Abstract
Large carnivores are important ecosystem components but are extinction prone due to small populations, slow growth rates and large area requirements. Consequently, there has been a surge of carnivore conservation efforts. Such efforts typically target local populations, with limited attention to the effects on the ecosystem function of predator guilds. Also, there is no framework for prioritizing these efforts globally. We compared taxonomic and functional diversity of continental carnivore guilds, compared them with the corresponding guilds during the Late Pleistocene and synthesized our results into suggestions for global prioritizations for carnivore conservation. Recent extinctions have caused taxonomically and functionally depleted carnivore guilds in Europe and North and South America, contrasting with guilds in Africa and Asia, which have retained a larger proportion of their carnivores. However, Asia is at higher risk of suffering further extinctions than other continents. We suggest three priorities of contrasting urgency for global carnivore conservation: (i) to promote recovery of the threatened Asian species, (ii) to prevent species in the depleted guilds in Europe and North and South America from becoming threatened, and (iii) to reconstruct functionally intact sympatric guilds of large carnivores at ecologically effective population sizes.
Keywords: carnivore, conservation, biodiversity, restoration
1. Introduction
Large terrestrial carnivores are important ecological components in a wide range of biomes (Terborgh 2005). However, large carnivores are also often extinction prone due to low population densities, slow growth rates and large area requirements (Purvis et al. 2000). Carnivores have also suffered a high level of human persecution, both historically and recently (Woodroffe 2001). This has led to a recent surge of carnivore conservation efforts (see Gittleman et al. 2001; Ray et al. 2005). However, despite suggestions that the composition of predator assemblages alter their ecological effects (Sih et al. 1998), carnivore conservation typically targets protection or restoration of local populations and success is often measured in terms of viability of the protected or reintroduced populations (Breitenmoser et al. 2001; Hayward et al. 2007). Limited attention has been given to the effects of carnivore conservation on the ecosystem function of predator guilds (e.g. Dalerum et al. 2008), except the bold suggestion to repopulate North America with non-native species to restore ecologically functional relationships among large vertebrates (Donlan et al. 2006). Furthermore, although regional plans for carnivore conservation and restoration have been proposed (Carroll et al. 2001; Mills et al. 2001; Enserink & Vogel 2006), there is no current framework to make global prioritizations for such efforts.
We evaluated taxonomic and functional diversity of continental carnivore guilds and their taxonomic and functional losses since the Late Pleistocene. We chose this point in time since large mammal extinction rates since the Late Pleistocene have grossly exceeded previous extinction rates, and that this has probably been caused by human interference (Koch & Barnosky 2006). Species compositions during the Late Pleistocene can therefore be regarded as the last existing record of the state of ecosystems largely undisturbed by modern human activities. Finally, we synthesize these patterns into suggestions for global prioritizations for carnivore conservation.
2. Material and methods
We searched relevant literature to determine the approximate number of species of large carnivores at the end of the Pleistocene epoch, as well as species composition of contemporary continental carnivore guilds (table S1 in the electronic supplementary material). To evaluate functional diversity and integrity of carnivore guilds, we adopted an ecomorphological classification of carnivores suggested by Werdelin (1996) as a proxy for carnivore functional groups. This classification includes scavengers/omnivores, bone crushers, bone crackers, stalk and ambush carnivores, ambush and slash carnivores, and pursuit carnivores (see Werdelin (1996) for formal definitions and characteristics). Each species could only belong to one functional group. We included carnivores larger than approximately 10 kg in body mass. This classification includes the large carnivores, which frequently rely on prey larger than themselves and the mesocarnivores, but excludes smaller species that have a different ecological niche.
We used the most recent International Union for Conservation of Nature and Natural Resources (IUCN) red list of threatened species (IUCN 2007) to estimate how many of the extant species that were regarded as threatened or non-threatened. Following IUCN (2007), we regarded a species as threatened if categorized as ‘Vulnerable’, ‘Endangered’ or ‘Critically endangered’ and as non-threatened if categorized as ‘Least concern’ or ‘Near threatened’. When necessary, we estimated threat category for each continent from regional and country-specific assessments in the published IUCN action plans (table S1 in the electronic supplementary material). We regarded Europe and Asia (delimited by the Caspian and Black Seas and the Ural Mountains) as separate continental regions due to contrasting fauna and socio-economy.
We used a modified version of the Shannon diversity index (Buckland et al. 2005) to quantify taxonomic and functional diversity of carnivore guilds. We applied the index to the number of species within each family to evaluate taxonomic diversity, and to the number of species within each functional group to evaluate functional diversity. The index can be expressed as
where ni is the present number of species in group i; n′ is the total number of species in the original guild (i.e. Late Pleistocene guilds); and N is the number of groups within the present guild.
We calculated the percentage of extinct species since the Late Pleistocene for each continent, and evaluated taxonomic and functional integrity by scaling the taxonomic and functional diversity indices in relation to the index values of the Late Pleistocene guilds. This gave us an easily interpreted value ranging from 0 (no integrity left) to 1 (complete integrity in relation to the reference guild). We can define this normalized index as
where is the diversity index for the reference guild, or in its equivalent form
where ni is the number of species within group i in the present guild; is the equivalent number of species in the benchmark guild; and N is the number of groups.
3. Results
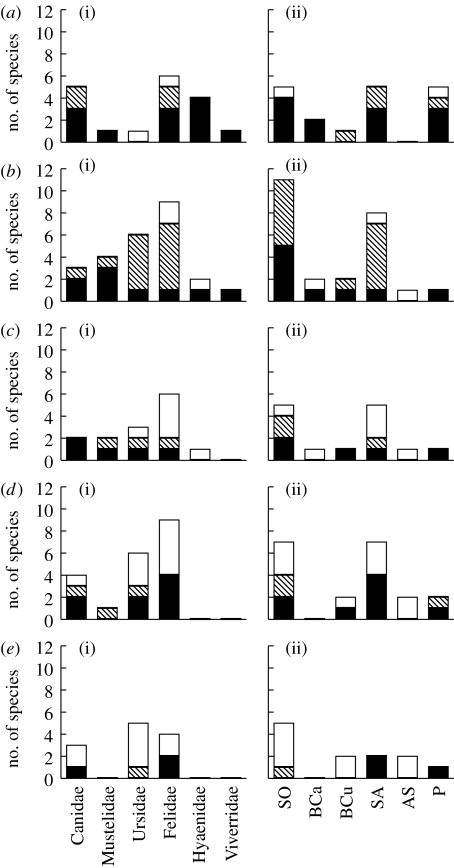
Carnivore guilds in Africa and Asia are more species rich and have higher taxonomic diversity than those in Europe and North and South America. The African guild contains 16 species from five families (M′=1.37), the Asian guild 22 species from five families (M′=1.50), the European guild 8 species from four families (M′=1.11), the North American guild 11 species from four families (M′=0.73) and the South American guild 4 species from three families (M′=0.71) (figure 1). However, the number of threatened species is higher in Asia than in the other continents (table 1).
Figure 1.
The number of extant species of large (more than 10 kg in body weight) terrestrial carnivores in each family as well as the approximate number of species that has gone extinct since the Late Pleistocene on the major continents and the number of extant and approximate number of extinct species of different functional groups. (a) Africa, (b) Asia, (c) Europe, (d) North America, (e) South America. (a(i))–(e(i)) Taxonomic family and (a(ii))–(e(ii)) ecomorph. White bars, extinct; hatched bars, threatened; black bars, non-threatened. Species records underlying the figure are given in table S1 in the electronic supplementary material. Functional groups are defined by ecomorphs suggested by Werdelin (1996): SO, scavenger/omnivores; BCa, bone crackers; BCu, bone crushers; SA, stalk and ambush; AS, ambush and slash; P, pursuit.
Table 1.
Continental patterns of taxonomic and functional diversity within Late Pleistocene and contemporary carnivore guilds. (Taxonomic and functional diversity were quantified by the modified Shannon index calculated on the number of species within taxonomic families and functional groups, respectively, and taxonomic and functional integrity was calculated by normalizing the present guild in relation to the Pleistocene guild.)
| taxonomic diversity | |||||||
|---|---|---|---|---|---|---|---|
| Pleistocene guild | present guild | ||||||
| species | taxonomic diversity | species | taxonomic diversity (M′) | taxonomic integrity | extinct species (%) | threatened species (%) | |
| Africa | 18 | 1.54 | 16 | 1.37 | 0.89 | 11 | 25 |
| Asia | 25 | 1.59 | 22 | 1.50 | 0.95 | 12 | 64 |
| Europe | 14 | 1.44 | 8 | 1.11 | 0.77 | 43 | 25 |
| North America | 20 | 1.19 | 11 | 0.73 | 0.61 | 45 | 27 |
| South America | 12 | 1.07 | 4 | 0.71 | 0.66 | 67 | 25 |
| functional diversity | |||||||
|---|---|---|---|---|---|---|---|
| Pleistocene guild | present guild | ||||||
| groups | functional diversity | groups | functional diversity (M′) | functional integrity | extinct groups (%) | threatened groups (%) | |
| Africa | 6 | 1.68 | 5 | 1.45 | 0.89 | 17 | 20 |
| Asia | 6 | 1.39 | 5 | 1.18 | 0.85 | 17 | 0 |
| Europe | 6 | 1.49 | 4 | 1.01 | 0.68 | 33 | 0 |
| North America | 5 | 1.38 | 4 | 1.02 | 0.74 | 20 | 0 |
| South America | 5 | 1.47 | 3 | 0.71 | 0.48 | 40 | 33 |
Africa has the most functionally diverse carnivore guild (M′=1.45), followed by Asia (M′=1.18), North America (M′=1.02), Europe (M′=1.01) and South America (M′=0.71). In both Africa and Asia, all functional groups except the globally extinct ‘slash and ambush’ group (the sabretoothed felids) still exist, while Europe and North America is lacking one additional group (‘bone crackers’, primarily represented by contemporary hyaenas) and South America is lacking two additional groups (‘bone crackers’ and ‘bone crushers’, primarily represented by contemporary large canids) (figure 1). However, three out of five extant functional groups in Asia and Africa contain threatened species while two out of four groups in Europe and North America and only one group in South America contains threatened species (figure 1). It is only in Africa and South America that functional groups are threatened with continental extinction (table 1).
The continental variation in taxonomic and functional diversity appear to have been caused by recent extinctions; only 2 out of 18 species have gone extinct in Africa, 3 out of 25 species in Asia, 6 out of 14 species in Europe, 9 out of 20 in North America and 8 out of 12 in South America (table 1). Since Pleistocene carnivore guilds were similar taxonomically and functionally, extinctions have resulted in lower taxonomic and functional integrity of the extant guilds in North and South America relative to Eurasia and Africa (table 1).
4. Discussion
Three conservation categories of continental carnivore guilds appear from our analyses. First, the African guild has many species, has suffered limited extinctions and has few threatened species. Second, the Asian guild similarly has many species and has suffered limited extinctions, but in contrast has many threatened species. Third, the guilds in Europe and North and South America have few species and have suffered substantial extinctions, but have proportionally few threatened species. These patterns indicate that two separate priorities ought to be set for global carnivore conservation, with contrasting urgency. The first and most urgent one should be to recover the threatened species in Asia, and the second and less urgent one should be to prevent species from becoming threatened in the already depleted guilds in Europe and North and South America.
On a continental scale, both Africa and Asia contain functionally intact carnivore guilds, while the guilds in Europe and North and South America have been functionally, as well as taxonomically, depleted. It is only in Africa that we find sympatric species assemblages of carnivores that form functionally intact guilds on a local scale, and there only in larger protected areas (Mills 2005). Furthermore, many species, particularly in Asia, are occurring at population sizes that probably are below what is ecologically effective to provide their ecosystem function. Therefore, we also suggest a third conservation priority, to reconstruct as functionally complete sympatric carnivore guilds as possible, given extant species on each continent, at ecologically effective population sizes.
We used a simple diversity index to quantify taxonomic and functional diversity of continental carnivore guilds. Although this index has been recommended for biodiversity monitoring (Buckland et al. 2005), we applied it to historical species occurrences, including extinct species. Therefore, discrepancies in the fossil record as well as our interpretation of it could affect our quantification of continental carnivore diversity. Furthermore, we used a palaeontological definition of carnivore ecomorphology (Werdelin 1996) as a crude proxy of carnivore functional groups, and thus carnivore species functionality. Although functional groups have been suggested as useful additions to conservation panning (Blondel 2003), their definition will strongly determine how well they represent functional contributions of species. Despite these caveats, however, we have for the first time presented a comparison of the taxonomic and functional diversity as well as integrity of continental carnivore guilds. Such analyses are paramount for our ability to make informed prioritizations regarding an ever-increasing demand to preserve species and ecosystems, and we suggest that similar analyses are carried out on other organism groups as well.
Acknowledgments
This research was made possible by a UP postdoctoral fellowship to F.D. Matt Hayward and an anonymous reviewer provided valuable comments on the manuscript.
Supplementary Material
Table S1. Species included in analyses of continental carnivore guilds. Extant species is based on Nowak (1999) and extinct species on Martin & Klein 1989, Turner (1997), Koch & Barnosky (2006), and Prevosti & Vazcaino (2006)
References
- Blondel J. Guilds or functional groups, does it matter? Oikos. 2003;100:223–231. doi:10.1034/j.1600-0706.2003.12152.x [Google Scholar]
- Breitenmoser U., Breitenmoser-Wursten C., Carbyn L.N., Funk S.M. Assessment of carnivore reintroductions. In: Gittleman J.L., Funk S.M., Macdonald D.W., Wayne R.K., editors. Carnivore conservation. Cambridge University Press; Cambridge, UK: 2001. pp. 241–281. [Google Scholar]
- Buckland S.T., Magurran A.E., Green R.E., Fewster R.M. Monitoring change in biodiversity through composite indexes. Phil. Trans. R. Soc. B. 2005;360:243–254. doi: 10.1098/rstb.2004.1589. doi:10.1098/rstb.2004.1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll C., Noss R.F., Paquet P.C. Carnivores as focal species for conservation planning in the Rocky Mountain region. Ecol. Appl. 2001;11:961–980. doi:10.1890/1051-0761(2001)011[0961:CAFSFC]2.0.CO;2 [Google Scholar]
- Dalerum F., Somers M.J., Kunkel K.E., Cameron E.Z. The potential for large carnivores to act as biodiversity surrogates in southern Africa. Biodivers. Conserv. 2008;17:2939–2949. doi:10.1007/s10531-008-9406-4 [Google Scholar]
- Donlan C.J., et al. Pleistocene rewilding: an optimistic agenda for twenty-first century conservation. Am. Nat. 2006;168:660–681. doi: 10.1086/508027. doi:10.1086/508027 [DOI] [PubMed] [Google Scholar]
- Enserink M., Vogel G. The carnivore comeback. Science. 2006;314:746–748. doi: 10.1126/science.314.5800.746. doi:10.1126/science.314.5800.746 [DOI] [PubMed] [Google Scholar]
- Gittleman J.L., Funk S.M., Macdonald D.W., Wayne R.K., editors. Carnivore conservation. Cambridge University Press; Cambridge, UK: 2001. [Google Scholar]
- Hayward M.W., et al. The reintroduction of large carnivores to the Eastern Cape, South Africa: an assessment. Oryx. 2007;41:205–214. doi:10.1017/S0030605307001767 [Google Scholar]
- IUCN 2007 IUCN red list of threatened species. See www.iucnredlist.org
- Koch P.L., Barnosky A.D. Late quaternary extinctions: state of the debate. Annu. Rev. Ecol. Evol. Syst. 2006;37:215–250. doi:10.1146/annurev.ecolsys.34.011802.132415 [Google Scholar]
- Mills M.G.L. Large carnivores and biodiversity in African savanna ecosystems. In: Ray J.C., Redford K.H., Steneck R.S., Berger J., editors. Large carnivores and the conservation of biodiversity. Island Press; Washington, DC: 2005. pp. 208–228. [Google Scholar]
- Mills M.G.L., Freitag S., Van Jaarsveld A.S. Geographic priorities for carnivore conservation in Africa. In: Gittleman J.L., Funk S.M., Macdonald D.W., Wayne R.K., editors. Carnivore conservation. Cambridge University Press; Cambridge, UK: 2001. pp. 467–480. [Google Scholar]
- Purvis A., Gittleman J.L., Cowlishaw G., Mace G.M. Predicting extinction risks in declining species. Proc. R. Soc. B. 2000;267:1947–1952. doi: 10.1098/rspb.2000.1234. doi:10.1098/rspb.2000.1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray J.C., Redford K.H., Steneck R.S., Berger J., editors. Large carnivores and the conservation of biodiversity. Island Press; Washington, DC: 2005. [Google Scholar]
- Sih A., Englund G., Wooster D. Emergent impacts on multiple predators on prey. Trends Ecol. Evol. 1998;13:350–355. doi: 10.1016/s0169-5347(98)01437-2. doi:10.1016/S0169-5347(98)01437-2 [DOI] [PubMed] [Google Scholar]
- Terborgh J. The green world hypothesis revisited. In: Ray J.C., Redford K.H., Steneck R.S., Berger J., editors. Large carnivores and the conservation of biodiversity. Island Press; Washington, DC: 2005. pp. 82–99. [Google Scholar]
- Werdelin L. Carnivoran ecomorphology: a phylogenetic perspective. In: Gittleman J.L., editor. Carnivore behavior, ecology and evolution. vol. 2. Cornell University Press; Ithaca, NY: 1996. pp. 582–624. [Google Scholar]
- Woodroffe R. Strategies for carnivore conservation: lessons from contemporary extinction. In: Gittleman J.L., Funk S.M., Macdonald D.W., Wayne R.K., editors. Carnivore conservation. Cambridge University Press; Cambridge, UK: 2001. pp. 61–92. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Species included in analyses of continental carnivore guilds. Extant species is based on Nowak (1999) and extinct species on Martin & Klein 1989, Turner (1997), Koch & Barnosky (2006), and Prevosti & Vazcaino (2006)