Abstract
Why have some animals evolved large brains despite substantial energetic and developmental costs? A classic answer is that a large brain facilitates the construction of behavioural responses to unusual, novel or complex socioecological challenges. This buffer effect should increase survival rates and favour a longer reproductive life, thereby compensating for the costs of delayed reproduction. Although still limited, evidence in birds and mammals is accumulating that a large brain facilitates the construction of novel and altered behavioural patterns and that this ability helps dealing with new ecological challenges more successfully, supporting the cognitive-buffer interpretation of the evolution of large brains.
Keywords: brain evolution, cognitive ecology, life history, neurobiology
Ever since Darwin, scientists have wondered why some animals—including ourselves—have evolved brains that are substantially larger than that expected for their body size. In The descent of man, Darwin (1871) argued that ‘No one, I presume, doubts that the large proportion which the size of man's brain bears to his body, compared to the same proportion in the gorilla or orang, is closely connected with his higher mental powers’. However, what evidence do we have that the disproportionally larger brains of some animals are connected to ‘higher mental powers’, whatever that means? And, why should a ‘higher mental power’ facilitate the survival and/or reproduction of animals in the wild? After more than a century of research, controversy remains regarding the selective advantages that enlarged brains provide in the wild, although some theories have started receiving a degree of supporting evidence.
Here, I consider one of these theories, the cognitive buffer hypothesis (CB hypothesis, hereafter), which suggests that the primary adaptive function of a large brain is to buffer individuals against environmental challenges by facilitating the construction of behavioural responses (Allman et al. 1993; Deaner et al. 2003). This buffer effect should increase survival rates and favour a longer reproductive life, thereby partially compensating for the costs of delayed reproduction associated with the need to grow a large brain (Iwaniuk & Nelson 2003). As a follow-up of Deaner et al.'s (2003) classical review, I show that the basic assumptions and predictions of the CB theory have recently received important empirical support, providing a solid basis for developing a more general theory on brain size evolution.
1. Are large-brained animals better at constructing behavioural responses?
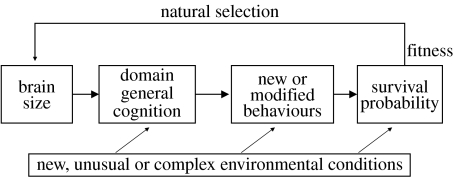
The basic assumption of the CB hypothesis is that a large brain facilitates the construction of novel or altered behavioural patterns through domain general cognitive processes such as innovation and learning (figure 1). However, this assumption has proved difficult to validate, in part because of the problem of quantifying ecologically relevant behavioural responses in species with very different life styles. As a way to overcome this problem, Lefebvre et al. (1997) suggested quantifying how often new behavioural patterns are observed in nature and relate their frequency to the size of the species' brains. The literature is full of observations of animals using new behaviours to solve ecological problems. Classic examples are the milk-opening behaviour of blue tits Cyanistes caeruleus and the potato-washing behaviour of Japanese macaques Macaca fuscata. A systematic documentation of similar observations has revealed that some animals are more capable of producing new or modified behaviours than others, and that highly innovative species tend to have relatively larger brains (reviewed in Lefebvre et al. 2004), a pattern first described in birds (Lefebvre et al. 1997) and then in primates (Reader & Laland 2002; Byrne & Corp 2004). The reasons why a large brain should be functionally better than a smaller one in those tasks are not well understood yet, although several reasons have been proposed (Lefebvre & Sol 2008). For example, large brains contain more neurons (Herculano-Houzel et al. 2006), which should provide higher capacity for gathering, storing and integrating information.
Figure 1.
Schematic representation of the CB hypothesis (see text for details; modified from Ricklefs & Wikelski 2002).
2. Why should behavioural changes be useful to survive in the wild?
Assuming that the pay-off for increasing brain size is enhanced performance in constructing flexible behavioural patterns, why should this facilitate the survival of animals in the wild? One important reason is that in nature animals often have to deal with problems for which they must devise novel or flexible solutions (Ricklefs 2004). Although the fitness benefits have rarely been quantified within any wild population, field observations and laboratory experiments suggest that the construction of new or altered behaviours can be advantageous in a broad array of contexts, facilitating for instance that animals can track resource variation, use hard-to-eat foods, exploit new ecological opportunities, deal with environmental complexity, avoid unfamiliar predators and gather information from conspecifics (reviewed in Godfrey-Smith 2001; Dukas 2004; Sol in press). New or altered behaviours are generally thought to be particularly advantageous in complex environments or in those that are novel or likely to change, which continually challenge the animal with problems and ecological opportunities. However, the CB hypothesis does not put the emphasis on the exact context that selects for enhanced behavioural flexibility. Although some contexts are likely to be more important than others, a variety of selective pressures can lead to brain increases, provided that the response entails the construction of new or altered behavioural patterns. Thus, the CB hypothesis allows the integration of a variety of hypotheses on brain size evolution (see Sol et al. 2005).
3. Is a large brain useful to survive in the wild?
Showing that large brains facilitate the construction of behavioural responses to socioecological challenges is necessary but insufficient to demonstrate the CB hypothesis. To validate the hypothesis, we also need to demonstrate that larger brains reduce mortality in the wild. The observation that species with larger brains live longer (Allman et al. 1993; Ricklefs & Scheuerlein 2001; Deaner et al. 2003) is often interpreted as an evidence for the CB hypothesis, as longevity is negatively correlated with adult mortality. However, an association between brain size and lifespan across species is also predicted by other theories of brain size evolution, including the growth regulation and the neuronal investment hypotheses (Deaner et al. 2003). The crucial finding that would differentiate the CB hypothesis from the alternatives is that larger brains reduce mortality rate during adulthood, when the brain is fully functional, but this has proven difficult to demonstrate. Fortunately, in the past years, an unparalleled amount of data on mortality has been assembled for many wild bird populations, making it possible to test the prediction. As predicted by the CB hypothesis, species with disproportionally larger brains consistently show lower rates of adult mortality in the wild when compared with the species with smaller brains, at least in birds (Sol et al. 2007).
However, the strongest evidence currently available that a large brain reduces extrinsic mortality comes from studies examining whether large-brained species survive better than small-brained species when introduced by humans to new environments. A species that is exposed to a novel environment will generally face many novel environmental challenges, and success will depend on whether individuals can rapidly develop behavioural responses to these new challenges (Sol 2003). In birds, larger brained species are more likely to be successful when introduced in novel regions than are small-brained species, a pattern first described in New Zealand and then globally (Sol et al. 2005). Successful invaders are also characterized by a high propensity of innovative behaviour in their native ranges, and path models suggest that the brain influences success by enhancing innovation propensity rather than through non-cognitive mechanisms (Sol et al. 2005). These findings fit well with the recent discoveries that larger brained birds are less likely to experience population decline as a result of habitat alterations (Shultz et al. 2005) and are more tolerant to a higher degree of climatic variability (Schuck-Paim et al. 2008).
Nevertheless, critical to developing a theory is establishing common, repeated patterns in different animal taxa. In fishes, Drake (2007) found no evidence that brain size affected the likelihood of establishment in introduced species. One reason may be that in fishes the brain areas thought to be involved in innovation and learning are generally small compared with other brain areas, and hence they are poorly represented by the whole brain size. Mammals provide a more crucial challenge to the CB hypothesis, as they have larger telencephalons and exhibit more sophisticated behavioural patterns. A recent analysis of over 400 introduction events has revealed that mammal species with larger brains do tend to be more successful at establishing themselves in novel environments (Sol et al. 2008). The finding that brain size is statistically associated with establishment success in mammals and birds, the animals with the largest of brains, provides important support for the CB hypothesis.
4. Future directions
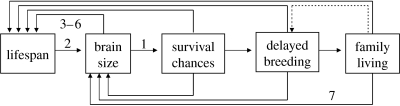
Despite the enormous difficulties in assembling evidence, there is now some support for the idea that large brains confer advantages to individuals in the form of behavioural flexibility to deal with novel environmental challenges. Current evidence is clearly insufficient to conclude that the CB hypothesis is a major explanation for the evolution of large brains (Sol in press), but provides a good starting point from which to build a more general theory. Following Deaner et al. (2003) and Ricklefs (2004), I argue below that a main priority should be to better integrate the theory into a broader life-history framework (figure 2).
Although the CB hypothesis argues that the increase in the brain drives an increase in lifespan, the reverse can also be true. When a species has a long-life strategy, individuals should gain greater fitness benefits by investing in adult survivorship rather than in current reproduction (Ricklefs & Wikelski 2002), implying that selection will favour the evolution of adaptations (such as larger brains) that increase survival over reproduction. Animals with a longer life are also more likely to be exposed to changes during their life and hence gain greater benefits from information acquisition and flexible behaviours (Deaner et al. 2003). Thus, a large brain is more beneficial, and thereby should be preferentially selected, in long-lived species than in short-lived ones.
As already noted, a large brain can be associated with a longer lifespan by mechanisms other than by increasing survival (Deaner et al. 2003). For example, the physiological regulator hypothesis argues that because larger brained animals have a more precise regulation of function, they are able to live longer. These mechanisms have received little attention from researchers, but they are important because of their potential for generating positive feedbacks and accelerate brain size evolution.
Growing a larger brain and learn the skills needed for survival require a longer developmental period (Iwaniuk & Nelson 2003), which suggests that the evolution of large brains can be constrained in short-lived lineages. The observation that precocial birds tend to have smaller brains than altricial birds (Bennett & Harvey 1985) supports this possibility, but understanding the importance of evolutionary constraints on brain size evolution requires employing phylogenetic techniques for character reconstructions.
Longevity can favour a delayed onset of reproduction, which should give parents the opportunity of a prolonged investment in offspring and promote family living (Covas & Griesser 2007). This can facilitate an increase in brain size if, as the social intelligence hypothesis suggests, individuals living in stable social groups face higher cognitive demands that individuals living alone (Byrne & Corp 2004; Dunbar & Shultz 2007). The social environment might in turn further buffer inexperienced individuals from the challenges posed by the environment, reducing the age of first breeding and hence the costs of delayed reproduction (Allman & Hasenstaub 1999). These ideas remain largely untested.
Figure 2.
Framework to integrate the CB hypothesis into a more general life-history-based theory on brain evolution. The positive and negative effects are represented by the solid and dashed lines, respectively. 1, CB hypothesis; 2, delayed maturation hypothesis; 3, physiological regulator hypothesis; 4, growth regulation hypothesis; 5, neuronal investment hypothesis; 6, maturational constraints hypothesis; 7, social intelligence hypothesis.
Understanding how and why brain size covaries with other life-history traits is an enormous challenge, but one that is critical to developing a general theory on brain size evolution. A fruitful avenue for future research would be the use of modelling approaches and phylogenetic reconstructions to elucidate the complex causal links and feedbacks that may link brain size with other life-history traits.
Acknowledgments
The author would like to thank Tom Smulders for the invitation to contribute to this special issue, and especially Louis Lefebvre and Simon Reader for many of their fruitful discussions. This project was supported by a Proyecto de Investigación (CGL2007-66257) from MEC (Spain).
Footnotes
One contribution of 10 to a Special Feature on ‘Brain evolution’.
References
- Allman J., Hasenstaub A. Brains, maturation times, and parenting. Neurobiol. Aging. 1999;20:447–454. doi: 10.1016/s0197-4580(99)00076-7. doi:10.1016/S0197-4580(99)00076-7 [DOI] [PubMed] [Google Scholar]
- Allman J.M., McLaughlin T., Hakeem A. Brain-weight and life-span in primate species. Proc. Natl Acad. Sci. USA. 1993;90:118–122. doi: 10.1073/pnas.90.1.118. doi:10.1073/pnas.90.1.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett P.M., Harvey P.H. Relative brain size and ecology in birds. J. Zool. Lond. 1985;207:151–169. [Google Scholar]
- Byrne R.W., Corp N. Neocortex size predicts deception rate in primates. Proc. R. Soc. B. 2004;271:1693–1699. doi: 10.1098/rspb.2004.2780. doi:10.1098/rspb.2004.2780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covas R., Griesser M. Life history and the evolution of family living in birds. Proc. R. Soc. B. 2007;274:1349–1357. doi: 10.1098/rspb.2007.0117. doi:10.1098/rspb.2007.0117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwin C. John Murray; London, UK: 1871. The descendent of man, and selection in relation to sex. [Google Scholar]
- Deaner R.O., Barton R.A., Svan Schaik C.P. Primate brains and life histories: renewing the connection. In: Kappeler P.M., Pereira M.E., editors. Primate life histories and socioecology. The University of Chicago Press; Chicago, IL: 2003. pp. 233–265. [Google Scholar]
- Drake J.M. Parental investment and fecundity, but not brain size, are associated with establishment success in introduced fishes. Funct. Ecol. 2007;21:963–968. doi:10.1111/j.1365-2435.2007.01318.x [Google Scholar]
- Dukas R. Evolutionary biology of animal cognition. Annu. Rev. Ecol. Syst. 2004;35:347–374. doi:10.1146/annurev.ecolsys.35.112202.130152 [Google Scholar]
- Dunbar R.I.M., Shultz S. Evolution in the social brain. Science. 2007;317:1344–1347. doi: 10.1126/science.1145463. doi:10.1126/science.1145463 [DOI] [PubMed] [Google Scholar]
- Godfrey-Smith P. Environmental complexity and the evolution of cognition. In: Sternberg R., Kaufman J., editors. The evolution of intelligence. Lawrence Erlbaum Associates; London, UK: 2002. pp. 233–249. [Google Scholar]
- Herculano-Houzel S., Mota B., Lent R. Cellular scaling rules for rodent brains. Proc. Natl Acad. Sci. USA. 2006;103:12138–12143. doi: 10.1073/pnas.0604911103. doi:10.1073/pnas.0604911103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaniuk A.N., Nelson J.E. Developmental differences are correlated with relative brain size in birds: a comparative analysis. Can. J. Zool. 2003;81:1913–1928. doi:10.1139/z03-190 [Google Scholar]
- Lefebvre L., Sol D. Brains, lifestyles and cognition: are there general trends? Brain Behav. Evol. 2008;72:135–144. doi: 10.1159/000151473. doi:10.1159/000151473 [DOI] [PubMed] [Google Scholar]
- Lefebvre L., Whittle P., Lascaris E., Finkelstein A. Feeding innovations and forebrain size in birds. Anim. Behav. 1997;53:549–560. doi:10.1006/anbe.1996.0330 [Google Scholar]
- Lefebvre L., Reader S.M., Sol D. Brains, innovations and evolution in birds and primates. Brain Behav. Evol. 2004;63:233–246. doi: 10.1159/000076784. doi:10.1159/000076784 [DOI] [PubMed] [Google Scholar]
- Reader S.M., Laland K.N. Social intelligence, innovation, and enhanced brain size in primates. Proc. Natl Acad. Sci. USA. 2002;99:4436–4441. doi: 10.1073/pnas.062041299. doi:10.1073/pnas.062041299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricklefs R.E. The cognitive face of avian life histories. Wilson Bull. 2004;116:119–196. doi:10.1676/04-054 [Google Scholar]
- Ricklefs R.E., Scheuerlein A. Comparison of aging-related mortality among birds and mammals. Exp. Gerontol. 2001;36:845–857. doi: 10.1016/s0531-5565(00)00245-x. doi:10.1016/S0531-5565(00)00245-X [DOI] [PubMed] [Google Scholar]
- Ricklefs R.E., Wikelski M. The physiology/lifehistory nexus. Trends Ecol. Evol. 2002;17:462–468. doi:10.1016/S0169-5347(02)02578-8 [Google Scholar]
- Schuck-Paim C., Alonso W.J., Ottoni E.B. Cognition in an ever-changing world: climatic variability is associated with brain size in Neotropical parrots. Brain Behav. Evol. 2008;71:200–215. doi: 10.1159/000119710. doi:10.1159/000119710 [DOI] [PubMed] [Google Scholar]
- Shultz S., Bradbury R., Evans K., Gregory R.D., Blackburn T.M. Brain size and resource specialisation predict long-term population trends in British birds. Proc. R. Soc. B. 2005;272:2305–2311. doi: 10.1098/rspb.2005.3250. doi:10.1098/rspb.2005.3250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sol D. Behavioural flexibility: a neglected issue in the ecological and evolutionary literature? In: Reader S.M., Laland K.N., editors. Animal innovation. Oxford University Press; Oxford, UK: 2003. pp. 63–82. [Google Scholar]
- Sol, D. In press. The cognitive-buffer hypothesis for the evolution of large brains. In Cognitive ecology II (eds R. Dukas & R. M. Ratcliffe), Chicago, IL: Chicago University Press.
- Sol D., Duncan R.P., Blackburn T.M., Cassey P., Lefebvre L. Big brains, enhanced cognition, and response of birds to novel environments. Proc. Natl Acad. Sci. USA. 2005;102:5460–5465. doi: 10.1073/pnas.0408145102. doi:10.1073/pnas.0408145102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sol D., Szekely T., Liker A., Lefebvre L. Big-brained birds survive better in nature. Proc. R. Soc. B. 2007;274:763–769. doi: 10.1098/rspb.2006.3765. doi:10.1098/rspb.2006.3765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sol D., Bacher S., Reader S.M., Lefebvre L. Brain size predicts the success of mammal species introduced into novel environments. Am. Nat. 2008;172:S63–S71. doi: 10.1086/588304. doi:10.1086/588304 [DOI] [PubMed] [Google Scholar]