Abstract
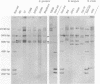
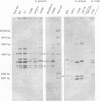
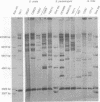
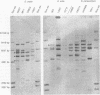
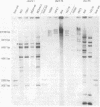
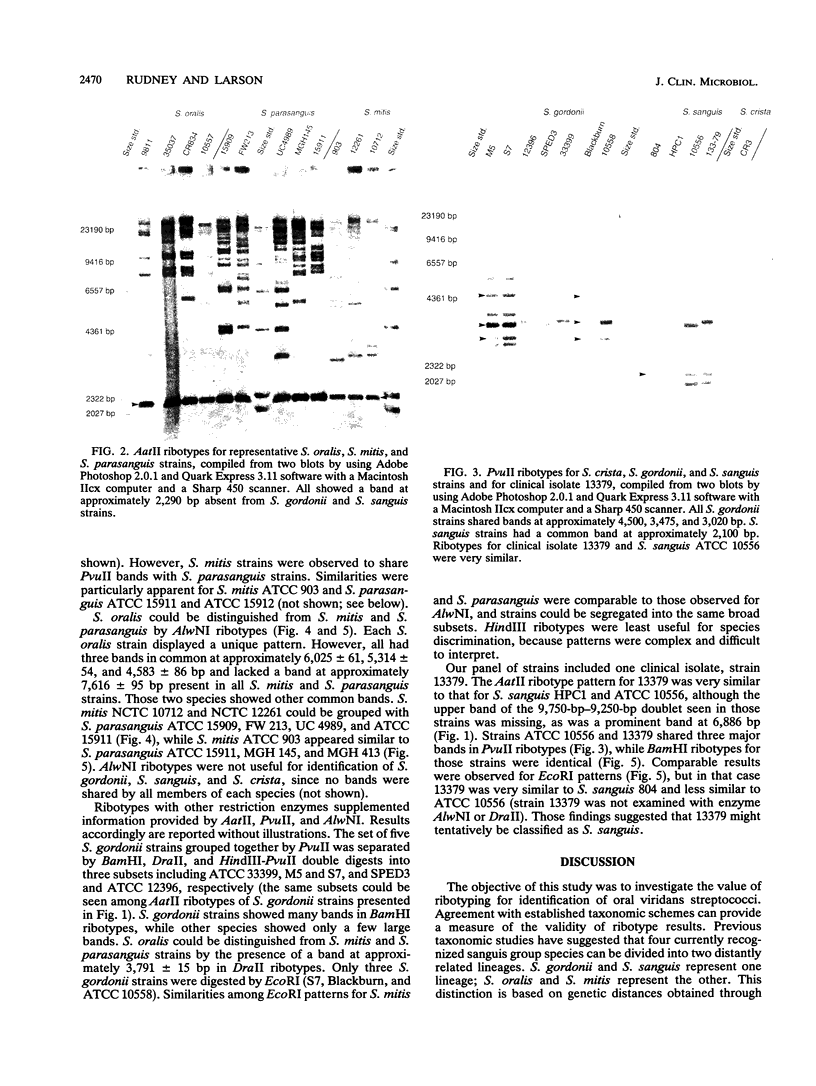
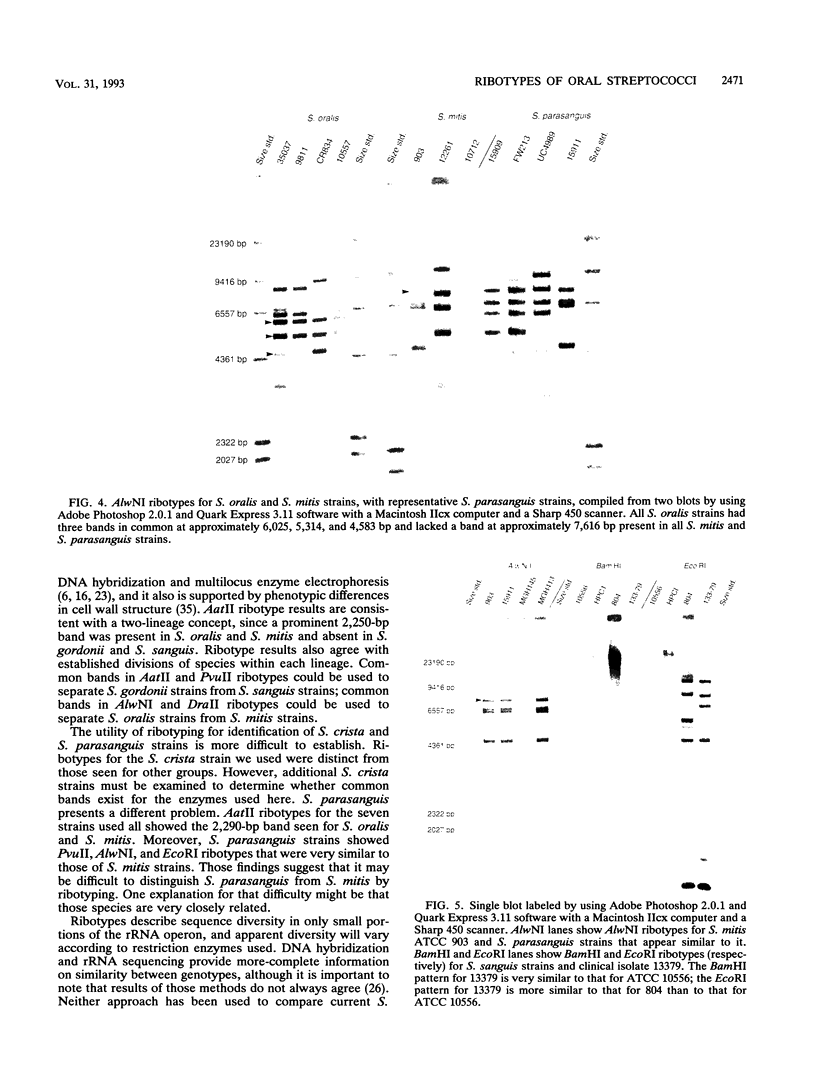
Oral streptococci formerly classified as Streptococcus sanguis have been divided into six genetic groups. Methods to identify those species by genotype are needed. This study compared restriction fragment polymorphisms of rRNA genes (ribotypes) for seven S. gordonii, three S. sanguis, four S. oralis, three S. mitis, one S. crista, and seven S. parasanguis strains classified in previous DNA hybridization studies, as well as one clinical isolate. DNA was digested with HindIII, PvuII, HindIII and PvuII combined, EcoRI, BamHI, AatII, AlwNI, and DraII. DNA fragments were hybridized with a digoxigenin-labeled cDNA probe obtained by reverse transcription of Escherichia coli 16S and 23S rRNA. S. oralis, S. mitis, and S. parasanguis all showed an isolated 2,290-bp band in AatII ribotypes that was absent from S. gordonii, S. sanguis, and S. crista. The last three groups showed species-specific bands with AatII and also with PvuII. S. oralis could be distinguished from S. mitis and S. parasanguis in AlwNI and DraII ribotypes. S. mitis and S. parasanguis could not be distinguished, since they shared multiple bands in PvuII, AlwNI, and EcoRI patterns. The clinical isolate in the panel was very similar to S. sanguis by all enzymes used. Our findings suggest that ribotyping may be useful for genotypic identification of oral viridans streptococci. Initial digests of clinical isolates might be made with AatII, followed by PvuII or AlwNI. Isolates then could be identified by comparing ribotype patterns with those of reference strains. This approach could facilitate clinical studies of these newly defined species.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baloga A. O., Harlander S. K. Comparison of methods for discrimination between strains of Listeria monocytogenes from epidemiological surveys. Appl Environ Microbiol. 1991 Aug;57(8):2324–2331. doi: 10.1128/aem.57.8.2324-2331.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beighton D., Hardie J. M., Whiley R. A. A scheme for the identification of viridans streptococci. J Med Microbiol. 1991 Dec;35(6):367–372. doi: 10.1099/00222615-35-6-367. [DOI] [PubMed] [Google Scholar]
- Bingen E. H., Denamur E., Lambert-Zechovsky N. Y., Elion J. Evidence for the genetic unrelatedness of nosocomial vancomycin-resistant Enterococcus faecium strains in a pediatric hospital. J Clin Microbiol. 1991 Sep;29(9):1888–1892. doi: 10.1128/jcm.29.9.1888-1892.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg H. M., Rimland D., Kiehlbauch J. A., Terry P. M., Wachsmuth I. K. Epidemiologic typing of Staphylococcus aureus by DNA restriction fragment length polymorphisms of rRNA genes: elucidation of the clonal nature of a group of bacteriophage-nontypeable, ciprofloxacin-resistant, methicillin-susceptible S. aureus isolates. J Clin Microbiol. 1992 Feb;30(2):362–369. doi: 10.1128/jcm.30.2.362-369.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook P. R. A general method for preparing intact nuclear DNA. EMBO J. 1984 Aug;3(8):1837–1842. doi: 10.1002/j.1460-2075.1984.tb02056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coykendall A. L. Classification and identification of the viridans streptococci. Clin Microbiol Rev. 1989 Jul;2(3):315–328. doi: 10.1128/cmr.2.3.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coykendall A. L., Specht P. A. DNA base sequence homologies among strains of Streptococcus sanguis. J Gen Microbiol. 1975 Nov;91(1):92–98. doi: 10.1099/00221287-91-1-92. [DOI] [PubMed] [Google Scholar]
- De Buyser M. L., Morvan A., Grimont F., el Solh N. Characterization of Staphylococcus species by ribosomal RNA gene restriction patterns. J Gen Microbiol. 1989 Apr;135(4):989–999. doi: 10.1099/00221287-135-4-989. [DOI] [PubMed] [Google Scholar]
- Demuth D. R., Golub E. E., Malamud D. Streptococcal-host interactions. Structural and functional analysis of a Streptococcus sanguis receptor for a human salivary glycoprotein. J Biol Chem. 1990 May 5;265(13):7120–7126. [PubMed] [Google Scholar]
- Douglas C. W., Pease A. A., Whiley R. A. Amylase-binding as a discriminator among oral streptococci. FEMS Microbiol Lett. 1990 Jan 1;54(1-3):193–197. doi: 10.1016/0378-1097(90)90281-t. [DOI] [PubMed] [Google Scholar]
- Dowson C. G., Hutchison A., Woodford N., Johnson A. P., George R. C., Spratt B. G. Penicillin-resistant viridans streptococci have obtained altered penicillin-binding protein genes from penicillin-resistant strains of Streptococcus pneumoniae. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5858–5862. doi: 10.1073/pnas.87.15.5858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenno J. C., LeBlanc D. J., Fives-Taylor P. Nucleotide sequence analysis of a type 1 fimbrial gene of Streptococcus sanguis FW213. Infect Immun. 1989 Nov;57(11):3527–3533. doi: 10.1128/iai.57.11.3527-3533.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frandsen E. V., Pedrazzoli V., Kilian M. Ecology of viridans streptococci in the oral cavity and pharynx. Oral Microbiol Immunol. 1991 Jun;6(3):129–133. doi: 10.1111/j.1399-302x.1991.tb00466.x. [DOI] [PubMed] [Google Scholar]
- Ganeshkumar N., Hannam P. M., Kolenbrander P. E., McBride B. C. Nucleotide sequence of a gene coding for a saliva-binding protein (SsaB) from Streptococcus sanguis 12 and possible role of the protein in coaggregation with actinomyces. Infect Immun. 1991 Mar;59(3):1093–1099. doi: 10.1128/iai.59.3.1093-1099.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour M. N., Whittam T. S., Kilian M., Selander R. K. Genetic relationships among the oral streptococci. J Bacteriol. 1987 Nov;169(11):5247–5257. doi: 10.1128/jb.169.11.5247-5257.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimont F., Lefèvre M., Ageron E., Grimont P. A. rRNA gene restriction patterns of Legionella species: a molecular identification system. Res Microbiol. 1989 Nov-Dec;140(9):615–626. doi: 10.1016/0923-2508(89)90193-9. [DOI] [PubMed] [Google Scholar]
- Hall L. M., Duke B., Guiney M., Williams R. Typing of Enterococcus species by DNA restriction fragment analysis. J Clin Microbiol. 1992 Apr;30(4):915–919. doi: 10.1128/jcm.30.4.915-919.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handley P., Coykendall A., Beighton D., Hardie J. M., Whiley R. A. Streptococcus crista sp. nov., a viridans streptococcus with tufted fibrils, isolated from the human oral cavity and throat. Int J Syst Bacteriol. 1991 Oct;41(4):543–547. doi: 10.1099/00207713-41-4-543. [DOI] [PubMed] [Google Scholar]
- Höpfl P., Ludwig W., Schleifer K. H., Larsen N. The 23S ribosomal RNA higher-order structure of Pseudomonas cepacia and other prokaryotes. Eur J Biochem. 1989 Nov 6;185(2):355–364. doi: 10.1111/j.1432-1033.1989.tb15123.x. [DOI] [PubMed] [Google Scholar]
- Kiehlbauch J. A., Plikaytis B. D., Swaminathan B., Cameron D. N., Wachsmuth I. K. Restriction fragment length polymorphisms in the ribosomal genes for species identification and subtyping of aerotolerant Campylobacter species. J Clin Microbiol. 1991 Aug;29(8):1670–1676. doi: 10.1128/jcm.29.8.1670-1676.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian M., Reinholdt J., Nyvad B., Frandsen E. V., Mikkelsen L. IgA1 proteases of oral streptococci: ecological aspects. Immunol Invest. 1989 Jan-May;18(1-4):161–170. doi: 10.3109/08820138909112235. [DOI] [PubMed] [Google Scholar]
- Lane D. J., Pace B., Olsen G. J., Stahl D. A., Sogin M. L., Pace N. R. Rapid determination of 16S ribosomal RNA sequences for phylogenetic analyses. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6955–6959. doi: 10.1073/pnas.82.20.6955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Murcia A. J., Benlloch S., Collins M. D. Phylogenetic interrelationships of members of the genera Aeromonas and Plesiomonas as determined by 16S ribosomal DNA sequencing: lack of congruence with results of DNA-DNA hybridizations. Int J Syst Bacteriol. 1992 Jul;42(3):412–421. doi: 10.1099/00207713-42-3-412. [DOI] [PubMed] [Google Scholar]
- Moureau P., Derclaye I., Gregoire D., Janssen M., Cornelis G. R. Campylobacter species identification based on polymorphism of DNA encoding rRNA. J Clin Microbiol. 1989 Jul;27(7):1514–1517. doi: 10.1128/jcm.27.7.1514-1517.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyvad B., Kilian M. Comparison of the initial streptococcal microflora on dental enamel in caries-active and in caries-inactive individuals. Caries Res. 1990;24(4):267–272. doi: 10.1159/000261281. [DOI] [PubMed] [Google Scholar]
- Nyvad B., Kilian M. Microbiology of the early colonization of human enamel and root surfaces in vivo. Scand J Dent Res. 1987 Oct;95(5):369–380. doi: 10.1111/j.1600-0722.1987.tb01627.x. [DOI] [PubMed] [Google Scholar]
- Pitcher D., Johnson A., Allerberger F., Woodford N., George R. An investigation of nosocomial infection with Corynebacterium jeikeium in surgical patients using a ribosomal RNA gene probe. Eur J Clin Microbiol Infect Dis. 1990 Sep;9(9):643–648. doi: 10.1007/BF01964264. [DOI] [PubMed] [Google Scholar]
- Pérolat P., Grimont F., Regnault B., Grimont P. A., Fournié E., Thevenet H., Baranton G. rRNA gene restriction patterns of Leptospira: a molecular typing system. Res Microbiol. 1990 Feb;141(2):159–171. doi: 10.1016/0923-2508(90)90025-l. [DOI] [PubMed] [Google Scholar]
- Rudney J. D., Krig M. A., Neuvar E. K., Soberay A. H., Iverson L. Antimicrobial proteins in human unstimulated whole saliva in relation to each other, and to measures of health status, dental plaque accumulation and composition. Arch Oral Biol. 1991;36(7):497–506. doi: 10.1016/0003-9969(91)90142-h. [DOI] [PubMed] [Google Scholar]
- Rudney J. D., Neuvar E. K., Soberay A. H. Restriction endonuclease-fragment polymorphisms of oral viridans streptococci, compared by conventional and field-inversion gel electrophoresis. J Dent Res. 1992 May;71(5):1182–1188. doi: 10.1177/00220345920710051001. [DOI] [PubMed] [Google Scholar]
- Smith C. J., Callihan D. R. Analysis of rRNA restriction fragment length polymorphisms from Bacteroides spp. and Bacteroides fragilis isolates associated with diarrhea in humans and animals. J Clin Microbiol. 1992 Apr;30(4):806–812. doi: 10.1128/jcm.30.4.806-812.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snipes K. P., Hirsh D. C., Kasten R. W., Hansen L. M., Hird D. W., Carpenter T. E., McCapes R. H. Use of an rRNA probe and restriction endonuclease analysis to fingerprint Pasteurella multocida isolated from turkeys and wildlife. J Clin Microbiol. 1989 Aug;27(8):1847–1853. doi: 10.1128/jcm.27.8.1847-1853.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stull T. L., LiPuma J. J., Edlind T. D. A broad-spectrum probe for molecular epidemiology of bacteria: ribosomal RNA. J Infect Dis. 1988 Feb;157(2):280–286. doi: 10.1093/infdis/157.2.280. [DOI] [PubMed] [Google Scholar]
- Tardif G., Sulavik M. C., Jones G. W., Clewell D. B. Spontaneous switching of the sucrose-promoted colony phenotype in Streptococcus sanguis. Infect Immun. 1989 Dec;57(12):3945–3948. doi: 10.1128/iai.57.12.3945-3948.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tudor J. J., Marri L., Piggot P. J., Daneo-Moore L. Size of the Streptococcus mutans GS-5 chromosome as determined by pulsed-field gel electrophoresis. Infect Immun. 1990 Mar;58(3):838–840. doi: 10.1128/iai.58.3.838-840.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiley R. A., Fraser H. Y., Douglas C. W., Hardie J. M., Williams A. M., Collins M. D. Streptococcus parasanguis sp. nov., an atypical viridans Streptococcus from human clinical specimens. FEMS Microbiol Lett. 1990 Mar 1;56(1-2):115–121. doi: 10.1111/j.1574-6968.1990.tb04133.x. [DOI] [PubMed] [Google Scholar]
- Woods T. C., Helsel L. O., Swaminathan B., Bibb W. F., Pinner R. W., Gellin B. G., Collin S. F., Waterman S. H., Reeves M. W., Brenner D. J. Characterization of Neisseria meningitidis serogroup C by multilocus enzyme electrophoresis and ribosomal DNA restriction profiles (ribotyping). J Clin Microbiol. 1992 Jan;30(1):132–137. doi: 10.1128/jcm.30.1.132-137.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]