Abstract
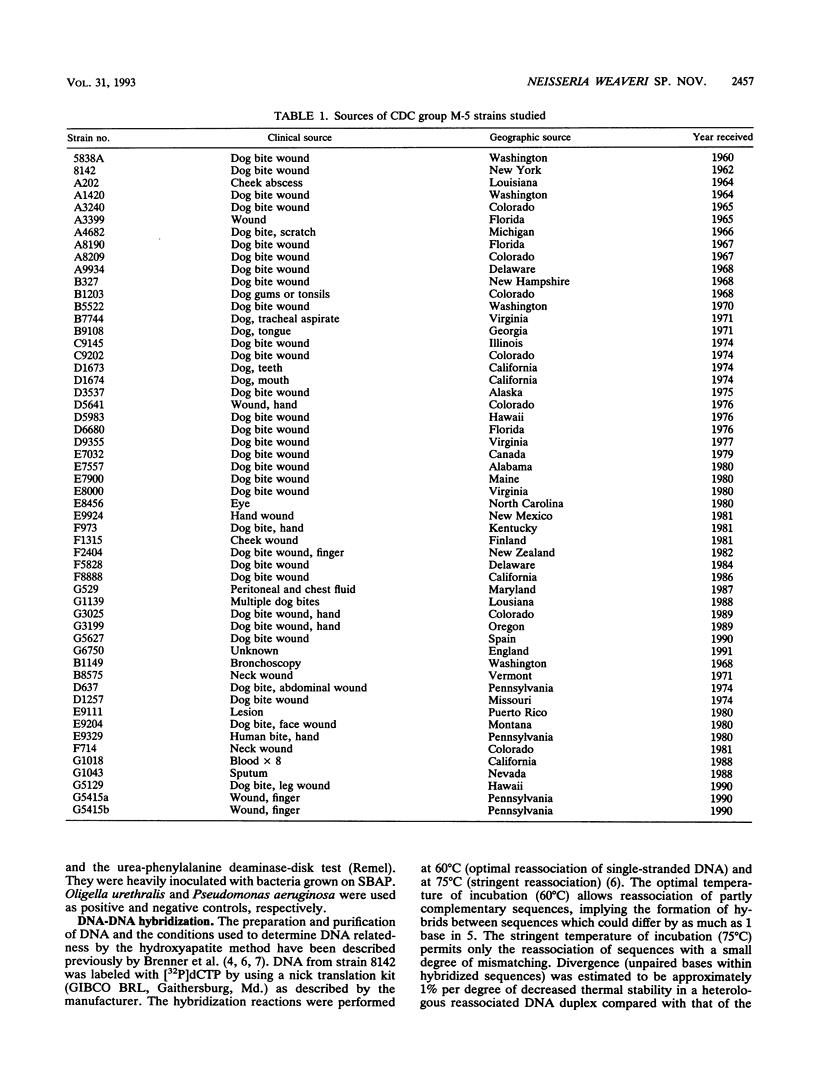
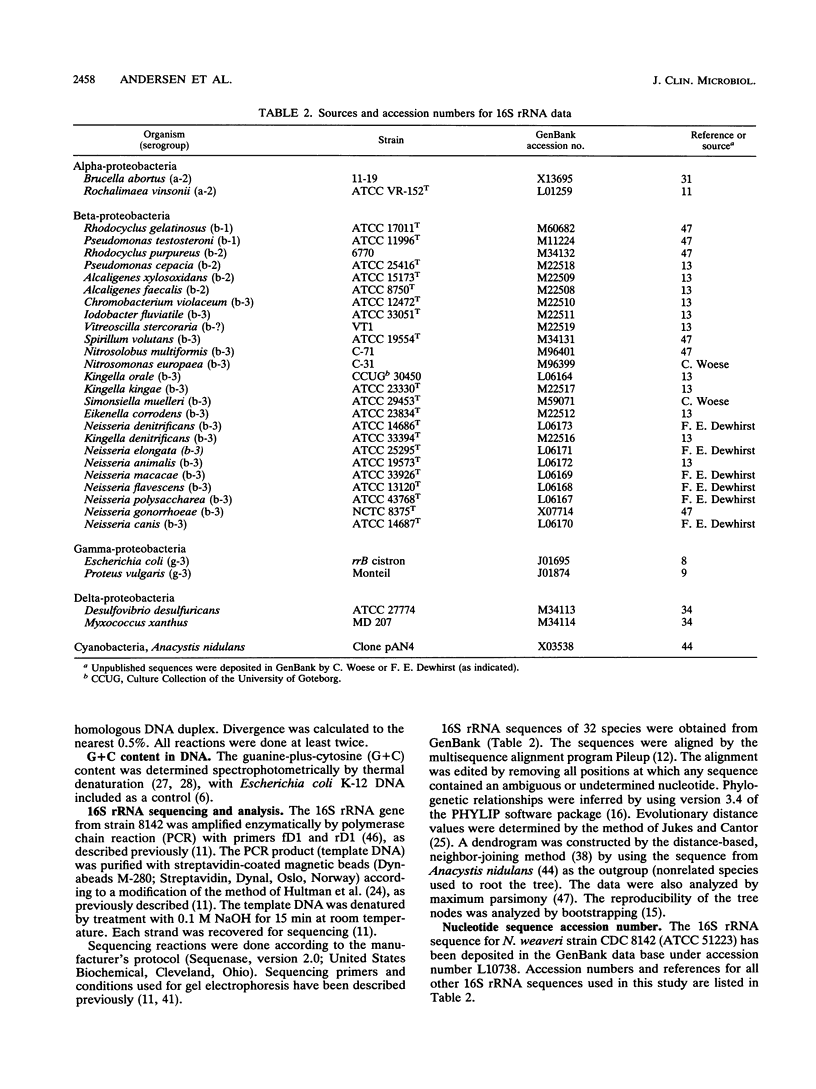
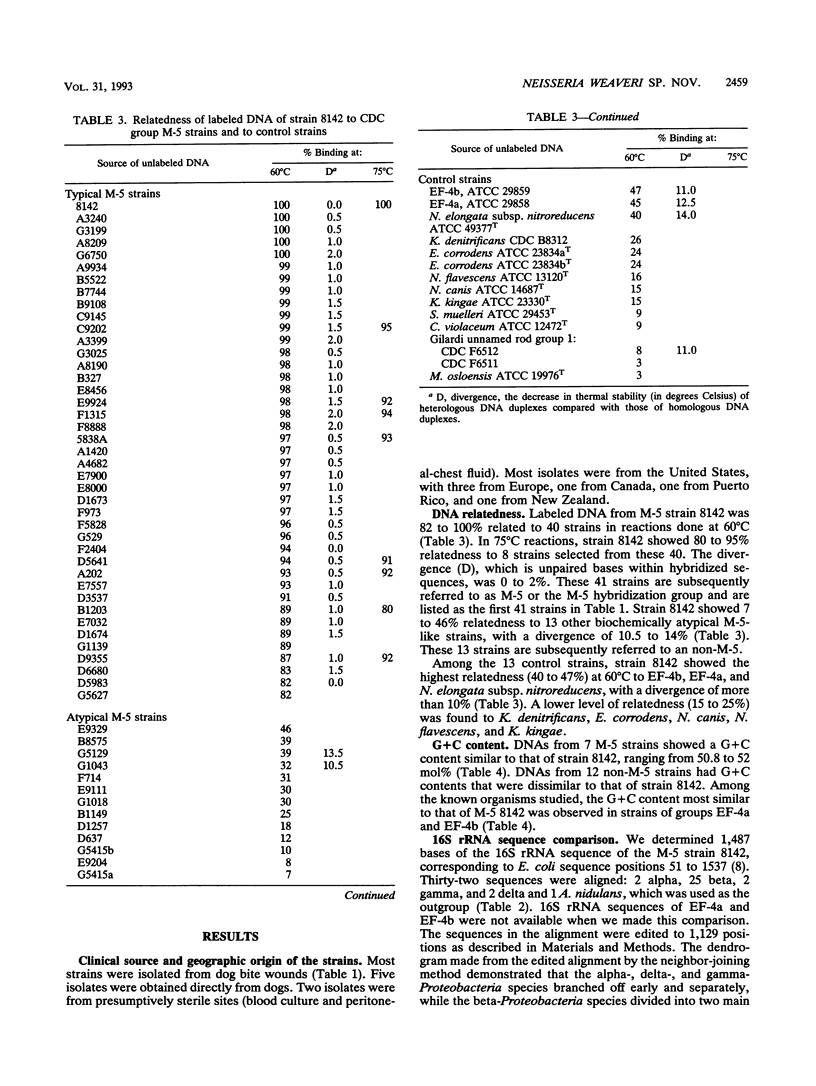
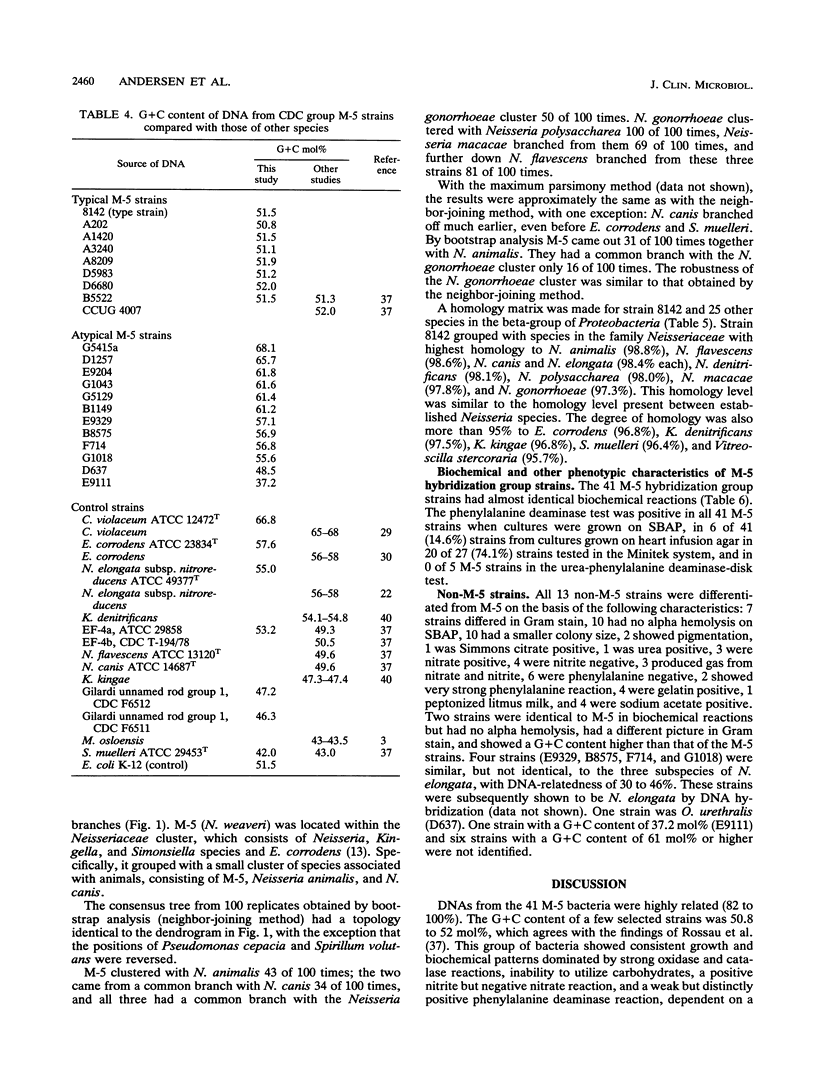
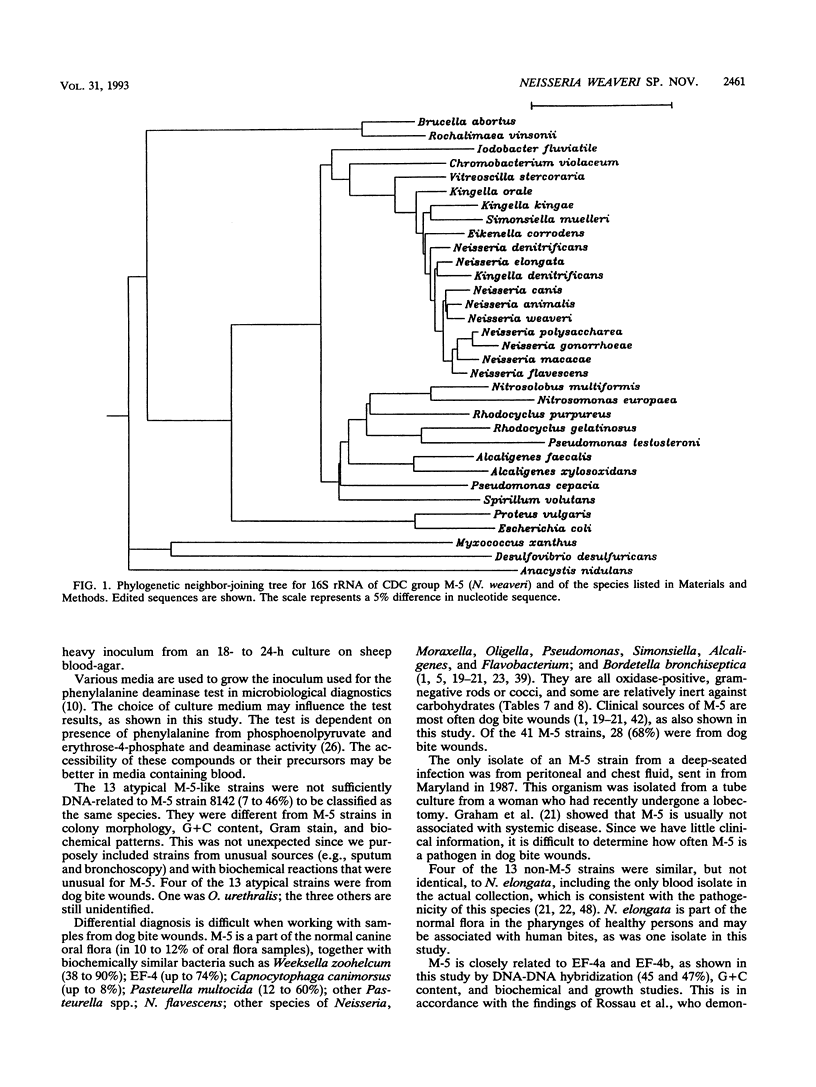
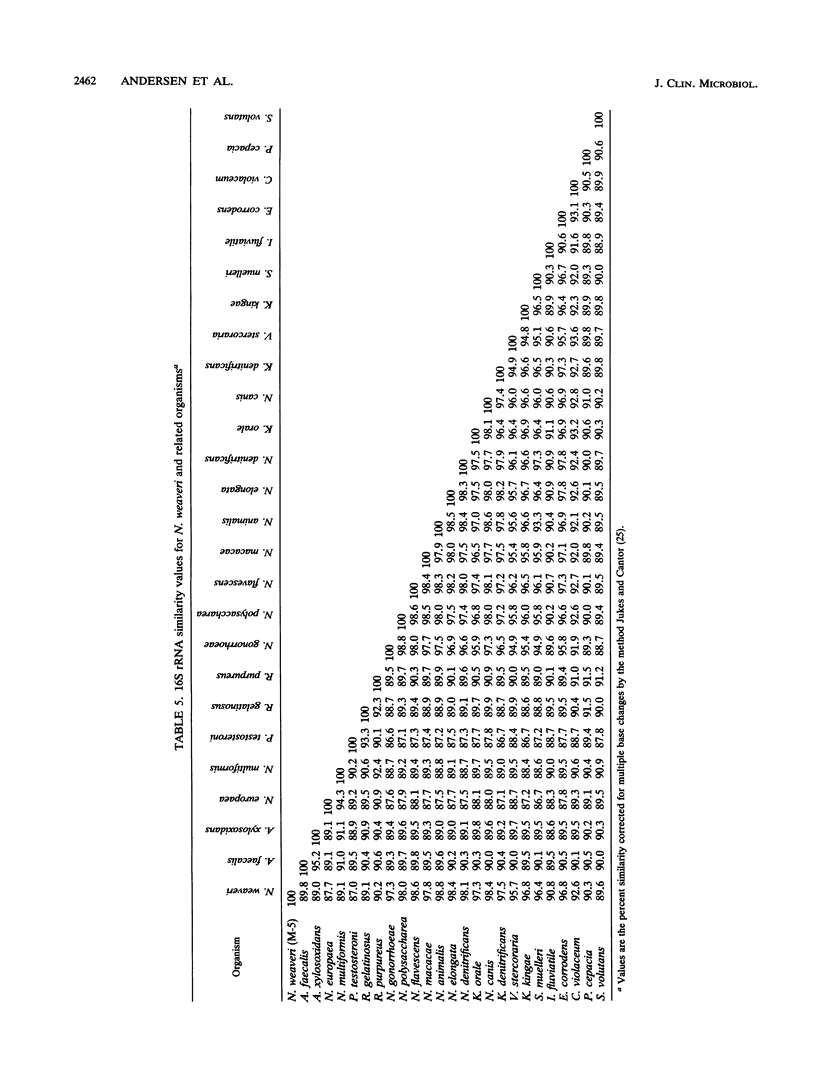
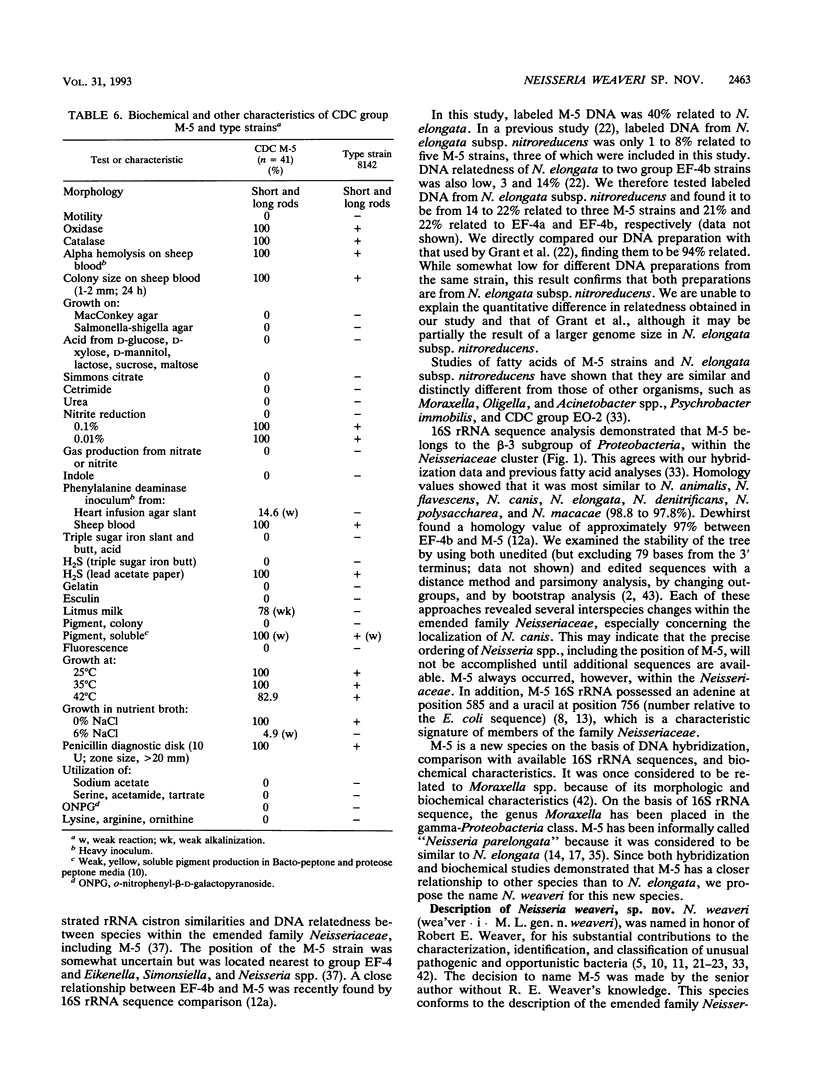
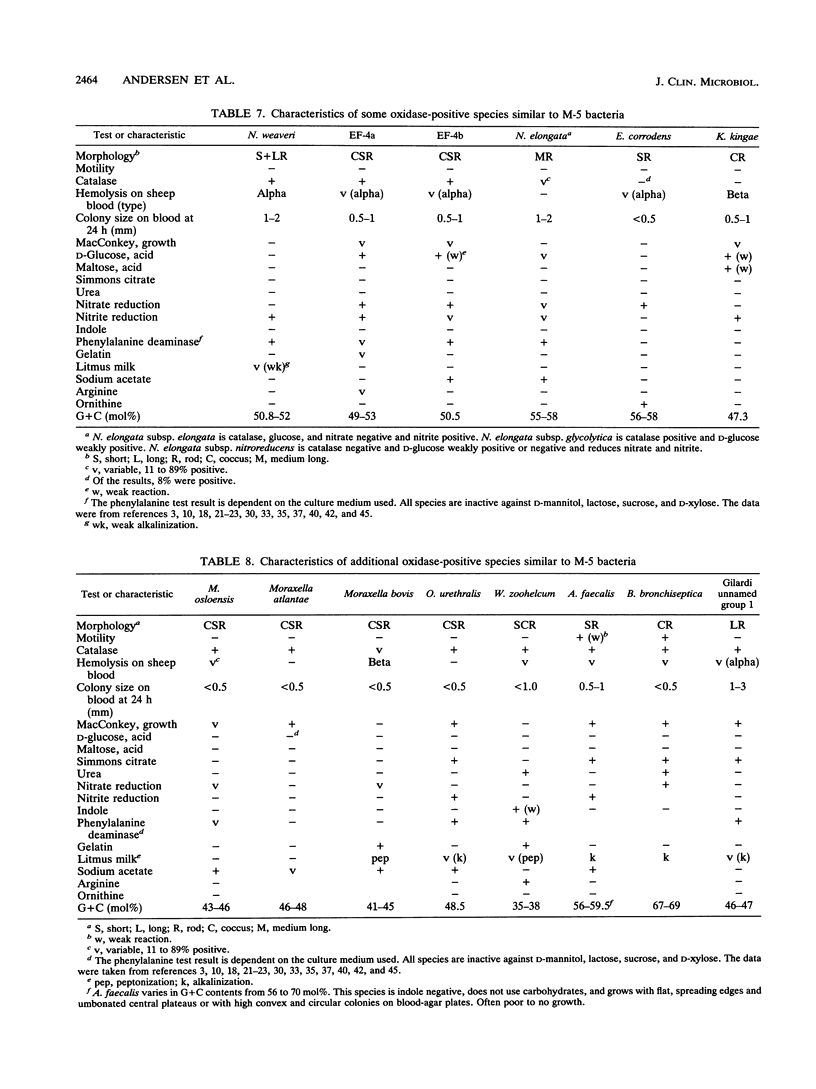
CDC group M-5 is a rod-shaped, gram-negative, nonmotile bacterium associated with dog bite wounds. DNA-DNA relatedness and biochemical and growth characteristics were studied for 54 strains from the collection at the Centers for Disease Control and Prevention. One typical M-5 strain, 8142, was further studied by 16S rRNA sequencing. DNA from 40 of 53 strains showed 82 to 100% relatedness (hydroxyapatite method) to labeled DNA from strain 8142. The guanine-plus-cytosine (G + C) content in 8 of the 41 highly related M-5 strains was 50.5 to 52 mol%. These 41 strains were oxidase and catalase positive, nonfermentative, nitrite positive, nitrate negative, weakly phenylalanine deaminase positive, aerobic, and alpha-hemolytic (sheep blood). DNA from the 13 remaining strains showed only 7 to 46% DNA relatedness to strain 8142. These 13 non-M-5 strains differed from the M-5 strains in G + C content, growth characteristics, and biochemical profiles. DNA from M-5 strain 8142 was most closely related to DNA from groups EF-4b (47%) and EF-4a (45%). 16S rRNA sequence analysis placed M-5 strain 8142 in the Neisseriaceae cluster of the beta-3 subgroup of the class Proteobacteria. It was most homologous (98.4 to 98.8%) to Neisseria animalis, Neisseria flavescens, Neisseria canis, and Neisseria elongata. All data are consistent with M-5 being a new species of Neisseria, for which we propose the name Neisseria weaveri.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailie W. E., Stowe E. C., Schmitt A. M. Aerobic bacterial flora of oral and nasal fluids of canines with reference to bacteria associated with bites. J Clin Microbiol. 1978 Feb;7(2):223–231. doi: 10.1128/jcm.7.2.223-231.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beanland T. J., Howe C. J. The inference of evolutionary trees from molecular data. Comp Biochem Physiol B. 1992 Aug;102(4):643–659. doi: 10.1016/0305-0491(92)90061-u. [DOI] [PubMed] [Google Scholar]
- Brenner D. J., Fanning G. R., Rake A. V., Johnson K. E. Batch procedure for thermal elution of DNA from hydroxyapatite. Anal Biochem. 1969 Apr 4;28(1):447–459. doi: 10.1016/0003-2697(69)90199-7. [DOI] [PubMed] [Google Scholar]
- Brenner D. J., Hollis D. G., Fanning G. R., Weaver R. E. Capnocytophaga canimorsus sp. nov. (formerly CDC group DF-2), a cause of septicemia following dog bite, and C. cynodegmi sp. nov., a cause of localized wound infection following dog bite. J Clin Microbiol. 1989 Feb;27(2):231–235. doi: 10.1128/jcm.27.2.231-235.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner D. J., McWhorter A. C., Knutson J. K., Steigerwalt A. G. Escherichia vulneris: a new species of Enterobacteriaceae associated with human wounds. J Clin Microbiol. 1982 Jun;15(6):1133–1140. doi: 10.1128/jcm.15.6.1133-1140.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosius J., Palmer M. L., Kennedy P. J., Noller H. F. Complete nucleotide sequence of a 16S ribosomal RNA gene from Escherichia coli. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4801–4805. doi: 10.1073/pnas.75.10.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbon P., Ebel J. P., Ehresmann C. The sequence of the ribosomal 16S RNA from Proteus vulgaris. Sequence comparison with E. coli 16S RNA and its use in secondary model building. Nucleic Acids Res. 1981 May 25;9(10):2325–2333. doi: 10.1093/nar/9.10.2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly J. S., Worthington M. G., Brenner D. J., Moss C. W., Hollis D. G., Weyant R. S., Steigerwalt A. G., Weaver R. E., Daneshvar M. I., O'Connor S. P. Rochalimaea elizabethae sp. nov. isolated from a patient with endocarditis. J Clin Microbiol. 1993 Apr;31(4):872–881. doi: 10.1128/jcm.31.4.872-881.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein E. J., Citron D. M., Wield B., Blachman U., Sutter V. L., Miller T. A., Finegold S. M. Bacteriology of human and animal bite wounds. J Clin Microbiol. 1978 Dec;8(6):667–672. doi: 10.1128/jcm.8.6.667-672.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham D. R., Band J. D., Thornsberry C., Hollis D. G., Weaver R. E. Infections caused by Moraxella, Moraxella urethralis, Moraxella-like groups M-5 and M-6, and Kingella kingae in the United States, 1953-1980. Rev Infect Dis. 1990 May-Jun;12(3):423–431. doi: 10.1093/clinids/12.3.423. [DOI] [PubMed] [Google Scholar]
- Grant P. E., Brenner D. J., Steigerwalt A. G., Hollis D. G., Weaver R. E. Neisseria elongata subsp. nitroreducens subsp. nov., formerly CDC group M-6, a gram-negative bacterium associated with endocarditis. J Clin Microbiol. 1990 Dec;28(12):2591–2596. doi: 10.1128/jcm.28.12.2591-2596.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultman T., Ståhl S., Hornes E., Uhlén M. Direct solid phase sequencing of genomic and plasmid DNA using magnetic beads as solid support. Nucleic Acids Res. 1989 Jul 11;17(13):4937–4946. doi: 10.1093/nar/17.13.4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its thermal denaturation temperature. J Mol Biol. 1962 Jul;5:109–118. doi: 10.1016/s0022-2836(62)80066-7. [DOI] [PubMed] [Google Scholar]
- Mandel M., Igambi L., Bergendahl J., Dodson M. L., Jr, Scheltgen E. Correlation of melting temperature and cesium chloride buoyant density of bacterial deoxyribonucleic acid. J Bacteriol. 1970 Feb;101(2):333–338. doi: 10.1128/jb.101.2.333-338.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno E., Stackebrandt E., Dorsch M., Wolters J., Busch M., Mayer H. Brucella abortus 16S rRNA and lipid A reveal a phylogenetic relationship with members of the alpha-2 subdivision of the class Proteobacteria. J Bacteriol. 1990 Jul;172(7):3569–3576. doi: 10.1128/jb.172.7.3569-3576.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss C. W., Daneshvar M. I., Hollis D. G. Biochemical characteristics and fatty acid composition of Gilardi rod group 1 bacteria. J Clin Microbiol. 1993 Mar;31(3):689–691. doi: 10.1128/jcm.31.3.689-691.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rarick H. R., Riley P. S., Martin R. Carbon substrate utilization studies of some cultures of Alcaligenes denitrificans, Alcaligenes faecalis, and Alcaligenes odorans isolated from clinical specimens. J Clin Microbiol. 1978 Sep;8(3):313–319. doi: 10.1128/jcm.8.3.313-319.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou N., Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987 Jul;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Saphir D. A., Carter G. R. Gingival flora of the dog with special reference to bacteria associated with bites. J Clin Microbiol. 1976 Mar;3(3):344–349. doi: 10.1128/jcm.3.3.344-349.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stackebrandt E., Charfreitag O. Partial 16S rRNA primary structure of five Actinomyces species: phylogenetic implications and development of an Actinomyces israelii-specific oligonucleotide probe. J Gen Microbiol. 1990 Jan;136(1):37–43. doi: 10.1099/00221287-136-1-37. [DOI] [PubMed] [Google Scholar]
- Thorne J. L., Kishino H., Felsenstein J. Inching toward reality: an improved likelihood model of sequence evolution. J Mol Evol. 1992 Jan;34(1):3–16. doi: 10.1007/BF00163848. [DOI] [PubMed] [Google Scholar]
- Weisburg W. G., Barns S. M., Pelletier D. A., Lane D. J. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1991 Jan;173(2):697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R. Bacterial evolution. Microbiol Rev. 1987 Jun;51(2):221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J. D., Janda J. M. Association of an important Neisseria species, Neisseria elongata subsp. nitroreducens, with bacteremia, endocarditis, and osteomyelitis. J Clin Microbiol. 1992 Mar;30(3):719–720. doi: 10.1128/jcm.30.3.719-720.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]



