Abstract
The genetic status of wolves in the western Great Lakes region has received increased attention following the decision to remove them from protection under the US Endangered Species Act. A recent study of mitochondrial DNA has suggested that the recovered wolf population is not genetically representative of the historic population. We present microsatellite genotype data on three historic samples and compare them with extant populations, and interpret published genetic data to show that the pre-recovery population was admixed over a century ago by eastern wolf (Canis lycaon) and grey wolf (Canis lupus) hybridization. The DNA profiles of the historic samples are similar to those of extant animals in the region, suggesting that the current Great Lakes wolves are representative of the historic population.
Keywords: hybridization, mitochondrial haplotype, microsatellite genotype, eastern wolf
1. Introduction
The ongoing debate over the evolutionary history and genetics of Canis populations in northeastern North America has become of immediate relevance to the conservation and management of wolves, given the recent US federal delisting of the western Great Lakes distinct population segment (FWS 2007). Various studies over the last two decades have focused on the genetic composition of Canis in the Great Lakes Region (GLR), and genetic data have shown that wolves in this region contain genetic material of Old World (OW) and New World (NW) evolved species (Lehman et al. 1991), yet much uncertainty remains about their relationship with other populations.
Recently, Leonard & Wayne (2008) have reported on mitochondrial DNA (mtDNA) analyses of the historic GLR wolves, suggesting that pre-recovery wolves were dominated by haplotypes distinct from grey wolves (C. lupus) and western coyotes (C. latrans) which they propose are from an endemic North American wolf referred to as the ‘Great Lakes wolf’. They interpreted the current population to be admixed, deriving primarily from lupus/latrans hybridization, with minor contributions from the Great Lakes wolf, and concluded that recently delisted GLR wolves are not genetically representative of the pre-recovery population. Their interpretation fails to recognize extensive genetic data on the NW evolved eastern wolf (C. lycaon), the mtDNA sequences of which are close to those of C. latrans (Wilson et al. 2000, 2003). The eastern wolf has been shown to be a distinct species (Wilson et al. 2000) that is capable of hybridizing with both coyotes and grey wolves across its range (see Kyle et al. 2006), acting as the conduit of Canis hybridization in northeastern North America. We do not agree with the suggestion that the current GLR population contains animals derived from lupus/latrans hybridization, as these sympatric species do not hybridize in western North America (see Kyle et al. 2006).
We present mtDNA and nuclear microsatellite data from three pre-recovery samples from the western GLR, and also compare mtDNA haplotypes from Leonard & Wayne (2008) to those reported by Wilson et al. (2000, 2003) as evidence that the present and pre-recovery wolf populations in the western GLR are genetically similar and are derived from lupus/lycaon hybridization.
2. Material and methods
We extracted DNA from three historic Canis samples provided by the University of Wisconsin Zoological Museum (table 1) using a DNeasy Blood & Tissue Kit (Qiagen Inc., Mississauga, ON) in a dedicated ancient DNA laboratory. A 343–347 bp fragment of the mtDNA control region was amplified using the primers described in Wilson et al. (2003) and conditions similar to theirs, except that we included 0.1 μg/μl BSA in the reaction and used an annealing temperature of 60°C. Contamination was monitored during extraction and PCR using negative controls. PCR products were cleaned with ExoSAP-IT (USB Corporation, Cleveland, OH) prior to sequencing on a MegaBACE 1000 (GE Healthcare). The sequence of sample 11 856 was confirmed from an independent amplification. The sequences were edited, aligned and compared with known haplotypes in Bioedit (Hall 1999). Refer to Wilson et al. (2000, 2003) for a description of the sequences. Amplification of eight nuclear microsatellite loci was attempted for each sample (Wilson et al. 2000), and homozygous genotypes were confirmed by repeated amplification. Two samples were genotyped at eight loci and the remaining sample at six loci.
Table 1.
mtDNA haplotypes and admixture proportions of historic Canis samples.
| admixture proportions | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| museum catalogue no. | sex | state | county | date | haplotype | no. of loci | P1 | P2 | P3 | P4 | P5 |
| 8626 | M | MN | Itasca | Spring 1900 | C13 | 8 | 0.009 | 0.012 | 0.180 | 0.791 | 0.008 |
| 8627 | M | MN | Itasca | February 1899 | C13 | 6 | 0.027 | 0.027 | 0.351 | 0.564 | 0.030 |
| 11 856 | – | WI | Ashland | Winter 1907/08 | C1 | 8 | 0.020 | 0.056 | 0.250 | 0.618 | 0.057 |
Alleles were scored in GeneMarker (v. 1.7, SoftGenetics LLC 2004) and the data were analysed using Structure (v. 2.2, Pritchard et al. 2000), including samples from studies by Grewal (2001) and Wilson et al. (submitted): Northwest Territories (n=67); Manitoba (n=41); Minnesota (n=9); northwestern Ontario (n=30); northeastern Ontario (n=34); Algonquin Provincial Park (n=49); Frontenac Axis (n=74); Adirondacks (n=66); Saskatchewan (n=36); and Texas (n=24). The admixture model of Structure was run for K=1 to K=10 with five repetitions of 106 iterations following a burn-in period of 250 000 iterations for each K. The number of populations K was determined to be five, based on the criteria outlined by Pritchard et al. (2000) and Evanno et al. (2005). The three historic samples were assigned a proportional membership to each of the five genetic clusters.
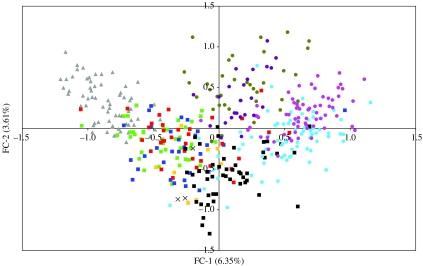
To supplement the results from Structure, a non-model-based factorial correspondence analysis (FCA) was performed on the microsatellite data for individual canids using Genetix (v. 4.05, Belkhir et al. 2004). Two-factorial components FC-1 and FC-2, which accounted for 6.35 and 3.61 per cent of the total inertia, respectively, were plotted to visualize the clustering of the historic samples in relation to the other sample groups.
3. Results
We compared the informative variable approximately 230 bp region of the Great Lakes wolf mtDNA control region haplotypes within Leonard & Wayne (2008), denoted as GL(X), to those within Wilson et al. (2000, 2003), denoted as C(X). As previously identified by Leonard & Wayne (2008), haplotype GL1 was identical to C. lycaon haplotype C1. However, we found other similarities among haplotypes from both studies (table 2), including two GL(X) haplotypes identical to C. lycaon haplotype C3. It is of interest that three GL(X) haplotypes were identical to a coyote-clustering sequence, haplotype C13, which has not been found in extant coyote populations but is present throughout the distribution of C. lycaon (Grewal 2001).
Table 2.
Comparison of variable sites between GL(X) and C(X) haplotypes: dot indicates same base as the uppermost row; dash indicates no base present. (Superscripts indicate identical haplotypes within approximately 230 bp region of comparison. Note C(X) haplotypes do not span entire alignment of GL(X) haplotypes.)
| variable site within GL(X) haplotypes | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| haplotype | 100 | 159 | 170 | 230 | 231 | 232 | 247 | 249 | 253 | 264 | 265 | 266 | 268 | 271 | 301 |
| GL1a | A | C | C | T | C | C | T | G | C | T | T | C | C | A | T |
| GL2 and GL19b | · | T | · | · | · | · | C | · | · | C | · | · | · | · | · |
| GL10, GL17 and GL18c | G | · | · | · | T | T | C | A | T | · | · | · | · | · | C |
| GL11d | G | · | – | C | · | T | C | A | T | · | · | · | · | · | C |
| GL12e | G | · | · | C | · | T | C | A | · | · | · | · | · | G | C |
| GL13f | G | · | · | C | · | T | C | A | T | · | · | · | · | G | C |
| GL16 g | G | · | – | · | · | T | C | A | T | C | C | T | T | G | C |
| C1a | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · |
| C3b | · | T | · | · | · | · | C | · | · | C | · | · | · | · | · |
| C9 g | G | · | – | · | · | T | C | A | T | C | C | T | T | G | C |
| C13c | G | · | · | · | T | T | C | A | T | · | · | · | · | · | C |
| C14f | G | · | · | C | · | T | C | A | T | · | · | · | · | G | C |
| C17e | G | · | · | C | · | T | C | A | · | · | · | · | · | G | C |
| C19d | G | · | – | C | · | T | C | A | T | · | · | · | · | · | C |
We sequenced the three historic samples at the mtDNA control region (294–322 bp) and assigned the haplotypes based on the approximately 230 bp region (table 1). The two haplotypes we observed in the three samples, C13 (n=2) and C1 (n=1), were identical to haplotypes found by Leonard & Wayne (2008) (table 2).
Based on the genotypes at the microsatellite loci, five groups were identified by Structure: Texas and Saskatchewan (western coyotes)=P1; Frontenac Axis and Adirondacks (eastern coyotes)=P2; Algonquin (eastern wolves)=P3; Manitoba, Minnesota and northwestern/northeastern Ontario (eastern/grey wolves)=P4; and Northwest Territories (grey wolves)=P5. The admixture proportions of the three historic samples revealed that their highest proportional memberships were to the P4 and P3 groups (table 1). The individual-based FCA clustered the historic samples among the Manitoba, Minnesota, northwestern/northeastern Ontario and Algonquin clusters (figure 1).
Figure 1.
FCA of microsatellite loci for Canis sample groups. Symbol type of sample group indicates mtDNA haplotypes present: squares, OW and NW (bright green, MB; gold, MINN; dark blue, NWON; red, NEON; black, ALG); triangles, OW (grey, NWT); circles, NW (sky blue, FRAX; pink, ADIR; dark yellow, SASK; violet, TXS); crosses, HIST.
4. Discussion
The genetic analyses show that the three historic samples from the western GLR have a mixed ancestry deriving primarily from groups representing eastern and grey wolves (i.e. P3 and P4). The historic samples did not cluster significantly with either of the two groups composed of coyote-like animals (i.e. P1 and P2). The results of the FCA were concordant with the results from Structure with the historic samples clustering with wolves and not coyotes. Both OW and NW mtDNA haplotypes occur in P3 and P4 (Grewal 2001), whereas only OW haplotypes occur in P5 (Wilson et al. 2003) and only NW haplotypes occur in P1 and P2 (Wilson et al. submitted) (figure 1). Given that OW and NW haplotypes occur in the groups for which the historic samples had their highest proportional memberships, we conclude that the historic samples represent animals containing genetic material derived from both grey (OW) and eastern wolves (NW), and not coyotes.
The occurrence of haplotype C13 in two historic samples that clustered with non-coyote groups based on nuclear microsatellite data (table 1) supports our interpretation that C13 is a C. lycaon haplotype, as does its apparent absence from extant coyote populations in the regions with no evidence of wolf–coyote hybridization, specifically Texas and Saskatchewan (Wilson et al. submitted, data not shown). The occurrence of haplotype C13 in wolves 100 yr ago is probably the result of one of three possible scenarios: (i) C13 evolved in the common ancestor of coyotes and eastern wolves and was perpetuated in both species when they diverged (i.e. incomplete lineage sorting), (ii) lycaon/latrans hybridization occurred earlier, i.e. pre-European settlement, whereby C13 was introgressed into C. lycaon and subsequently lost from the source C. latrans population, and (iii) an ancestral coyote haplotype was introgressed into the C. lycaon lineage during the Pleistocene or sometime prior to European settlement and subsequently diverged to become eastern wolf specific. The latter scenario would explain why C13 clusters closer to coyote sequences than eastern wolf sequences (Wilson et al. 2003). The loss of an mtDNA haplotype from a source C. latrans population seems unlikely, given the rapid population expansion of the species and the apparent absence of C13 from non-hybridizing coyote populations. The divergence of haplotype C13 from the eastern wolf clade and its absence in coyote populations (Wilson et al. 2003) supports C13 as being of eastern wolf origin through introgressive hybridization and subsequent divergence, and not incomplete lineage sorting.
The GL(X) haplotypes that were identical to haplotypes C1, C3 and C13 (table 2) occurred in samples from the western Great Lakes states (Leonard & Wayne 2008), further supporting the presence of C. lycaon genetic material in animals in this region.
The ability of the eastern wolf to hybridize with both coyotes and grey wolves complicates species assignments based on mitochondrial sequences and leads to questions concerning their validity, because the possibility exists of NW haplotypes occurring in lupus/lycaon hybrids and OW haplotypes occurring in lycaon/latrans hybrids. This issue has important ramifications for previous taxonomic interpretations based solely on mtDNA.
The DNA profiles presented here indicate that the pre-recovery western GLR wolf population was probably composed of lupus/lycaon hybrids, suggesting that eastern and grey wolves hybridized historically (i.e. more than 100 yr ago). To date, no C. lupus mtDNA has been observed in the pre-recovery western GLR samples; however, based on the nuclear microsatellite data, these animals are genetically similar to present-day animals, which have both grey and eastern wolf mtDNA haplotypes. We suspect that limited sampling has failed to resolve the presence of C. lupus mtDNA in the western GLR during pre-recovery times (Nowak 2002), accepting that it may have been present at a lower frequency than in the current population. Several factors may have contributed to the observed absence or suspected lower abundance of grey wolf haplotypes in the pre-recovery western GLR wolves: (i) population bottleneck, (ii) genetic drift, and (iii) sex-biased lupus/lycaon hybridization.
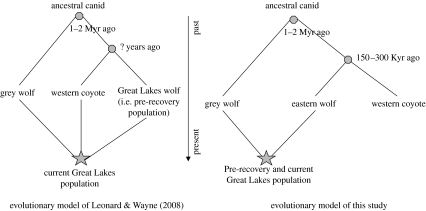
We suggest that recolonizing wolves originating from Minnesota, Manitoba and northwestern Ontario, containing C. lupus and C. lycaon mtDNA haplotypes, moved east into Wisconsin and continued into Michigan's Upper Peninsula. This may have resulted in the current wolf populations exhibiting C. lupus mtDNA in higher frequency than in the pre-recovery wolves from the western GLR. Previous research supports the presence of both C. lycaon and C. lupus haplotypes in Manitoba and northwestern Ontario (Wilson et al. 2003), which represents a potential and likely source of immigrants for the recovering Wisconsin and Michigan populations. Recent genetic analysis of Canis samples from the western GLR, based on a variety of genetic markers, supports lupus/lycaon hybridization (Wheeldon, unpublished data), as does previous research (Mech & Federoff 2002). The hypothesis that the current Great Lakes wolf population is derived from lupus/latrans hybridization is rejected by the data (figure 2).
Figure 2.
Comparison of Canis evolution hypotheses. Star, hybridization; circle, divergence.
The conclusion that the recovered Wisconsin and Michigan wolf populations are composed of lupus/lycaon hybrids has implications for the delisting of grey wolves in the GLR, and an important issue to consider is when the lupus/lycaon hybridization occurred. Given the genetic similarities between the pre-recovery and current western GLR wolves, the current and future conservation and management actions should focus on conserving the current wolf population and maintaining gene flow across its range, and not attempt to interfere with hybridization dynamics in the hope of achieving a ‘pure’ animal or desired phenotype.
Acknowledgments
We thank Jennifer Leonard for providing sequence data for our haplotype comparisons, and Paula Holahan and Adrian Wydeven for providing the historic samples for analysis. This research was made possible by the NSERC grants and funding from the Ontario Ministry of Natural Resources.
References
- Belkhir K., Borsa P., Chikhi L., Raufaste N., Bonhomme F. Université de Montpellier II; Montpellier, France: 1996–2004. Genetix 4.05, logiciel sous Windows TM pour la génétique des populations. Laboratoire Génome, Populations, Interactions, CNRS UMR 5171. [Google Scholar]
- Evanno G., Regnaut S., Goudet J. Detecting the number of clusters of individuals using the software Structure: a simulation study. Mol. Ecol. 2005;14:2611–2620. doi: 10.1111/j.1365-294X.2005.02553.x. doi:10.1111/j.1365-294X.2005.02553.x [DOI] [PubMed] [Google Scholar]
- Fish and Wildlife Service. Proposed rules. Fed. Regist. 2007;72:6052–6103. [Google Scholar]
- Grewal, S. K. 2001 A genetic analysis of the eastern timber wolf, pp. 1–173. MSc Thesis, McMaster University.
- Hall T.A. Bioedit: a user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucleic Acids Symp. Ser. 1999;41:95–98. [Google Scholar]
- Kyle C.J., Johnson A.R., Patterson B.R., Wilson P.J., Shami K., Grewal S.K., White B.N. Genetic nature of eastern wolves: past, present and future. Conserv. Genet. 2006;7:273–287. doi:10.1007/s10592-006-9130-0 [Google Scholar]
- Lehman N., et al. Introgression of coyote mitochondrial DNA into sympatric North American gray wolf populations. Evolution. 1991;45:104–119. doi: 10.1111/j.1558-5646.1991.tb05270.x. doi:10.2307/2409486 [DOI] [PubMed] [Google Scholar]
- Leonard J.A., Wayne R.K. Native Great Lakes wolves were not restored. Biol. Lett. 2008;4:95–98. doi: 10.1098/rsbl.2007.0354. doi:10.1098/rsbl.2007.0354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mech L.D., Federoff N.E. Alpha (1)—antitrypsin polymorphism and systematics of eastern North American wolves. Can. J. Zool. 2002;80:961–963. doi:10.1139/z02-066 [Google Scholar]
- Nowak R.M. The original status of wolves in eastern North America. Southeast. Nat. 2002;1:95–130. doi:10.1656/1528-7092(2002)001[0095:TOSOWI]2.0.CO;2 [Google Scholar]
- Pritchard J.K., Stephens M., Donnelly P. Inference of population structure from multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson P.J., et al. DNA profiles of the eastern Canadian wolf and the red wolf provide evidence for a common evolutionary history independent of the gray wolf. Can. J. Zool. 2000;78:2156–2166. doi:10.1139/cjz-78-12-2156 [Google Scholar]
- Wilson P.J., Grewal S., McFadden T., Chambers R.C., White B.N. Mitochondrial DNA extracted from eastern North American wolves killed in the 1800s is not of gray wolf origin. Can. J. Zool. 2003;81:936–940. doi:10.1139/z03-059 [Google Scholar]
- Wilson, P. J., Johnson, A., Grewal, S., Jakubas, W., Mullen, S., Chambers, R. E., Dumond, M. & White, B. N. Submitted. Genetic characterization of the eastern coyote.