Abstract
Recent advances in immunology have led to exciting new possibilities in medicine. We now understand how to design vaccine capable of resetting the balance between immune effector and regulatory cells in people suffering from autoimmune and allergic diseases. These approaches are effective while avoiding the side effects associated with non-specific immune suppression.
1. The immune system
The immune system has evolved to protect us from infection. Elements of our own immune system can be found in invertebrates. These so called ‘innate’ immune mechanisms include engulfment of microbes, their encapsulation and their lysis by chemicals. Analogous mechanisms, including phagocytosis by neutrophils and macrophages, microbial lysis by lysozyme and complement components and the process of chronic inflammation are found in all vertebrates. Vertebrates face additional microbial challenges when compared with invertebrates and have evolved increasingly sophisticated immune mechanisms. These include a spectacular array of antigen receptors generated by a unique gene rearrangement process. These receptors equip lymphocytes (B and T cells) with the receptors required to respond to virtually any antigen that nature can throw at them. The ‘adaptive’ immune system cooperates with the innate immune system to provide effective protection from infection. However, the evolution of such a diverse and powerful immune system has considerable drawbacks.
2. The challenge of self–non-self discrimination
How does the immune system distinguish between the antigens expressed in its own body (self antigens) from those found in microbes (non-self antigens)? Furthermore, how does the immune system know how to distinguish between infectious agents and otherwise innocuous foreign substances such as food and airborne antigens such as pollen? The immune system uses various devices to ensure that its cells tolerate self-antigens.
Many of the antigens found in the body are expressed in the thymus. Their expression leads to deletion of those T lymphocytes carrying receptors specific for self-antigens. No system is perfect, however, and a small number of potentially self-reactive cells escape deletion in the thymus. This is true for all people whether or not they suffer from an autoimmune disease. Most people remain healthy, however, because there are additional mechanisms that control the activity of self-reactive cells in their lymphoid organs (Fig. 1). Among these mechanisms the function of regulatory T cells is arguably the most important.
Fig. 1.
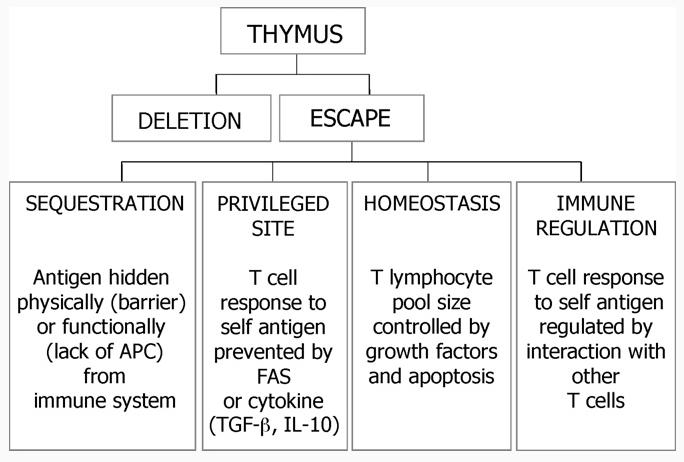
T lymphocytes differentiate in the thymus where the majority of self-reactive cells are deleted. Those cells that escape deletion remain quiescent in peripheral lymphoid organs by various processes including sequestration, privileged sites, homeostasis and immune regulation.
3. Regulatory T cells
An unregulated immune system would face two problems. First, it would respond to infections so violently that the resulting immune pathology would cause irreversible damage to the infected tissue. Second, an unregulated immune system would allow those T cells that have escaped deletion in the thymus to attack the individuals own tissues and cause widespread autoimmune disease. This is just what happens when crucial molecules involved in the function of regulatory T (Treg) cells, such as FoxP3 and CTLA-4, are rendered non-functional through deletion or mutation. An important subset of regulatory cells expresses the high affinity receptor for interleukin 2 (CD25) and the transcriptional regulator FoxP3. Additional subsets of regulatory cells include Tr1 and Th3 cells that depend on the secretion of hormone like substances (cytokines) such as interleukin 10 (IL-10) and transforming growth factor for their regulatory function [1]. Mutations of the FoxP3 gene are associated with a broad range of autoimmune and allergic conditions in both mouse and man. Furthermore, mice deficient in IL-10 suffer from heightened susceptibility to hypersensitivity conditions such as inflammatory bowel disease.
4. Harnessing Treg cells for treatment of autoimmune and allergic conditions
We now appreciate that the immune system of a healthy person represents a fine balance between different cells of the immune system (Fig. 2). On the one hand are cells that protect us from infection but that also have the potential to cause autoimmune and allergic disorders. On the other are cells that regulate immune responses and prevent excessive reactivity. The recent characterization of regulatory T cells has stimulated a drive to discover safe and effective ways to induce these cells. Induction of regulatory T cells is a major goal in immunotherapy since this would confer specificity to treatment and hence override the current use of non-specific drugs. Monoclonal antibodies directed to helper T cells enhance the generation of Treg cells. For example, treatment of the non-obese diabetic mouse with CD3-specific antibodies induces T cell mediated tolerance involving CD25+ cells [1]. Natural CD25+ Treg cells can be expanded in vitro in the presence of high concentrations of IL-2 and a small number of these cells reverse diabetes in the non-obese diabetic mouse. Furthermore, antigen-pulsed dendritic cells induce proliferation of CD25+ cells in vitro. The resulting CD25+ cells are expanded in the presence of antigen and IL-2 and are significantly more potent than unselected CD25+ cells for treatment of experimental diabetes [1].
Fig. 2.
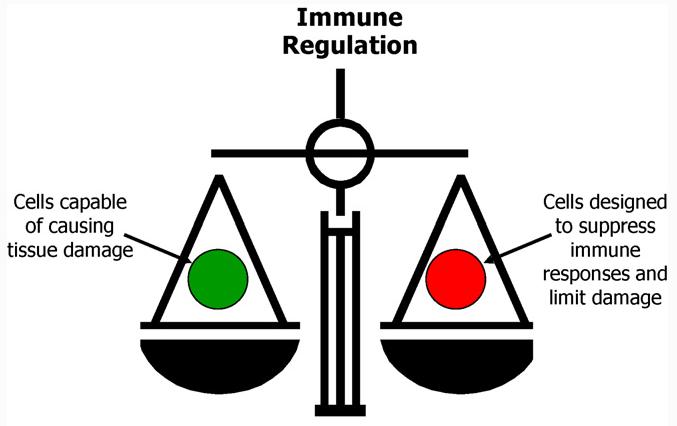
It is now clear that the immune system is a delicate balance between the pathogenic factor/helper T-cells and natural/induced Treg cells.
IL-10 is an immune suppressive cytokine that has direct effects on lymphocytes and APC such as dendritic cells. Repeated stimulation of lymphocytes in vitro in the presence of high amounts of IL-10 leads to the differentiation of T regulatory type 1 (Tr1) cells [2]. These cells secrete IL-10 at high levels and also produce TGF-beta, interferon gamma and interleukin 5. Similarly, repeated antigenic stimulation in the presence of immune suppressive drugs such as vitamin D3 and dexamethasone leads to differentiation of a similar subset of cells with regulatory properties. Can such cells be induced in vivo? Several groups have shown that T cells with a regulatory phenotype can be induced following interaction with antigen-pulsed immature DCs. Wakkach described the generation of Tr1-like T cells following culture with bone-marrow derived DCs cultured in vitro with IL-10. This group also discovered a natural population of DCs with the same phenotype as the IL-10 treated cells; this natural population was also able to induce Tr1 cells in vivo [3]. Lutz revealed that repeated injection of peptide-loaded immature bone-marrow DCs, generated with TNFα, was protective against subsequent induction of autoimmune disease, once again through the generation of IL-10 producing CD4+ T cells [4]. Treatment of DCs with vitamin D3 or its analogues was also found to stop progression of diabetes in mouse models. It becomes clear that presentation of antigen by immature DC leads to the generation of regulatory T cells and that these function through secretion of immune suppressive cytokines. Therefore, it may be possible to develop therapeutic strategies for autoimmune and allergic diseases either by targeting antigen to DC in vivo or by expanding the cells in vitro, pulsing with antigen and injecting them back into patients. Antigens coupled to antibodies specific for dendritic cells have been used to deliver antigens to DC in vivo resulting in antigen specific tolerance [5].
5. Induction of Treg cells with specific antigen
It is now almost a century since the first description of antigen specific immunotherapy (SIT). In 1911 Noon described how hay fever could by treated by inoculation with allergen. This well accepted practice leads to immune deviation and the induction of allergen specific Treg cells. The approach is inherently dangerous since intact antigens can crosslink mast cell bound IgE antibodies leading to anaphylaxis. Recent studies have shown, however, that synthetic peptides, based on the structure of T cell epitopes within antigens, can replace whole antigens for SIT [6]. T cell epitopes are the peptide fragments of antigens that are generated by antigen processing, bind to MHC proteins and hence stimulate responses among antigen-specific T cells. Allergies to bee venom or cat dander have been successfully treated by subcutaneous or intradermal administration of the appropriate T cell epitopes. Injected peptides seek out and bind to immature dendritic cells and this leads to the induction of allergen specific, IL-10 secreting Treg cells. The direct administration of antigenic peptides supersedes the use of antigen-loaded dendritic cells and no longer requires the complex process of dendritic cell selection in vitro.
6. How do therapeutic peptide vaccines work?
The administration of synthetic peptides based on T cell epitopes from self-antigens has proved to be a highly effective means of preventing and treating experimental autoimmune disease in animal models. As yet, however, there is little understanding of the mechanisms involved. Although anergy and deletion have been observed, these mechanisms cannot account for the fact that treatment with a peptide from antigen A can inhibit the immune response to antigen B. This phenomenon of bystander suppression, first described by Weiner and colleagues in oral tolerance [7], has been observed following peptide therapy in experimental autoimmunity [8]. Peptide therapy in a mouse model of CNS autoimmune disease has proved to be IL-10 dependent [9]. Treatment of the Tg4 mouse, bearing a T cell receptor specific for the immunodominant peptide from myelin basic protein, with soluble peptide similarly renders the mice resistant to EAE in an IL-10 dependent manner [10]. Subsequent studies revealed that repetitive antigen administration led to the induction of IL-10 secreting regulatory cells capable of bystander suppression in vivo [11]. These potent suppressor cells are derived from naïve T cells but fail to express FoxP3 [12,13]. The induced regulatory cells have properties consistent with Th1reg cells and express Tbet and EGR-2 at high levels [14]. Th1reg cells arise during chronic infections and are believed to dampen the immune response and hence prevent immune pathology [15].
7. Designing peptides for therapy
Peptides for immunotherapy can be selected by various approaches. Epitopes can be predicted using sophisticated algorithms. Alternatively epitopes may be eluted from the MHC proteins of antigen-fed APC [16]. While this identifies the naturally processed epitopes from an antigen it does not guarantee that these will serve as effective peptides for SIT. Eluted peptides may bind inappropriately to MHC proteins when added back to APC [17]. As a result, the peptides fail to engage T cells specific for the naturally processed antigen and are in effect ‘cryptic’ epitopes. Work from our own laboratory has shown that peptides must mimic the naturally processed epitope to serve as tolerogens in autoimmune disease. We propose that repetitive antigen encounter following peptide administration mimics the effect of chronic infection and that the induction of peptide induced regulatory T cells will prove to be an effective means of preventing and treating autoimmune disease in man. A cocktail of four myelin-derived peptides is currently being tested in a phase IIa trial in multiple sclerosis. Peptide therapy of autoimmune and allergic diseases will greatly improve the safety of SIT while reducing the reliance of patients on non-specific immune suppressive drugs.
References
- 1.Wraith DC, Nicolson KS, Whitley NT. Regulatory CD4+ T cells and the control of autoimmune disease. Curr Opin Immunol. 2004;16:695–701. doi: 10.1016/j.coi.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 2.O'Garra A, Vieira PL, Vieira P, Goldfeld AE. IL-10-producing and naturally occurring CD4+ Tregs: limiting collateral damage. J Clin Invest. 2004;114:1372–8. doi: 10.1172/JCI23215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wakkach A, Fournier N, Brun V, Breittmayer JP, Cottrez F, Groux H. Characterization of dendritic cells that induce tolerance and T regulatory 1 cell differentiation in vivo. Immunity. 2003;18:605–17. doi: 10.1016/s1074-7613(03)00113-4. [DOI] [PubMed] [Google Scholar]
- 4.Menges M, Rossner S, Voigtlander C, Schindler H, Kukutsch NA, Bogdan C, et al. Repetitive injections of dendritic cells matured with tumor necrosis factor α induce antigen-specific protection of mice from autoimmunity. J Exp Med. 2002;195:15–21. doi: 10.1084/jem.20011341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 6.Larche M, Wraith DC. Peptide-based therapeutic vaccines for allergic and autoimmune diseases. Nat Med. 2005;11:S69–76. doi: 10.1038/nm1226. [DOI] [PubMed] [Google Scholar]
- 7.Faria AM, Weiner HL. Oral tolerance: mechanisms and therapeutic applications. Adv Immunol. 1999;73:153–264. doi: 10.1016/s0065-2776(08)60787-7. [DOI] [PubMed] [Google Scholar]
- 8.Anderton SM, Wraith DC. Hierarchy in the ability of T cell epitopes to induce peripheral tolerance to antigens from myelin. Eur J Immunol. 1998;28:1251–61. doi: 10.1002/(SICI)1521-4141(199804)28:04<1251::AID-IMMU1251>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 9.O'Neill EJ, Day MJ, Wraith DC. IL-10 is essential for disease protection following intranasal peptide administration in the C57BL/6 model of EAE. J Neuroimmunol. 2006;178:1–8. doi: 10.1016/j.jneuroim.2006.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burkhart C, Liu GY, Anderton SM, Metzler B, Wraith DC. Peptide-induced T cell regulation of experimental autoimmune encephalomyelitis: a role for IL-10. Int Immunol. 1999;11:1625–34. doi: 10.1093/intimm/11.10.1625. [DOI] [PubMed] [Google Scholar]
- 11.Sundstedt A, O'Neill EJ, Nicolson KS, Wraith DC. Role for IL-10 in suppression mediated by peptide-induced regulatory T cells in vivo. J Immunol. 2003;170:1240–8. doi: 10.4049/jimmunol.170.3.1240. [DOI] [PubMed] [Google Scholar]
- 12.Vieira PL, Christensen JR, Minaee S, O'Neill EJ, Barrat FJ, Boonstra A, et al. IL-10-secreting regulatory T cells do not express Foxp3 but have comparable regulatory function to naturally occurring CD4+ CD25+ regulatory T cells. J Immunol. 2004;172:5986–93. doi: 10.4049/jimmunol.172.10.5986. [DOI] [PubMed] [Google Scholar]
- 13.Nicolson KS, O'Neill EJ, Sundstedt A, Streeter HB, Minaee S, Wraith DC. Antigen-induced IL-10+ regulatory T cells are independent of CD25+ regulatory cells for their growth, differentiation, and function. J Immunol. 2006;176:5329–37. doi: 10.4049/jimmunol.176.9.5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson PO, Manzo BA, Sundstedt A, Minaee S, Symonds A, Khalid S, et al. Persistent antigenic stimulation alters the transcription program in T cells, resulting in antigen-specific tolerance. Eur J Immunol. 2006;36:1374–85. doi: 10.1002/eji.200635883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trinchieri G. Interleukin-10 production by effector T cells: Th1 cells show self control. J Exp Med. 2007;204:239–43. doi: 10.1084/jem.20070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peakman M, Stevens EJ, Lohmann T, Narendran P, Dromey J, Alexander A, et al. Naturally processed and presented epitopes of the islet cell autoantigen IA-2 eluted from HLA-DR4. J Clin Invest. 1999;104:1449–57. doi: 10.1172/JCI7936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Viner NJ, Nelson CA, Deck B, Unanue ER. Complexes generated by the binding of free peptides to class II MHC molecules are antigenically diverse compared with those generated by intracellular processing. J Immunol. 1996;156:2365–8. [PubMed] [Google Scholar]


