Abstract
Background
Clinical characterization of bladder carcinomas is still inadequate using the standard clinico-pathological prognostic markers. We assessed the correlation between nm23-H1, Rb, EGFR and p53 in relation to the clinical outcome of patients with muscle invasive bilharzial bladder cancer (MI-BBC).
Methods
nm23-H1, Rb, EGFR and p53 expression was assessed in 59 MI-BBC patients using immunohistochemistry and reverse transcription (RT-PCR) and was correlated to the standard clinico-pathological prognostic factors, patient's outcome and the overall survival (OS) rate.
Results
Overexpression of EGFR and p53 proteins was detected in 66.1% and 35.6%; respectively. Loss of nm23-H1and Rb proteins was detected in 42.4% and 57.6%; respectively. Increased EGFR and loss of nm23-H1 RNA were detected in 61.5% and 36.5%; respectively. There was a statistically significant correlation between p53 and EGFR overexpression (p < 0.0001), nm23 loss (protein and RNA), lymph node status (p < 0.0001); between the incidence of local recurrence and EGFR RNA overexpression (p= 0.003) as well as between the incidence of metastasis and altered Rb expression (p = 0.026), p53 overexpression (p < 0.0001) and mutation (p = 0.04). Advanced disease stage correlated significantly with increased EGFR (protein and RNA) (p = 0.003 & 0.01), reduced nm23-H1 RNA (p = 0.02), altered Rb (p = 0.023), and p53 overexpression (p = 0.004). OS rates correlated significantly, in univariate analysis, with p53 overexpression (p = 0.011), increased EGFR (protein and RNA, p = 0.034&0.031), nm23-H1 RNA loss (p = 0.021) and aberrations of ≥ 2 genes. However, multivariate analysis showed that only high EGFR overexpression, metastatic recurrence, high tumor grade and the combination of ≥ 2 affected markers were independent prognostic factors.
Conclusion
nm23-H1, EGFR and p53 could be used as prognostic biomarkers in MI-BBC patients. In addition to the standard pathological prognostic factors, a combination of these markers (≥ 2) has synergistic effects in stratifying patients into variable risk groups. The higher is the number of altered biomarkers, the higher will be the risk of disease progression and death.
Background
In Egypt, Schistosoma-associated bladder cancer represents the commonest malignancy in all diagnosed cancer cases according to the registry of the National Cancer Institute, Cairo [1]. To date, several studies have attempted to identify the spectrum of genetic changes that occurs during urothelial transformation of bilharzial bladder cancer (BBC) and to elucidate in detail the natural history of tumors with different clinical outcome. A wealth of information about the molecular pathogenesis of BBC has emerged, including cytogenetic and molecular genetic analysis via comparative studies on schistosoma- and non-schistosoma- associated bladder cancers which demonstrate different clinicopathologic features, pathogenetic mechanisms and a unique genetic make-up. However, the clinical significance of these defects either singular or in combination, is still not clear [2-4].
Some of these studies documented a significant reduction in disease free survival (DFS) for p53 positive tumors in BBC and transitional cell carcinoma (TCC) of the western countries [3,5] Similarly, absence of Rb protein was found more frequently in tumors with high grade and stage and was clearly associated with poor clinical outcome [6-9] However, the examination of data for single markers is not sufficient to direct clinical decisions for individual patients [4].
The nm23-H1 gene (NME1), localized on chromosome 17q21.3 was first isolated as a metastasis suppressor gene by differential screening of cDNA library from high and low metastatic clones of a murine melanoma cell line [10]. The clinical relevance of the nm23-H1 as a metastasis suppressor for human cancers including bladder is still controversial. Some studies demonstrated that nm23-H1 is inversely correlated with tumor staging, histological differentiation and clinical outcome [11,12], others showed a positive relationship to histological grading and muscle invasion [13].
Epidermal growth factor receptor (EGFR) is a member of the tyrosine kinase receptor family, a group of receptors which are all encoded by the c-erbB oncogenes. It is situated on chromosome 7 and is a 170 kDa protein that consists of three distinct structural parts. It has been proved that epidermal growth factor signaling plays a pivotal role in tumorigenesis and disease progression. Overexpression of EGFR leads to uncontrolled cell proliferation, increased angiogenesis and reduced apoptosis, processes necessary for continuing malignant growth [14]. The role of EGFR in urothelial tumors was supported by the observation that 40%–60% of human bladder tumors overexpress EGFR mRNA and protein [15]. In addition, some studies showed a strong correlation between EGFR positivity and high grade, late stage, tumor progression and poor clinical outcome in the classic TCC of the bladder [16-18].
The present study was conducted to assess the prognostic impact of altered expression of nm23-H1, EGFR,Rb and p53 gene status either singular or in combination in Egyptian cases of muscle invasive-BBC (MI-BBC). Aberrations involving these markers will be correlated to the standard prognostic factors of bladder cancer, patients' response to treatment, and overall survival (OS).
Methods
Patients and tissue samples
The study included 59 cystectomy specimens obtained from operable cases of MI-BBC that were diagnosed and treated at the National Cancer Institute (NCI), Cairo University during the years 1996–1999. From each case, a section was obtained from the paraffin block of the tumor, stained with hematoxylin and eosin and examined under microscope by two independent pathologists (MNM & BAA) to confirm the diagnosis, typing and grading of the tumor as well as to calculate tumor: normal ratio. Only cases in which the neoplastic cells constituted ≥ 75% were included in the study. Also included are 15 normal urothelial tissue samples which were used as a control. These were obtained from morphologically normal areas as far away as possible from tumors not exceeding 1.5 cm in diameter and were confirmed to be normal by microscopic examination of hematoxylin and eosin- stained slides.
None of the cases have received neo-adjuvant or adjuvant therapy prior to surgery. All Clinico-pathological data of the patients including patients' age, sex, tumor type, grade and stage, lymph node status, local recurrence, distant metastasis and overall survival (OS) rates were retrospectively collected from the pathology reports and clinical files of the patients. All studied tumors were graded according to The World Health Organization (WHO) classification and staged according to the TNM pathological staging system [19]. Patients were followed up for local recurrence (LR), distant metastasis (DM) and overall survival (OS). The follow-up period ranged from 1–94 months with a median of 19 months. The ethical committee of the NCI approved the protocol which was in accordance with the ethical guidelines of the 1975 Declaration of Helsinki.
From each case (tumor and normal), 4 um thick sections (4) were cut onto positive-charged slides for immunohistochemistry and three 12 um thick sections were cut into Eppendorf tubes for RNA extraction.
Immunohistochemistry
Sections were deparaffinized in xylene, hydrated through a series of graded alcohol, washed in distilled water and 0.01 PBS (pH 7.4), immersed in 10 mmol/L citrate buffer (pH 6.0) and put in a microwave for 5 min at 60°C for antigen retrieval. Then they were placed in methanol containing 3% H2O2 for 30 min at 4°C to block endogenous peroxidase activity and incubated with rabbit serum for 10 min to block non-specific antibody binding sites. The primary antibody was applied at a working concentration and incubated for 2 hours at 4°C. The monoclonal antibodies used are: antihuman Rb1 (Rb1, 1:80, DAKO), anti-human nm23-H1 (1:50; Santa Cruz, CA, USA) and anti-human EGFR (clone H11, 1:50, DAKO). The secondary antibody and the avidin-biotin complex (ABC) were applied to slides. Diaminobenzidine (DAB) was used as a chromogen and sections were counterstained with Mayer's hematoxylin. Negative controls were obtained by replacing the primary antibody by non-immunized rabbit or mouse serum [20].
A distinct brown nuclear staining was scored positive for Rb. The staining pattern of nm23-H1 was cytoplasmic and EGFR was membranocytoplasmic. Altered expression of Rb was defined as absence of nuclear staining in all sections examined [20]. Evaluation of nm23-H1 was performed as previously described [21]. Cases were scored negative with < 5% positive cells, +; with ≥ 5%–<25%, ++; with ≥ 25%–<70%, and +++; with ≥ 70% immunostaining of tumor cells. The cutoff point for EGFR was 10% and the intensity of staining was scored as weak; 10–<25%, moderate; 25–<50% and marked >50% (15). At least 500 cells were counted at 200× magnification.
RNA extraction and PCR amplification
Total RNA was extracted from paraffin-embedded tissue sections according to the described methodology of pure script RNA isolation kit, (Gentra, USA). The extracted total RNA was assessed for degradation, purity and DNA contamination by spectrophotometry and electrophoresis in 1.0% ethidium bromide-stained agarose gel. Reverse transcription (RT) of the isolated total RNA was done using the Superscript One-Step RT-PCR Kit with Platinum Taq (Life Technologies, Inc.) in a 50 ul reaction volume containing 1 μl of Superscript II RT enzyme, 2.5 ul 10× RT-buffer [250 mM Tris-HCl pH8.3, 375 mM KCl, 15 mM MgCl2], 0.25 μl of 100 mM dithiothreitol, 1 μl of 25 ng from random primer, 1.5 μl of 10 mM deoxynucleotide triphosphates, 0.5 μl RNAsin (Promega, USA.), and 1.0 μg of the extracted total RNA (each sample as well as the controls). Samples were then incubated at 50°C for 60 min, followed by 4°C until the PCR amplification reaction. PCR amplification was done as previously described by Raynor et al. [22] and Ayabe et al. [23]. The primers used and the PCR conditions for each marker gene are illustrated in table 1. Amplification of B-actin gene (621-bp fragment) was performed to test for the presence of artifacts and to asses the quality of RNA. A water control tube containing all reagents except c-DNA was also included in each batch of PCR assays to monitor contamination of genomic DNA in the PCR reagents. Negative RT-PCR control was used against each sample.
Table 1.
Primer sequences, expected product size in nucleotides (nt) and PCR conditions for each of the studied marker genes.
| Primer | Sequences | Fragment size | PCR conditions |
|---|---|---|---|
| nm23-H1 | 5'-CGCAGTTCAAACCTAAGCAGCAGCTGG-3' 5'-AGATCCAGTTCTGAGCACAGCTCG-3' |
483 bp | 95°C for 5 m followed by: 95°C (30s), 55°C(30s), 72°C(30s) for 32 cycles and 72°C (7 m) |
| EGFR | 5'-TGTGAGGTGGTCCTTGGGAATTTGG-3' 5'-TGCTGACTATGTCCCGCCACTGGA-3' |
322 bp | 95°C for 5 min followed by: 94°C (1 m), 66°C (1 m), 72°C (2 m) for 35 cycles and 72°C (7 m) |
| β-actin | 5'-ATCTGGCACCACACCTTCTACAATGAGGCTGCG-3' 5'-CGTCATACTCCTGCTTGCTGATCCACATCTGC-3' |
838 bp | 95°C for 5 min followed by: 94°C (45s), 60°C (45s), 72°C (2 m) for 35 cycles and 72°C (7 m) |
Quantization of the studied genes
Fifteen microliters of each PCR product were separated by electrophoresis through a 2.0% ethidium bromide-stained agarose gel and visualized with ultraviolet light. Gels were video-photographed the bands were scanned as digital peaks, areas of the peaks were then calculated in arbitrary units with a digital imaging system (Photo-documentation system, model IS-1000; Alpha Innotech Co., San Leandro, CA, USA). To evaluate the relative expression levels of target genes in the RT-PCR, the expression value of the normal pooled urothelial tissues (15 samples) was used as a normalizing factor and a relative value was calculated for each target gene amplified in the reaction. Loss of expression in any of the studied genes was considered if there was a complete absence or more than a 75% decrease in the intensity of the desired band, in comparison to the band of normal pooled liver tissue [24]. Samples were assayed in batches that included both cases and controls. The absence of bands was verified by repeating the RT-PCR twice at different days and consistent presence of B-actin gene amplification.
Statistical analysis
Numerical data were expressed as mean ± standard deviation (SD), median, minimum and maximum. Qualitative data were expressed as frequency and percentage. Chi square test (Fisher's exact test) was used to examine the relation between qualitative variables. For quantitative data, comparison between two groups was done using Mann-Whitney test. Survival analysis was done using Kaplan-Meier method. The OS duration of the patients was calculated from the date of diagnosis to the date of death. Comparison between two survival curves was done using log-rank test. Probability (p-value) ≤ 0.05 was considered significant. The multivariate analysis (MVA) was done using Cox proportional hazard model. Factors entered in the MVA were significant or of borderline significance in univariate analysis.
Results
In the present study, 59 well-characterized cases of MI-BBC were assessed for the expression level and the prognostic value of pRb, nm23, and EGFR proteins as well as RNA using immunohistochemistry and RT-PCR. The patients' age ranged from 30–76 years with a median of 50 years, the male: female ratio was 2.47:1.0. Twenty one out of the 54 patients for whom data regarding metastatic recurrence was available (38.8%) showed evidence of recurrence either as local (13), distant (8) or both (5). The median OS of the patients was 27 ± 6.6 months with the cumulative OS rate of 56% at 18 months. The presenting clinicopathological features and survival status of the whole group of patients are shown in table 2.
Table 2.
Clinicopathological features of the 59 patients studied
| Parameter | No of Cases (%) |
|---|---|
| Sex: | |
| Male | 42 (71.2) |
| Female | 17 (28.8) |
| Pathological Subtype: | |
| SCC | 27 (45.8) |
| TCC | 27 (45.8) |
| Adeno | 5 (8.5) |
| Grade: | |
| I | 5 (8.5) |
| II | 39 (66.1) |
| III | 15 (25.4) |
| Pathological Stage: | |
| P2 | 3 (5.08) |
| P3a | 24 (40.06) |
| P3b | 26 (44.06) |
| P4 | 6 (10.2) |
| Lymph Node Involvement: | |
| Positive | 10 (16.9) |
| Negative | 49 (83.1) |
| Local recurrence: | |
| No | 41 (69.5) |
| Yes | 13 (30.5)* |
| Metastasis: | |
| Negative | 46 (78) |
| Positive | 8 (22.03)* |
| Outcome: | |
| Dead | 32 (54.2) |
| Alive | 27 (45.8) |
* Data regarding recurrence and metastasis was available for 54 cases only
Expression of the studied markers
A positive cytoplasmic immunostaining for nm23-H1 as well as a positive nuclear immunostaining for Rb were detected in the 15 normal urothelium samples included in the study as a control. The nm23-H1 positive cells were restricted to the superficial layer only whereas Rb positivity was recognized in all layers. On the other hand, no immunorectivity for p53 or EGFR proteins was detected in the control samples.
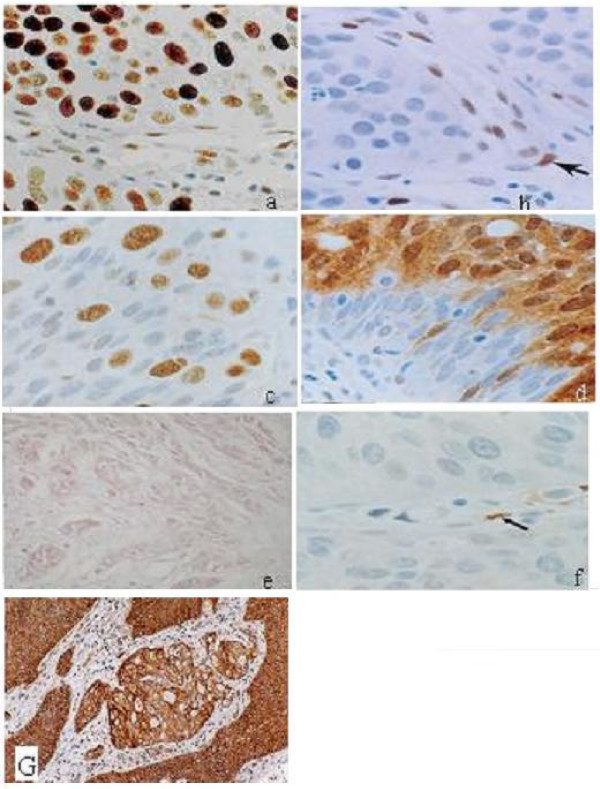
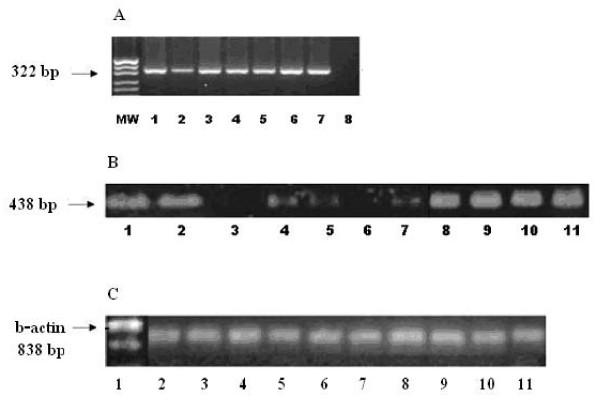
Loss of nm23 protein was detected in 25/59 cases (42.4%) whereas, positive cytoplasmic immunostaining was evident in 34/59 (57.6%) cases; 8 of them were minimally positive and 10 were markedly positive (Figure 1d, e, and 1f). RNA was successfully extracted from 52 out of the 59 cases included in the study whereas in the remaining 7 cases, either no RNA or a degraded RNA was present. This is explained by the difficulty in obtaining RNA from paraffin-embedded tissues even with the use of special kits available. Loss of nm23-H1 RNA was detected in 19/52 (36.5%) of the cases (Figure 2b). The concordance between the loss of nm23-H1 protein and nm23-H1 RNA expression was 84.6%.
Figure 1.
Protein expression of the studied markers in cases of muscle invasive bilharzial bladder cancer: Cases of BBC showing (a) positive immunostaining for p53, (b) negative immunostainning for Rb, (c) positive heterogeneous immunostaining for Rb (d) normal nm23-H1, (e) reduced nm23-H1 expression, (f) loss of nm23-H1 expression in neoplastic cells with positive expression in endothelial cells (arrow-positive control), (g) EGFR overexpression.
Figure 2.
RNA expression of EGFR, nm23-H1 and β actin in cases of muscle invasive bilharzial bladder cancer: (a) Expression of EGFR by RT-PCR: first lane; molecular weight marker, lanes 1–7: cases of BBC showing EGFR expression, lane 8: a negative control, (b) nm23-H1 RNA expression by RT-PCR: Lanes 1, 2, 8–11: normal expression of nm23-H1, lanes 3 and 6: loss of expression of nm23-H1, and lanes 4, 5, and 7: reduced expression of nm23-H1, and (c) Lanes 1–11: Expression of β actin in cases of BBC.
A positive membranous immunostaining for EGFR protein was detected in 39/59 (66.1%) cases; 20 of them were markedly positive, 9 were moderately positive and 10 were minimally positive (Figure 1g). Increased EGFR RNA was detected in 32 out of the 52 (61.5%) evaluable cases (Figure 2a). The concordance between the increased expression of EGFR protein and RNA was 98.1%.
Altered pRb expression, defined as negative nuclear staining, was detected in 34/59 (57.6%) cases whereas 25 cases (42.3%) were normal with either homogeneous or heterogeneous nuclear immunostaining (Figures 1b, and 1c).
We have previously assessed the same series of BBC for p53 protein expression and gene mutations [3]. Positive nuclear immunostaining for p53 was detected in 21 out of the 59 cases included in the present study (35.6%); of which 6 were minimally positive and 15 were markedly positive (Figure 1a). Mutations of the p53 gene (exons 5 through 10) were detected in 8 cases only (13.6%) with no mutational hotspots. Four cases showed a single mutational event and 4 revealed several mutations in more than one exon.
Statistical analysis revealed no significant relation between the expression level of the studied proteins except for the highly significant relation between p53 and EGFR overexpression where 20 out of the 21 (95.2%) p53 positive cases revealed EGFR overexpression while a single case only (4.8%) showed normal EGFR protein expression (p < 0.0001).
Correlation between the studied markers and standard prognostic factors
As shown in table 3, there was a significant correlation between positive lymph node status and reduced nm23-H1 (protein &RNA) (p < 0.0001) and p53 overexpression (p = 0.02). Also, there was a significant correlation between advanced disease stage (p2& p3a vs. p3b& p4) and reduced nm23-H1 RNA expression (p = 0.02), increased EGFR (protein & RNA) (p = 0.003 & 0.01 respectively), altered Rb expression (p = 0.023) and p53 overexpression (p = 0.004). Similarly, there was a significant correlation between the incidence of recurrence and increased EGFR RNA (p = 0.003) and a marginally significant correlation with EGFR protein overexpression (p = 0.064) as well as between metastasis and p53 overexpression (p < 0.0001), p53 mutation (p = 0.04) and altered Rb expression (p = 0.026).
Table 3.
The correlation between aberrant expression level of the studied markers and the clinicopathological features of the patients
| Parameter |
nm23-H1 IHC(25) |
nm23-H1 RNA (19) |
EGFR IHC(39) |
EGFR RNA (32) |
Rb IHC(34) |
P53 IHC(21) |
|---|---|---|---|---|---|---|
| Age(mean ± SD) | 50.2 ± 11 | 54.o ± 8.6 | 51.7 ± 11 | 48.5 ± 10.2 | 49.5 ± 11.4 | 53.9 ± 11.3 |
| p value | 0.41 | 0.63 | 0.73 | 0. 41 | 0.144 | 0.175 |
| Sex | ||||||
| Male (42) | 19 | 13 | 28 | 25 | 22 | 17 |
| Female (17) | 6 | 4 | 11 | 7 | 12 | 4 |
| p value | 0.48 | 0.42 | 0.88 | 0.62 | 0.2 | 0.22 |
| Pathology: | ||||||
| SCC | 14 | 9 | 16 | 9 | 16 | 7 |
| TCC | 10 | 8 | 20 | 20 | 16 | 12 |
| AC | 1 | 2 | 3 | 3 | 2 | 2 |
| P value | 0.536 | 0.661 | 0.21 | 0.365 | 0.85 | 0.36 |
| Grade: | ||||||
| I | 1 | 2 | 3 | 3 | 4 | 0 |
| II | 17 | 15 | 25 | 20 | 22 | 16 |
| III | 7 | 2 | 11 | 9 | 8 | 5 |
| P value | 0.916 | 0.713 | 1.0 | 0.785 | 0.71 | 0.224 |
| Stage: | ||||||
| p3a | 15 | 4 | 26 | 17 | 25 | 17 |
| p3b | 9 | 10 | 7 | 9 | 6 | 2 |
| p4 | 1 | 5 | 6 | 6 | 3 | 2 |
| P value | 0.13 | 0.02 | 0.003 | 0.01 | 0.023 | 0.004 |
| Lymp nodes: | ||||||
| Positive | 9 | 6 | 8 | 4 | 4 | 6 |
| Negative | 16 | 13 | 31 | 28 | 30 | 15 |
| P value | <0.0001 | <0.0001 | 0.31 | 0.431 | 0.216 | 0.02 |
| Recurrence: | ||||||
| Negative | 16 | 15 | 26 | 24 | 23 | 13 |
| Positive | 9 | 4 | 13 | 8 | 11 | 8 |
| P value | 0.43 | 0.51 | 0.06 | 0.003 | 0.72 | 0.347 |
| Metastasis: | ||||||
| Negative | 20 | 15 | 31 | 23 | 30 | 13 |
| Positive | 5 | 4 | 8 | 9 | 4 | 8 |
| P value | 0.75 | 0.51 | 0.78 | 0.511 | 0.026 | <0.0001 |
SCC = Squamous Cell Carcinoma; TCC = Transitional Cell Carcinoma; Adeno = Adenocarcinoma
Survival analysis
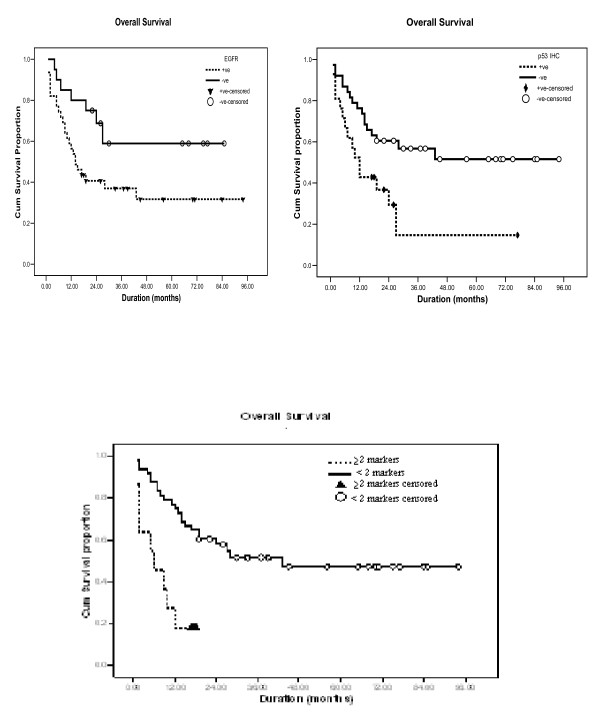
On univariate analysis there was a statistically significant correlation between reduced OS rates and tumor type (SCC) (p = 0.043), high tumor grade (p = 0.008), local recurrence (p = 0.0001) and distant metastasis (p = 0.0001). A borderline significance was reported between advanced disease stage (3b+4 vs 2+3a) and reduced OS. Similarly reduced OS rates was significantly associated with loss of nm23-H1 RNA (p = 0.021), increased EGFR protein (Figure 3a) and EGFR RNA expression (p = 0.034 &p = 0.025) and p53 overexpression (p = 0.011) (Figure 3b). The cumulative survival for cases with normal nm23-H1 RNA expression was 64.8% whereas it was 35.5% for cases with reduced/lost nm23-H1 RNA. Likewise, the cumulative survival for EGFR positive patients was 41.0% compared to 69.0% for EGFR negative patients and 29% for patients with p53 overexpression vs 60% for patients with no overexpression. On the other hand, the cumulative survival for nm23-H1 protein overexpression, altered Rb and p53 mutation did not differ significantly between negative and positive groups (p values 0.345, 0.287 and 0.539; respectively). On multivariate analysis, only increased EGFR expression (protein or RNA), the presence of metastasis and/or recurrence, a high tumor grade (GII &GIII) and a combination of aberrant markers (≥ 2) were significantly associated with reduced OS rates (p = 0.022&0.025, p = 0.000, p = 0.001, p = 0.001). On the other hand, p53 overexpression and advanced tumor stage (3b &4 vs 2&3a) showed a borderline significance (table 4)
Figure 3.
Kaplan-Meier analysis for cases of BBC. Overall survival is significantly lower in patients with: (a) increased EGFR expression, (b) p53 overexpression, (c) combined (nm23 -ve and EGFR +ve), (d) combined (nm23 -ve and p53 +ve),
Table 4.
Overall survival in relation to standard clinicopathological prognostic factors and the studied markers
| Univariate analysis | Multivariate analysis | ||||||
|---|---|---|---|---|---|---|---|
| Predictive variables | CS | HR | CI | p | HR | CI | p |
| Age | |||||||
| <47 | 68% | 1 | 0.99, 1.02 | 0.880 | |||
| ≥ 47 * | 66% | ||||||
| Sex: | |||||||
| Male (42) | 59% | 1.1 | 0.85, 1.04 | 0.652 | |||
| Female (17) | 64% | ||||||
| Tumor type | |||||||
| SCC (27) | 60% | 1.212, | 0.538, 2.734 | 0.643 | |||
| TCC (27) | 65% | ||||||
| Adenocarcinoma (5) | 60% | ||||||
| Tumor grade | |||||||
| I (5) | 66% | 1.96 | 1.66, 21.331 | <0.000 | 4.350 | 1.786, 10.591 | 0.001 |
| II (39) | 32% | ||||||
| III (15) | 11% | ||||||
| Lymph Node Involvement: | 1.099 | 0.423, 2.859 | 0.846 | ||||
| Positive (10) | 49% | ||||||
| Negative (49) | 55% | ||||||
| Metastatic recurrence: | |||||||
| No (33) | 58% | 14.149 | 5.117, 39.123 | 0.000 | 14.903 | 5.315, 41.785 | 0.000 |
| Yes (21) | 25% | ||||||
| Pathological Stage: | |||||||
| p2 & pp3a (27) | 49% | 1.341 | 0.575, 3.127 | 0.498 | |||
| p3b &p4 (32) | 35% | ||||||
| Nm23 (IHC) | |||||||
| Positive (34) | 66.5% | 1.62 | 0.535, 2.244 | 0.345 | |||
| Negative (25) | 59.6% | ||||||
| nm23(RNA) | |||||||
| Positive (33) | 64.8% | 1.34 | 1.20, 1.50 | 0.021 | 0.695 | 0.586, 1.975 | 0.444 |
| Negative (19) | 35.5% | ||||||
| EGFR (IHC) | |||||||
| Positive (39) | 41% | 1.61 | 1.04, 2.29 | 0.034 | 3.576 | 1.206, 10.602 | 0.022 |
| Negative (20) | 69% | ||||||
| EGFR (RNA) | |||||||
| Positive (32) | 39% | 1.69 | 1.16, 2.74 | 0.031 | 3.911 | 1.148, 8.511 | 0.025 |
| Negative (20) | 69% | ||||||
| Rb | |||||||
| Positive (25) | 54% | 1.1 | 0.27, 1.76 | 0.287 | |||
| Negative (34) | 49% | ||||||
| p53 (IHC) | |||||||
| Positive (21) | 29% | 3.9 | 2.281, 12.42 | 0.011 | 2.512 | 0.948, 6.662 | 0.64 |
| Negative (38) | 60% | ||||||
| P53 (mutations) | |||||||
| Positive (8) | 0.539 | ||||||
| Negative (51) | |||||||
| Number of abnormal genes | 1.70 | 1.20, 5.90 | 0.022 | 7.331 | 2.696, 12.940 | 0.001 | |
| <2 | |||||||
| ≥ 2 | |||||||
* The median age for the studied patients
** Data regarding metastatic recurrence was available for 54 cases only
The OS rates were also assessed in relation to combined genetic aberrations involving more than one gene (Table 5). Cumulative survival for altered expression of nm23-H1 and p53 (combined) was 11.0% as compared to 57.0% for other combinations (p = 0.0004), while that for altered expression of nm23-H1 and EGFR (combined) was 28.0% as compared to 60.0% for other combinations (p = 0.029). The cumulative survival for altered expression of EGFR and p53 (combined) was 18.0% as compared to 58% for other combinations (p = 0.0002) (Figure 3c–e). However, combined nm23-H1 loss and altered Rb, altered Rb and p53 overexpression as well as altered Rb and EGFR overexpression did not show an impact on survival (p = 0.438, 0.174, and 0.875; respectively). A statistically significant correlation was also present between OS and aberrations involving the 4 studied markers (p = 0.0001).
Table 5.
Correlation between survival and combined marker expression
| No. | Cumulative survival (%) | Median ± SE | 95% CI | p. value | |
|---|---|---|---|---|---|
| Nm23(-)&p53(+) | 9 | 11 | 6 ± 1.49 | 3.08 – 8.92 | |
| Other combinations | 50 | 57 | 43 ± - | - | 0.0004 |
| Nm23(-)&EGFR(+) | 18 | 28 | 10 ± 3.18 | 3.76 – 16.24 | |
| Other combinations | 41 | 60 | 43 ± - | - | 0.029 |
| EGFR(+)&p53(+) | 11 | 18 | 6 ± 3.85 | 0 – 13.55 | |
| Other combinations | 48 | 58 | 43 ± - | - | 0.0002 |
| Nm23(-), p53(+),EGFR(+) | 6 | 15 | 5.8 ± 3.85 | 0.1–13.55 | 0.0001 |
| Other combinations | 53 | 59 | 43 ± - | - | |
| Nm23(-), p53(+),EGFR(+)&b (-) | 7 | 9 | 4 ± 5.62 | 1.45 – 20.21 | <0.0001 |
| Other combinations | 52 | 58 | 50 ± - | - | |
Discussion and Conclusion
Molecular studies of bladder tumors have identified several genetic alterations including self insufficiency in growth signals, evasion of apoptosis, sustained angiogenesis, acquisition of indefinite replication potential and metastasis. However, only few of these have proven to be potentially positive clinical targets in the prognosis and therapy of bladder cancer [25].
The present study addresses, for the first time, the role of nm23-H1 and EGFR aberrations (at the RNA and protein levels) in Egyptian cases of muscle invasive BBC in relation to RB, and p53 and illustrates the clinical significance of these aberrations either singular or in combination.
Reduced expression of nm23-H1 protein and nm23-H1 RNA was significantly associated with positive lymph nodes, advanced disease stage (p3a vs p3b&p4) and reduced overall survival (OS) rates. Taken together, it could be suggested that, nm23-H1 may be a suppressor for BBC progression like in some tumors of other organs e.g. breast, liver, stomach, ovary, and colon [21,26-28].
Data regarding nm23-H1 in TCC of the bladder in western countries are contradictory. Our results are consistent with several studies [12,29-31] which showed a significantly inverse correlation between reduced nm23-H1 expression and disease staging, histopathologic differentiation, tumor size, high risk of metastasis and reduced OS. However, some reports showed a positive correlation between nm23-H1 expression and tumor grading, muscle invasion or proliferating cell nuclear antigen expression, implying a positive growth regulatory role for nm23-H1 in bladder carcinogenesis [13,31,32].
This discordance in the reported results may be partly explained by differential specificity of the antibodies applied in the earlier reports. In our work as well as that of Chow and co workers [29], a monoclonal antibody reactive to purified nm23-H1 of human origin was used, whereas polyclonal nm23-H1 antibody [12,31] and monoclonal anti-NDP (nucleoside diphosphate) kinase antibody [32] were used in some prior studies. With respect to NDP kinase, the enzyme activity has been proved to be unrelated to tumor suppression [33]. The high sensitivity and specificity of the monoclonal antibody used in the present study was confirmed by the 100% concordance reported between the protein and RNA expression levels.
Our results regarding the frequency of increased EGFR expression (62.3% and 66.1%; protein and RNA respectively) is within the universally reported range (60–75%) in non- bilharzial bladder cancer cases [34,35]. In BBC, the role of EGFR was addressed in two studies only. In the first study [20], EGFR overexpression was detected in 67% of the cases with no significant relation reported with any of the clinicopathological prognostic factors or survival. In the second study (36) the authors reported EGFR loss in high grade SCC but not TCC.
We also found that increased expression of EGFR protein and/or RNA were significantly associated with advanced disease stage, increased incidence of local recurrence and reduced OS. The significant association reported in the current study between increased EGFR expression and high tumor stage is in agreement with several previous studies on TCC of the western countries [34,36]. However, Popove et al [16] demonstrated that EGFR expression had no additional prognostic value over clinical stage, grade or cell proliferation. The study of Memon et al. [37], provided an explanation for this controversial data since they found that in some cases EGFR expression alone shows no correlation with survival, yet a high expression of EGFR together with increased Her3 and Her4 correlate with a better survival compared to increased EGFR together with decreased Her3 and Her4. This denotes the existence of a cooperative/synergistic effect between EGFR and other ERB family members mainly Her3/4.
In a recent study, Calquhom et al. [38] found a trend towards improved bladder cancer-specific survival in EGFR negative patients. Moreover, a positive response to radiotherapy significantly correlated with a negative EGFR status in the studied patients. Similarly Munk et al. [39] illustrated that EGFR activation induces cell survival in RT4 and T24 bladder cancer cell lines treated with DNA damaging chemotherapeutic agents. Therefore, they concluded that combined treatment with these drugs and EGFR inhibitors might improve the efficacy of bladder cancer treatment.
Numerous studies have claimed that inactivation of p53 and Rb plays a pivotal role in the pathogenesis of BBC and none bilharzial bladder cancer [5,9,40-42]. Our results are similar to those of Loachim et al. [43] who found altered expression of Rb and p53 proteins in 55.2% and 33.3% of their studied cases. On the other hand most previous studies reported lower rates, varying from 16–39% for Rb expression. These studies included either superficial bladder tumors only [41] or a mixture of superficial and muscle invasive tumors [5,42], whereas the present study includs muscle invasive tumors only. The higher frequency found in BBC cases could also be explained by the high frequency of HPV infection (46%–100%) reported by several investigators in Egyptian cases of BBC [3,44,45].
The clinical significance of Rb loss is still conflicting. Our results are consistent with some previous studies [42,46] which showed that Rb negative tumors had a more aggressive biologic behavior with decreased patients' survival, and more liability to tumor progression than positive ones. Thus loss of Rb expression can be used as a prognostic factor in bladder cancer. However, some other studies found no correlation between altered Rb expression and any of the known prognostic variables [5,47].
Our results regarding p53 mutation and/or protein overexpression in BBC and its prognostic value are comparable to previous reports on BBC and some non bilharzial bladder cancer [3,5,7,44]. However, our frequency is lower than Helal et al [48] who detected p53 overexpression in 52% of in non bilharzial bladder cancer cases and in and 56.3% of BBC cases. The difference between our results and those of Helal et al [48] could be attributed to the presence of associated HPV infection in our studied cases which lead to degradation of the p53 protein (3) whereas Helal et al. were not able to find HPV-DNA by in situ hybridization in their studied cases. The prognostic value of p53 which was reported in the current study has been previously reported in several studies on bladder cancer [3,42,46,49].
An interesting and novel finding in the present study is the correlation between aberrant expression of the studied markers and the OS rates and the use of marker combination for the assessment of the clinical outcome of patients. According to our data patients were categorized into two groups with a statistically significant difference between these groups with respect to OS rates: patients with aberrations in a single gene and those with more than one abnormal gene (Tables 4&5). This denotes the significance of using a panel of genes for predicting patient's outcome rather than using a single gene. Similar results have been reported by Shariat et al. [42] who found in their study of superficial cases of TCC of the bladder that a combination of biomarkers (p53, Rb, p21 and p27) are useful in stratifying patients into different risk groups for disease recurrence and progression.
We conclude that aberrant expression of nm23-H1, EGFR, p53 and RB is a frequent event in BBC. A high EGFR and p53 expression could be added to the existing clinico-pathologic prognostic factors. Moreover, a combination of these markers or some of them has synergistic effects in stratifying patients into different risk groups: those with more than one abnormal gene have a higher risk of disease progression and a reduced OS whereas those with a single abnormal gene have a better clinical outcome and OS rates.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
HMK and NMM put the study design and revised the manuscript. In addition, HMK was responsible for collecting and checking the clinical data of the studied patients and NMM revised the immunohistochemistry data. AAB: participated in the study design, carried out the immunohistochemistry and molecular studies and participated in drafing the manuscript. A-RNZ and MSM participated in performing the molecular studies and in drafting the manuscript. AMR participated in the immunohistochemistry and in revising the manuscript. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Hussein M Khaled, Email: khaled@internetegypt.com.
Abeer A Bahnassy, Email: chaya2000@hotmail.com.
Amira A Raafat, Email: amira_raafat@hotmail.com.
Abdel-Rahman N Zekri, Email: ncizekri@yahoo.com.
Maha S Madboul, Email: chaya2000@hotmail.com.
Nadia M Mokhtar, Email: nmokhtar@hotmail.com.
Acknowledgements
The authors would like to thank Dr. Ghada Mahmoud Sherif and Dr. Manar Moneir, Ass. Prof. of Epidemiology & Biostatistics, National Cancer Institute, Cairo University for doing the statistical analysis of this work.
References
- National Cancer Institute registry. the national cancer registry newsletter, Ministry of Health and Population, Egypt. 2002.
- Gutierrez MI, Siraj AK, Khaled HM, Koon N, El-Rifai W, Bahita K. CpG island methylation in Schistosoma- and non-Shistosoma-associated bladder cancer. Modern pathology. 2004;17(10):1268–74. doi: 10.1038/modpathol.3800177. [DOI] [PubMed] [Google Scholar]
- Khaled HM, Bahnassi AA, Zekri AR, Kassem HA, Mokhtar N. Correlation between p53 mutations and HPV in bilharzial bladder cancer. Urol Oncol. 2003;21(5):334–41. doi: 10.1016/s1078-1439(03)00014-0. [DOI] [PubMed] [Google Scholar]
- Knowles MA. What we could do now: molecular pathology of bladder cancer. Molecular pathology. 2001;54(4):215–21. doi: 10.1136/mp.54.4.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niehans GA, Kratzke RA, Froberg MK, Aeppli DM, Nguyen PL, Geradts MK. G1 checkpoint protein and p53 abnormalities occur in most invasive transitional carcinomas of the urinary bladder. Br J Cancer. 1999;80(8):1175–84. doi: 10.1038/sj.bjc.6690483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara H, Zhou F, Yoshikawa K, Yazumi S, Ko K, Yamaoka Y, Nizukami T, Yoshida T, Akinaga A, Tamaoki T, Motoda H, Benedict WF, Takahashii R. Retinoblastoma protein- inhibited cellular growth arrest overcomes the ability of cotransfected wild-type p53 to induce apoptosis. Br J Cancer. 2000;83(8):1039–46. doi: 10.1054/bjoc.2000.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordon-Cardo C, Sheinfeld J, Dalbagni G. Genetic studies and molecular markers of bladder cancer. Sem Surg Oncol. 1997;13(5):319–27. doi: 10.1002/(SICI)1098-2388(199709/10)13:5<319::AID-SSU5>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Cote RJ, Dunn MD, Chaterjee SJ, Stein JP, Taylor CR, Benedict WF. Elevated and absent pRb expression is associated with bladder cancer progression and has cooperation effects with p53. Cancer Res. 1998;58:1090–94. [PubMed] [Google Scholar]
- Cordon-Cardo C, Zhang Z-F, Dalbagni G. Cooperative effects of p53 and Rb alterations in primary superficial bladder tumors. Cancer Res. 1997;57:1217–21. [PubMed] [Google Scholar]
- Chow NG, Lin HS, Chan SH. The role of nm23 in the progression of transitional cell bladder cancer. Clin Cancer Res. 2000;6(9):3595–9. [PubMed] [Google Scholar]
- Kanayama H, Takigawa H, Kagawa S. Analysis of nm23 gene expression in human bladder and renal cancers. Int J Urol. 1994;1:324–331. doi: 10.1111/j.1442-2042.1994.tb00058.x. [DOI] [PubMed] [Google Scholar]
- Alderisio M, Valli C, Russo A, Dardanoni G. nm23-H protein, DNA ploidy and S-phase fraction in relation to overall survival and disease free survival in transitional cell carcinoma of the bladder. Anticancer Res. 1998;18(6A):4225–309. [PubMed] [Google Scholar]
- Shiina H, Igawa M, Nagami H, Yagi H, Yoned T. Immunohistochemical analysis of proliferation cell nuclear antigen, p53 protein and nuclear DNA content in transitional cell carcinoma of the bladder. Cancer. 1996;78:1762–74. doi: 10.1002/(SICI)1097-0142(19961015)78:8<1762::AID-CNCR17>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Thogersen VB, Jorgensen PE, Sorensen BS, Bross P, Orntoft T, Wolf H, Nexo E. Expression of transforming growth factor _ and epidermal growth factor receptor in human bladder cancer. Scand J Clin Lab Invest. 1999;59:267–77. doi: 10.1080/00365519950185634. [DOI] [PubMed] [Google Scholar]
- Cheng J, Huang H, Zhang Z-T, Shapiro E, Pellicer A, Sun T-T, Wu Z-R. Overexpression of EGFR in urothelium elicits urothelial hyperplasia and promotes bladder tumor growth. Cancer Res. 2002;62:4157–63. [PubMed] [Google Scholar]
- Popov Z, Gil-Diez-De-Medina S, Ravery V, Hoznek A, Bastuji-Garin S, Lefrere-Belda MA, Abbou CC, Chopin DK. Prognostic value of EGF receptor and tumor cell proliferation in bladder cancer: therapeutic implications. Urol Oncol. 2004;22(2):93–101. doi: 10.1016/j.urolonc.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Amsellem-Quazana D, Bieche I, Tozlu S, Botto H, Debre B, Lidereau R. Gene expression profiling of ERBB receptors and ligands in human transitional cell carcinoma of the bladder. J Urol. 2006;175(3 Pt 1):1127–32. doi: 10.1016/S0022-5347(05)00317-4. [DOI] [PubMed] [Google Scholar]
- Rotterud R, Fossa SD, Nesland JM. Protein networking in bladder cancer. immunoreactivity for FGFR3, EGFR, ERBB2, KAI1, AND RAS in normal and malignant urothelium. Histol Histopathol. 2007;22(4):349–63. doi: 10.14670/HH-22.349. [DOI] [PubMed] [Google Scholar]
- Beahrs OH, Genson DE, Hutter RVP, editor. American Joint Committee on Cancer Urinary Bladder. 'manual for staging of cancer', chap. 32. Vol. 3. Philadelphia, Lippincott; 1998. pp. 193–198. [Google Scholar]
- Ramchurren N, Cooper K, Summerhay IC. Molecular events underlying schistosomiasis-related bladder cancer. Int J Cancer. 1995;62:237–244. doi: 10.1002/ijc.2910620302. [DOI] [PubMed] [Google Scholar]
- Mandai M, Konishi I, Koshiyama M, Fukunoto M. Expression of metastasis-related nm23-H1 and nm23-H2 genes in ovarian carcinomas: correlation with clinicopathology, EGFR, c-erbB-2, and C-erbB3 genes, and sex steroid receptor expression. Cancer Res. 1994;54:1825–30. [PubMed] [Google Scholar]
- Raynor M, Stephenson S-A, Walsh DCA, Pittman KB, Dobrovic A. Optimization of the RT-PCR detection of immunomagnetically enriched carcinoma cells. BMC Cancer. 2002;2:14. doi: 10.1186/1471-2407-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayabe T, Tomita M, Matsuzaki Y, Ninomiya H, Hara M, Shimizu T, Edagawa M, Onitsuka T, Hamada M. Micrometastasis and Expression of nm23 Messenger RNA of Lymph Nodes from Lung Cancer and the Postoperative Clinical Outcome. Ann Thorac Cardiovasc Surg. 2004;10(3):152–9. [PubMed] [Google Scholar]
- Zekri Abdel-Rahman N, Sabry Gelane M, Bahnassy Abeer A, Shalaby Kamal A, Abdel-Wahabh Sabrin A, Zakaria Serag. Mismatch repair genes (hMLH1, hPMS1, hPMS2, GTBP/hMSH6, hMSH2) in the pathogenesis of hepatocellular carcinoma. World J Gastroenterol. 2005;11:3020–3026. doi: 10.3748/wjg.v11.i20.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwast T van der. Molecular diagnosis of bladder cancer. Histopathology. 2002;41(suppl.2):403–23. [Google Scholar]
- Iizuka N, Oka M, Noma T, Nakazawa A, Hirose K, Suzuki T. NM23-H1 and NM-H2 messenger RNA abundance in hepatocellular carcinoma. Cancer Res. 1995;55:652–57. [PubMed] [Google Scholar]
- Kodera Y, Isobe KI, Yamauchi m, kondoh K, Kimura N, Akiyama S, Itoh K, Nakashima I, Takagi H. Expression of nm23-H1 RNA levels in human gastric cancer tissues. Cancer (Phila.) 1994;73:259–65. doi: 10.1002/1097-0142(19940115)73:2<259::AID-CNCR2820730205>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Cheah PY, Cao X, Eu KW, Seow-choen F. NM23-H1 immunostaining is inversely associated with tumor staging but not overall survival or disease recurrence in colorectal carcinomas. Br J Cancer. 1998;77:1164–8. doi: 10.1038/bjc.1998.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow NH, Cairns P, Eisenberger CF, Schoenberg MP, Taylor DC, Epstein JI, Sidransky D. Papillary urothelial hyperplasia is a clonal precursor to papillary transitional cell bladder cancer. Int J Cancer. 2000;89:514–518. doi: 10.1002/1097-0215(20001120)89:6<514::AID-IJC8>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Krause FS, Feil G, Bichler KH. Immunohistochemical examinations (Ki-67, p53, nm23) and DNA cytophotometry in bladder cancer. Anticancer Res. 2000;20(6D):5023–8. [PubMed] [Google Scholar]
- Shiina H, Igawa M, Urakami S, Shirakawa H, Ishibe T. Immunohistochemical analysis of nm23 protein in transitional cell carcinoma of the bladder. Br J Urol. 1995;76:708–13. doi: 10.1111/j.1464-410x.1995.tb00761.x. [DOI] [PubMed] [Google Scholar]
- Nakopoulou LL, Constandinises CA, Tzonou A, Dimopoulos CA. Immunohistochemical evaluation of nm23 gene product in transitional cell carcinoma of the bladder. Histopathol. 1996;28:429–35. doi: 10.1046/j.1365-2559.1996.336375.x. [DOI] [PubMed] [Google Scholar]
- MacDonald NJ, de al Rosa A, Benedict NA, Freije JM, Krutsch H, Steeg PS. A serine phosphrylation of Nm23 and not its nucleoside diphosphate kinase activity correlates with suppression of tumor metastatic potential. J Biol Chem. 1993;268:25780–89. [PubMed] [Google Scholar]
- Sriplakich S, Jahnson S, karlsson MG. Epidermal growth factor receptor expression: Predictive value for the outcome after cytstectomy for bladder cancer. BJU Int. 1999;83(4):498–503. doi: 10.1046/j.1464-410x.1999.00914.x. [DOI] [PubMed] [Google Scholar]
- Cardillo MR, Castagna G, Meneo L, De bernardinis E, Di Silverio F. EGFR, Muc-1 and Muc-2 in bladder cancer. J exp Clin Cancer Res. 2000;19(2):225–33. [PubMed] [Google Scholar]
- Hailel A, Posch B, El Baz M, Mokhtar AA, Susani M, Ghoneim MA, Marberger M. Bilharzial related organ confined muscle invasive bladder cancer: prognostic value of apoptosis markers, proliferation markers, p253, E-cadherin, epidermal growth factor receptor and c-erb B2. J Urol. 2001;165(5):1481–7. doi: 10.1016/S0022-5347(05)66332-X. [DOI] [PubMed] [Google Scholar]
- Memon AA, Sorensen BS, Meldgaard P, Fokdal L, Thykjaer T, Nexo E. The relation between survival and expression of HER1 and her2 depends on the expression of HER3 and HER4: A study in bladder cancer patients. Br J Cancer. 2006;94(11):1703–9. doi: 10.1038/sj.bjc.6603154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun AJ, Sundar F, Rajjavabun PH, Griffiths TR, Symonds RP, Mellon JK. Epidermal growth factor receptor status predicts local response to radical radiotherapy in muscle-invasive bladder cancer. Clin Oncol (R Coll Radiol) 2006;18(9):702–709. doi: 10.1016/j.clon.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Munk M, Memon AA, Nexo E, Sorensen BS. Inhibition of growth factor receptor in bladder cancer cells treated with the DNA-damaging drug etoposide markedly increases apoptosis. BJU Int. 2007;99(1):196–201. doi: 10.1111/j.1464-410X.2006.06510.x. [DOI] [PubMed] [Google Scholar]
- Steiner G, Bierhoff E, Schmidt D, Leissner J, Wolf HK, Albers P. p53 immunoreactivity in biopsy specimens of T1 G3 transitional cell carcinoma of the bladder, a helpful parameter in guiding the decision for or against cystectomy? Eur J Cancer. 2000;36:610–14. doi: 10.1016/S0959-8049(99)00325-1. [DOI] [PubMed] [Google Scholar]
- Tetu B, Fradet Y, Allard P, Veilleux C, Roberge N, Bernard p. Prevalence and clinical significance of HER2/Neu, p53 and Rb expression in primary superficial bladder cancer. J Urol. 1996;155(5):1784–8. doi: 10.1016/S0022-5347(01)66198-6. [DOI] [PubMed] [Google Scholar]
- Shariat SF, Ashfag R, Sagalowsky AI, Lotan Y. Predictive value of cell cycle biomarkers in nonmuscle invasive bladder transitional cell carcinoma. J Urol. 2007;177(2):481–7. doi: 10.1016/j.juro.2006.09.038. [DOI] [PubMed] [Google Scholar]
- Ioachim E, Charchanti A, Stavropoulos NE, Skopelitou A, Agnantis NJ. Immunohistochemical expression of retinoblastoma gene product, p53 protein, mdm2, C-erbB2, HLA-DR and proliferation indices in human urinary bladder carcinoma. Histol Histopathol. 2000;15(3):721–727. doi: 10.14670/HH-15.721. [DOI] [PubMed] [Google Scholar]
- Khaled HM, Raafat A, Mokhtar N, Zekri AR, Gaballah H. Human papilloma virus infection and overexpression of p53 protein in bilharzial bladder cancer. Tumori. 2001;87(4):256–61. doi: 10.1177/030089160108700409. [DOI] [PubMed] [Google Scholar]
- Yang H, Yang K, Khafagi A, Tang Y, Careg TE, Opipari AW, Lieberman R, Oeth PA, Lancaster W, Klinger HP, Kaseb AW, Metwali A, Khaled H, Kiernit DM. Sensitive detection of human papillomavirus in cervical, head and neck, and schistosomiasis-associated bladder malignancies. Proc Natl Acad Sci USA. 2005;102(21):7683–8. doi: 10.1073/pnas.0406904102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordon-Cardo C, Wartinger D, petrylak D, Dalbagni G, Reuter VE. Altered expression of the Rb gene product: prognostic indicator in bladder cancer. J Natl Cancer Inst. 1992;84:1251–56. doi: 10.1093/jnci/84.16.1251. [DOI] [PubMed] [Google Scholar]
- Logothetis CJ, Xu HJ, Ro HY, Hu SX, Benedict WF. Altered expression of retinoblastoma protein and known prognostic variables in locally advanced bladder cancer. J Natl Cancer Inst. 1992;84(16):1256–61. doi: 10.1093/jnci/84.16.1256. [DOI] [PubMed] [Google Scholar]
- Helal T, El A, Fadel MT, EL-Sayed NK. Human Papilloma Virus and p53 Expression in Bladder Cancer in Egypt. Relationship to schistosomiasis and Clinicopathologic Factors. Pathology Oncology Research. 2006;12(3):173–78. doi: 10.1007/BF02893365. [DOI] [PubMed] [Google Scholar]
- Krupski T, Moskaluk C, Boyd JC, Theodorescu D. A prospective pilot evaluation of urinary and immunhistochemical markers as predictors of clinical stage of urothelial carcinoma of the bladder. BJU Int. 2000;85(9):1027–32. doi: 10.1046/j.1464-410x.2000.00676.x. [DOI] [PubMed] [Google Scholar]