et al.'s selection experiment, we can ask why is there no evidence for an evolutionary trade-off when a physiological connection has been found repeatedly? Below we discuss three mutually non-exclusive possibilities.
First, it could be argued that we have incorrectly generalized antimicrobial response to some measure of overall immunocompetence. The various parts of the insect immune system (antimicrobial peptides, encapsulation, nitric oxide production, etc.) are known not to necessarily correlate (Mallon et al. 2003b). Hence, other parts of the immune response may show an evolutionary trade-off with memory if tested. However, as the physiological trade-off has been found repeatedly with ZOI measures, we felt this was the most likely place to find an evolutionary trade-off.
Second, there is the possibility that genetic variation exists not only in resource allocation but also in resource acquisition (Reznick et al. 2000). If there was more variation in allocation and less in acquisition, we would expect to see a negative correlation between any two life-history traits. Vice versa, we would expect to see a positive correlation (Van Noordwijk & Dejong 1986).
Third, Schmid-Hempel (2005) outlined the differences between the evolutionary and the activation cost of the immune system. Evolutionary costs are the fitness effects of possessing an immune system of a given strength. Evolutionary costs can occur due to the pleiotropic effects of resistance genes, linkage disequilibrium with deleterious alleles or changes in resource allocation during development. Activation costs are simply the effect on other physiological systems of generating the immune response from an organism's immune system. Although a physiological connection may lead us to look for an evolutionary trade-off there is no a priori reason to demand one.
Acknowledgements
We thank Oscar Ramos-Rodriguez for help with data collection, and Lars Chittka for useful comments. A.A. is funded by a Saudi government scholarship, and this work was supported by NERC grant (NER/A/S/2003/00469) to N.E.R.
Footnotes
The first two authors contributed equally to this work.
References
- Alghamdi A., Dalton L., Phillis A., Rosato E., Mallon E.B. Immune response impairs learning in free-flying bumble-bees. Biol. Lett. 2008;4:479–481. doi: 10.1098/rsbl.2008.0331. doi:10.1098/rsbl.2008.0331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chittka L., Ings T.C., Raine N.E. Chance and adaptation in the evolution of island bumblebee behaviour. Popul. Ecol. 2004;46:243–251. doi:10.1007/s10144-004-0180-1 [Google Scholar]
- Cremer S., Armitage S.A.O., Schmid-Hempel P. Social immunity. Curr. Biol. 2007;17:R693–R702. doi: 10.1016/j.cub.2007.06.008. doi:10.1016/i.cub.2007.06.008 [DOI] [PubMed] [Google Scholar]
- Dantzer R. Innate immunity at the forefront of psychoneuroimmunology. Brain Behav. Immun. 2004;18:1–6. doi: 10.1016/j.bbi.2003.09.008. doi:10.1016/j.bbi.2003.09.008 [DOI] [PubMed] [Google Scholar]
- Kolss M., Kraaijeveld A.R., Mery F., Kawecki T.J. No trade-off between learning ability and parasitoid resistance in Drosophila melanogaster. J. Evol. Biol. 2006;19:1359–1363. doi: 10.1111/j.1420-9101.2005.01068.x. doi:10.1111/j.1420-9101.2005.01068.x [DOI] [PubMed] [Google Scholar]
- König C., Schmid-Hempel P. Foraging activity and immunocompetence in workers of the bumble-bee Bombus terrestris L. Proc. R. Soc. B. 1995;260:225–227. doi:10.1098/rspb.1995.0084 [Google Scholar]
- Kraaijeveld A.R., Godfray H.C.J. Trade-off between parasitoid resistance and larval competitive ability in Drosophila melanogaster. Nature. 1997;389:278–280. doi: 10.1038/38483. doi:10.1038/38483 [DOI] [PubMed] [Google Scholar]
- Leadbeater E., Chittka L. Social learning in insects: from miniature brains to consensus building. Curr. Biol. 2007;17:R703–R713. doi: 10.1016/j.cub.2007.06.012. doi:10.1016/j.cub.2007.06.012 [DOI] [PubMed] [Google Scholar]
- Maier S.F., Watkins L.R. Cytokines for psychologists: implications of bi-directional immune to brain communication for understanding behaviour, mood, and cognition. Psychol. Rev. 1998;105:83–107. doi: 10.1037/0033-295x.105.1.83. doi:10.1037/0033-295X.105.1.83 [DOI] [PubMed] [Google Scholar]
- Mallon E.B., Brockmann A., Schmid-Hempel P. Immune response inhibits associative learning in insects. Proc. R. Soc. B. 2003;270:2471–2473. doi: 10.1098/rspb.2003.2456. doi:10.1098/rspb.2003.2456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallon E.B., Loosli R., Schmid-Hempel P. Specific versus nonspecific immune defense in the bumblebee, Bombus terrestris L. Evolution. 2003;57:1444–1447. doi: 10.1111/j.0014-3820.2003.tb00351.x. doi:10.1554/02-715 [DOI] [PubMed] [Google Scholar]
- Mery F., Kawecki T.J. A fitness cost of learning ability in Drosophila melanogaster. Proc. R. Soc. B. 2003;270:2465–2469. doi: 10.1098/rspb.2003.2548. doi:10.1098/rspb.2003.2548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moret Y., Schmid-Hempel P. Survival for immunity: the price of immune system activation for bumblebee workers. Science. 2000;290:1166–1168. doi: 10.1126/science.290.5494.1166. doi:10.1126/science.290.5494.1166 [DOI] [PubMed] [Google Scholar]
- Raine N.E., Chittka L. The correlation of learning speed and natural foraging success in bumble-bees. Proc. R. Soc. B. 2008;275:803–808. doi: 10.1098/rspb.2007.1652. doi:10.1098/rspb.2007.1652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine N.E., Ings T.C., Dornhaus A., Saleh N., Chittka L. Adaptation, genetic drift, pleiotropy, and history in the evolution of bee foraging behavior. Adv. Study Behav. 2006;36:305–354. doi:10.1016/S0065-3454(06)36007-X [Google Scholar]
- Raine N.E., Ings T.C., Ramos-Rodriguez O., Chittka L. Intercolony variation in learning performance of a wild British bumblebee population (Hymenoptera: Apidae: Bombus terrestris audax) Entomol. Generalis. 2006;28:241–256. [Google Scholar]
- Reznick D., Nunney L., Tessier A. Big houses, big cars, superfleas and the costs of reproduction. Trends Ecol. Evol. 2000;15:421–425. doi: 10.1016/s0169-5347(00)01941-8. doi:10.1016/S0169-5347(00)01941-8 [DOI] [PubMed] [Google Scholar]
- Riddell C.E., Mallon E.B. Insect psychoneuroimmunology: immune response reduces learning in protein starved bumblebees (Bombus terrestris) Brain Behav. Immun. 2006;20:135–138. doi: 10.1016/j.bbi.2005.06.008. doi:10.1016/j.bbi.2005.06.008 [DOI] [PubMed] [Google Scholar]
- Schmid-Hempel P. Evolutionary ecology of insect immune defenses. Ann. Rev. Entomol. 2005;50:529–551. doi: 10.1146/annurev.ento.50.071803.130420. doi:10.1146/annurev.ento.50.071803.130420 [DOI] [PubMed] [Google Scholar]
- Van Noordwijk A.J., Dejong G. Acquisition and allocation of resources: their influence on variation in life-history tactics. Am. Nat. 1986;128:137–142. doi:10.1086/284547 [Google Scholar]