Abstract
The water balance of tsetse flies (Diptera: Glossinidae) has significant implications for understanding biogeography and climate change responses in these African disease vectors. Although moisture is important for tsetse population dynamics, evolutionary responses of Glossina water balance to climate have been relatively poorly explored and earlier studies may have been confounded by several factors. Here, using a physiological and GIS climate database, we investigate potential interspecific relationships between traits of water balance and climate. We do so in conventional and phylogenetically independent approaches for both adults and pupae. Results showed that water loss rates (WLR) were significantly positively related to precipitation in pupae even after phylogenetic adjustment. Adults showed no physiology–climate correlations. Ancestral trait reconstruction suggests that a reduction in WLR and increased size probably evolved from an intermediate ancestral state and may have facilitated survival in xeric environments. The results of this study therefore suggest an important role for water balance physiology of pupae in determining interspecific variation and lend support to conclusions reached by early studies of tsetse physiology.
Keywords: climate change, geographical range, evolutionary physiology, trypanosomiasis
1. Introduction
Insects from dry environments generally lose water more slowly, tolerate greater loss of water, or carry more water, than insects from moist environments. This pattern occurs at small and large geographical scales among populations or species (Addo-Bediako et al. 2001; Hoffmann et al. 2003). However, studies investigating the significance of water balance and potential relationships with climate remain poorly explored for many taxa (Chown & Nicolson 2004). Of particular concern is whether changes in disease prevalence will occur with anthropogenic climate change (Kovats et al. 2001) especially if a direct link exists between the population dynamics of vectors and climate.
The transmission of protozoan parasites (Trypanosoma spp.) by tsetse (Diptera: Glossinidae) during feeding on vertebrate hosts makes them a significant socio-economic and health burden (Maudlin 2006). While it is clear that temperature and moisture are important predictors of geographical distribution and abundance, relationships with moisture are distinctly variable among Glossina species (Rogers & Randolph 1986; Rogers & Robinson 2004) thus warranting further investigation. Early physiological investigations suggested an important role for water balance in tsetse (Buxton & Lewis 1934; Bursell 1958). However, water balance was later argued to be unimportant as no relationship was found with habitat moisture in adults (Bursell 1959). Indeed, Bursell (1959, p. 219) finally concluded that ‘…the water balance of adult tsetse flies has been of negligible importance in the conquest of arid and semi-arid habitats’.
Here, we re-examine tsetse water balance physiology and potential relationships with climate variables for three principal reasons. First, early work was based mainly on proportional analyses (e.g. water loss as a fraction of body mass). However, this approach has been criticized for statistical reasons (Packard & Boardman 1999) and potentially confounds the interpretation of water balance trait variation with aridity (Chown 2002). Second, the non-independence of species violates critical statistical assumptions and correlations may be significantly biased when evolutionary history is not accounted for (e.g. Halsey et al. 2006). Third, previous studies did not have access to high-resolution GIS climate databases and often inferred environmental moisture variation. We therefore examine water balance physiology and potential relationships with climate in both conventional and phylogenetically adjusted analyses. In addition, we explore the ancestral physiological state for Glossina in order to better comprehend evolutionary responses to climate variation.
2. Material and methods
Species-level physiological data were compiled from Anglophone literature (electronic supplementary material S1 and S2). Physiological data were extracted for live, resting individuals only. We only included data for pupae between 10 and 25 days of age as there is substantial ontogenetic variation at other times (Bursell 1958). However, water loss rates (WLR) in this life stage are typically low and stable. We excluded less than 24-hour old adult flies for similar reasons. Evidence for sex effects on water balance of tsetse are limited (Bursell 1959, p. 213) and we thus assumed that these effects are negligible. Average body mass was obtained from colony-reared flies when unavailable in the literature.
Geographical locations for climate data extraction were selected based on ERGO/TALA distribution maps (http://ergodd.zoo.ox.ac.uk/tseweb/index.htm) and verified according to species habitat descriptions (Pollock 1996; electronic supplementary material S3 and S4). Sites used to obtain climate data were typically the centre of the geographical range. More locations were used for broadly distributed species to minimize potential bias arising from the site selection process. Climate data always included at least one site where physiological sampling occurred. Thereafter, climate data for mean annual precipitation (PP), mean annual temperature (MAT) and mean soil moisture (SM) was extracted for multiple locations per species (n=3–23) from WorldClim_2–5m database (Hijmans et al. 2005) and Dunne & Willmott (2000). Data were condensed to a single median value per climate variable per species.
A consensus species-level phylogeny was compiled for Glossina (electronic supplementary material S5) for use in phylogenetic generalized least-squares (PGLS) analyses with branch lengths set equal to one. In PGLS, an estimate of the phylogenetic covariance is given by λ, which indicates the strength of the phylogenetic effect (see Halsey et al. 2006). Phylogenetic and conventional (ordinary least squares, OLS) analyses examined models of the relationships between MAT, PP and SM (independent variables) on the physiological traits WLR, body water content (BWC) and survival time (ST) (dependent variables) for adults but, due to lack of available data, only WLR for pupae (electronic supplementary material S6–9). Relationships between body mass (Mb) and physiological traits for adults, and the effect of surface area (SA) on WLR for pupae, were also explored (electronic supplementary material S10–11). Habitat classification (xeric/mesic) was assigned according to Bursell (1958). Akaike weights (wi), the probability that the model is the correct one of those tested, were used to select the best model (Burnham & Anderson 2001). Survival of adult tsetse is probably directly influenced by PP, while pupae in the soil might only be indirectly influenced by PP and MAT. Therefore we investigated each of the climate variables separately and also in combined statistical models. Ancestral trait reconstruction was undertaken in Mesquite v. 2.5 (Maddison & Maddison 2008).
3. Results
For adults, WLR, followed by ST and BWC showed the strongest environmental models (table 1, electronic supplementary material S6). Phylogenetic information typically improved model fit substantially within each trait but these relationships all remained statistically non-significant (electronic supplementary material S6). The most likely, though non-significant model (wi=0.2665), was a negative relationship between WLR and MAT (table 1, electronic supplementary material S8).
Table 1.
The two best environmental models for water balance traits in Glossina. (WLR, water loss rate; PP, precipitation; SM, soil moisture; MAT, mean annual temperature. The significant result is shown in italics)
| life stage | model | λ | wi | estimate±s.e. | p-value |
|---|---|---|---|---|---|
| pupa | WLR∼PP+SM+MAT | 1.00 | 0.4690 | >0.05 | |
| WLR∼PP | 1.00 | 0.2247 | 0.0082±0.0029 | <0.05 | |
| adult | WLR∼MAT | 1.00 | 0.2665 | −0.0167±0.0175 | >0.05 |
| WLR∼PP | 1.00 | 0.1963 | −5.306×10−5±8.423×10−5 | >0.05 |
For pupae, the best-fit model included all three climatic variables (wi=0.4690) and incorporated phylogenetic effects on WLR (electronic supplementary material S7). However, this relationship was non-significant (table 1, electronic supplementary material S9). The next best model found a significant positive relationship between WLR and PP (wi=0.2247; table 1, electronic supplementary material S7) and included the effect of phylogeny (electronic supplementary material S9). This relationship was also significant (p=0.0428; electronic supplementary material S9) and positive in conventional regression (wi=0.0423; electronic supplementary material S7).
For adults, Mb was a significant predictor of WLR (p<0.031) and BWC (p<0.024) in conventional regression but not PGLS (p>0.05; electronic supplementary material S10). However, ΔAIC (Akaike information criterion) suggests that the relationship between WLR and Mb has a higher probability than WLR and BWC (electronic supplementary material S10). For pupae, SA was significantly related to WLR in conventional (p<0.015) but not PGLS analyses (p>0.05; electronic supplementary material S11).
Body mass-independent variation in WLR and BWC with habitat type was non-significant for adult flies (ANCOVA; p>0.05). However, ST varied significantly among xeric and mesic species (ANCOVA; p=0.036). Mesic pupae had significantly higher mass-adjusted WLR relative to xeric species (ANCOVA; p=0.011).
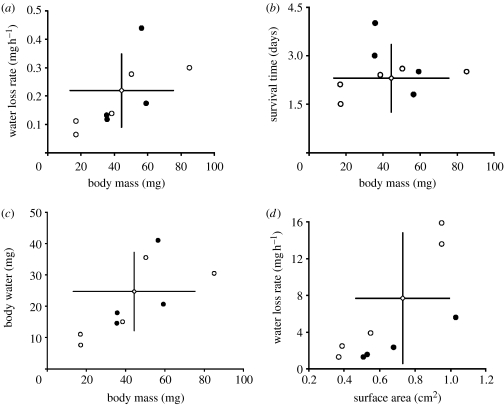
Ancestral reconstructions suggest Glossina evolved physiological tolerance to dry environments from an intermediate state, with traits of adults of several species falling outside the ancestral 95% confidence limits (CLs) on both trait axes (figure 1). Similarly, pupal WLR and SA for some mesic and xeric species fell outside ancestral 95% CLs suggesting that tsetse gained access to xeric environments predominantly through reductions in WLR and increases in body size, and may have responded in opposite directions for these traits in response to mesic conditions.
Figure 1.
Scatterplots of the relationship between (a) WLR, (b) ST, (c) BWC and Mb for adults and (d) WLR and SA for pupae, among Glossina species (n=9). The ancestral state is shown (diamond) with 95% CLs. Filled circles, xeric species; open circles, mesic species.
4. Discussion
The results of the present study are significant for two main reasons. First, in pupae, but not adults, we found a significant positive relationship between WLR and PP in the direction predicted by evolutionary physiology: species from dry environments have lower WLR than those from higher rainfall areas. Therefore, these results add novel support to Bursell's (1958) conclusion that there is a relationship between habitat moisture and pupal physiology, but not for adults (Bursell 1959; see also Hargrove 2004). Furthermore, the results for pupae appear relatively robust to a number of potentially confounding factors (e.g. statistical/phylogenetic, see §1). Similar responses to environmental moisture availability have been found among populations of adult Glossina pallidipes (Terblanche et al. 2006) and in other insect taxa (e.g. Addo-Bediako et al. 2001). In the present database, SM and PP were not correlated (p>0.534) and neither was PP and MAT (p>0.733) or SM and MAT (p>0.439). Therefore, these results are unlikely to be a consequence of autocorrelation of climate variables. However, only approximately one-third of Glossina species have been sampled to date and the lack of correlation in adults could indicate a type II statistical error.
These results may further be biased if the climate for a given location differs from the microclimate experienced by the population used to gather physiological data, particularly if populations show significant variation. Glossina inter-population variation has generally been poorly documented (though see Terblanche et al. 2006) and the extent to which this problem might influence the present results is thus unclear. Moreover, for each species in the present study the use of at least some climate data from locations where physiology was sampled should reduce this bias. Furthermore, phylogeny clearly affects these relationships since PGLS models were generally better than conventional analyses. Previous physiological studies of Glossina did not account for phylogenetic non-independence but evolutionary constraints should be considered in future.
Second, xeric and mesic species showed distinct differences in WLR for a given size, at least for pupae in the present database. These results therefore suggest that evolutionary changes in WLR and Mb in pupae may have resulted in the physiology–climate correlation. Glossina have a similar proportion of BWC (approx. 69–72%) but much greater variation of WLR and ST during desiccation (Bursell 1958, 1959). Adult ancestral trait reconstructions suggested variation in WLR, BWC or ST, may have been an evolutionary response to xeric environments (contra Bursell 1959). Indeed, ANCOVA showed significant variation in ST with habitat type; xeric species survive desiccation for longer than mesic species. These results generally support the view that water balance-related traits are labile in Glossina, as documented for other insects (e.g. Gibbs & Matzkin 2001; Chown & Klok 2003; but see Hoffmann et al. 2003).
In conclusion, this study provides additional support for a significant relationship between environmental moisture and WLR among Glossina pupae and thus strengthens earlier conclusions (e.g. Bursell 1958). Further work is required, however, examining responses to moisture availability in both life stages to determine potential climate change effects and whether these vary among mesic and xeric groups. Moreover, a wider range of Glossina species should be examined to see if the physiology–climate patterns hold more broadly.
Acknowledgments
The Faculty of AgriSciences (S.U.) and S. L. Chown (S.U.) provided partial financial and logistic support, respectively. Susana Clusella-Trullas and two anonymous referees provided constructive feedback.
Supplementary Material
Electronic supplementary material (S1–S11)
References
- Addo-Bediako A., Chown S.L., Gaston K.J. Revisiting water loss in insects: a large-scale view. J. Insect Physiol. 2001;47:1377–1388. doi: 10.1016/s0022-1910(01)00128-7. doi:10.1016/S0022-1910(01)00128-7 [DOI] [PubMed] [Google Scholar]
- Burnham K.P., Anderson D.R. Kullback–Leibler information as a basis for strong inference in ecological studies. Wildl. Res. 2001;28:111–119. doi:10.1071/WR99107 [Google Scholar]
- Bursell E. The water balance of tsetse pupae. Phil. Trans. R. Soc. B. 1958;241:179–210. doi:10.1098/rstb.1958.0002 [Google Scholar]
- Bursell E. The water balance of tsetse flies. Trans. R. Ent. Soc. Lond. 1959;111:205–235. [Google Scholar]
- Buxton P.A., Lewis D.J. Climate and tsetse flies: laboratory studies upon Glossina submorsitans and tachinoides. Phil. Trans. R. Soc. B. 1934;224:175–242. doi:10.1098/rstb.1934.0018 [Google Scholar]
- Chown S.L. Respiratory water loss in insects. Comp. Biochem. Physiol. A. 2002;133:791–804. doi: 10.1016/s1095-6433(02)00200-3. doi:10.1016/S1095-6433(02)00200-3 [DOI] [PubMed] [Google Scholar]
- Chown S.L., Klok C.J. Water balance characteristics respond to changes in body size in subantarctic weevils. Physiol. Biochem. Zool. 2003;76:634–643. doi: 10.1086/376919. doi:10.1086/376919 [DOI] [PubMed] [Google Scholar]
- Chown S.L., Nicolson S.W. Oxford University Press; Oxford, UK: 2004. Insect physiological ecology. Mechanisms and patterns. [Google Scholar]
- Dunne, K. A. & Willmott C. J. 2000 Global distribution of plant-extractable water capacity of soil (Dunne). Int. J. Climatol.16, 841–859. See http://www.daac.ornl.gov doi:10.3334/ORNLDAAC/545
- Gibbs A.G., Matzkin L.M. Evolution of water balance in the genus Drosophila. J. Exp. Biol. 2001;204:2331–2338. doi: 10.1242/jeb.204.13.2331. [DOI] [PubMed] [Google Scholar]
- Halsey L.G., Butler P.J., Blackburn T.M. A phylogenetic analysis of the allometry of diving. Am. Nat. 2006;167:276–287. doi: 10.1086/499439. doi:10.1086/499439 [DOI] [PubMed] [Google Scholar]
- Hargrove J.W. Tsetse population dynamics. In: Maudlin I., Holmes P.H., Miles M.A., editors. The trypanosomiases. CABI Publishing; Wallingford, UK: 2004. pp. 113–138. [Google Scholar]
- Hijmans R.J., Cameron S.E., Parra J.L., Jones P.G., Jarvis A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005;25:1965–1978. doi:10.1002/joc.1276 [Google Scholar]
- Hoffmann A.A., Hallas R.J., Dean J.A., Schiffer M. Low potential for climatic stress adaptation in a rainforest Drosophila species. Science. 2003;301:100–102. doi: 10.1126/science.1084296. doi:10.1126/science.1084296 [DOI] [PubMed] [Google Scholar]
- Kovats R.S., Campbell-Lendrum D.H., McMichael A.J., Woodward A., Cox J.S. Early effects of climate change: do they include changes in vector-borne disease? Phil. Trans. R. Soc. B. 2001;356:1057–1068. doi: 10.1098/rstb2001.0894. doi:10.1098/rstb2001.0894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddison, W. P. & Maddison, D. R. 2008 Mesquite: a modular system for evolutionary analysis. Version 2.5. See http://mesquiteproject.org
- Maudlin I. African trypanosomiasis. Ann. Trop. Med. Parasitol. 2006;100:679–701. doi: 10.1179/136485906X112211. doi:10.1179/136485906X112211 [DOI] [PubMed] [Google Scholar]
- Packard G.C., Boardman T.J. The use of percentages and size-specific indices to normalize physiological data for variation in body size: wasted time, wasted effort? Comp. Biochem. Physiol. A. 1999;122:37–44. doi:10.1016/S1095-6433(98)10170-8 [Google Scholar]
- Pollock J.N. Training manual for tsetse control personnel. vol. 2. FAO Publications; Rome, Italy: 1996. Ecological behaviour of tsetse. [Google Scholar]
- Rogers D.J., Randolph S.E. Distribution and abundance of tsetse flies (Glossina spp.) J. Anim. Ecol. 1986;55:1007–1025. doi:10.2307/4430 [Google Scholar]
- Rogers D.J., Robinson T.P. Tsetse distribution. In: Maudlin I., Holmes P.H., Miles M.A., editors. The trypanosomiases. CABI Publishing; Wallingford, UK: 2004. pp. 139–179. [Google Scholar]
- Terblanche J.S., Klok C.J., Krafsur E.S., Chown S.L. Phenotypic plasticity and geographic variation in thermal tolerance and water loss of the tsetse Glossina pallidipes (Diptera: Glossinidae): implications for distribution modelling. Am. J. Trop. Med. Hyg. 2006;74:786–794. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Electronic supplementary material (S1–S11)