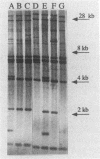
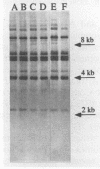
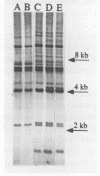
Abstract
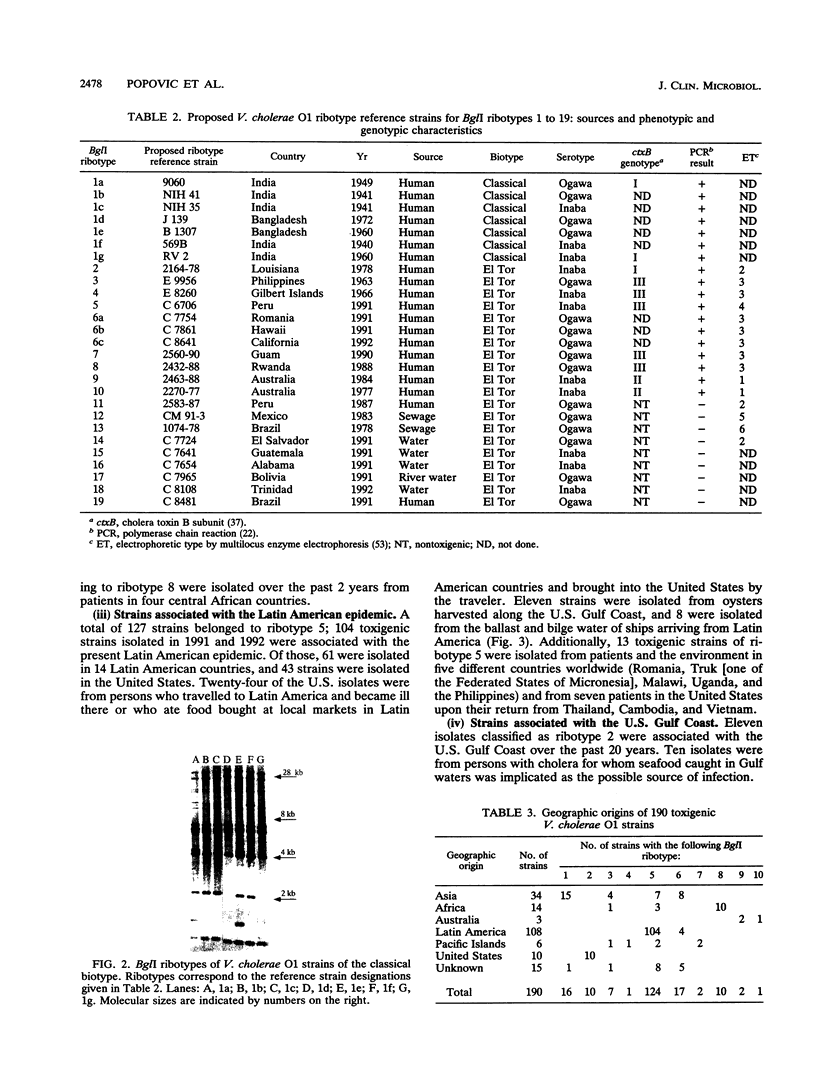
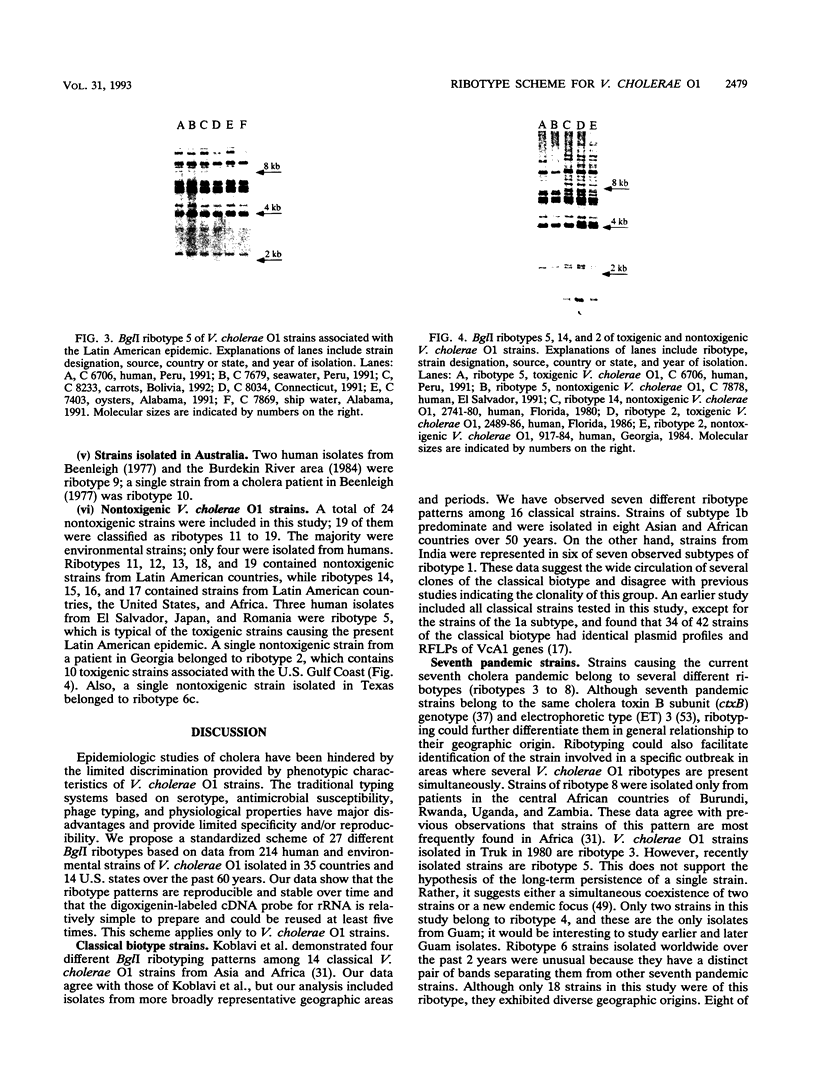
A standardized scheme of 27 different BglI ribotypes and subtypes of Vibrio cholerae O1 strains is proposed on the basis of data from 214 human and environmental strains isolated in 35 countries and 14 U.S. states over the past 60 years. The ribotype patterns obtained are reproducible and stable over time. Seven different but very similar ribotypes (1a to 1g) were observed among 16 strains of the classical biotype. Twenty ribotypes and subtypes were identified among 198 V. cholerae O1 strains of the El Tor biotype. Six different patterns were found among the strains causing the current seventh pandemic. Strains of ribotype 8 originated only in central African countries, while those of ribotype 3 originated mainly in Asia and the Pacific Islands. The most widely distributed strains were those of ribotype 6, which was subdivided into three very similar but still distinguishable subtypes. The present Latin American epidemic is caused by strains of ribotype 5. Strains of this ribotype were isolated from several other geographic locations but can be differentiated from the Latin American strains by other molecular methods. Strains associated with two documented environmental reservoirs exhibited three distinct ribotype patterns; those isolated from patients who ate food from the U.S. Gulf waters were all of ribotype 2, while the strains related to the northeast Australian rivers were of ribotypes 9 and 10. Nontoxigenic V. cholerae O1 strains originating in Latin America and the U.S. Gulf Coast did not form a specific cluster of ribotypes. Ribotyping in combination with other well-defined methods can assist in epidemiologic investigations, helping to trace the movement of strains and to identify their geographic origins.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almeida R. J., Cameron D. N., Cook W. L., Wachsmuth I. K. Vibriophage VcA-3 as an epidemic strain marker for the U.S. Gulf Coast Vibrio cholerae O1 clone. J Clin Microbiol. 1992 Feb;30(2):300–304. doi: 10.1128/jcm.30.2.300-304.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altwegg M., Hickman-Brenner F. W., Farmer J. J., 3rd Ribosomal RNA gene restriction patterns provide increased sensitivity for typing Salmonella typhi strains. J Infect Dis. 1989 Jul;160(1):145–149. doi: 10.1093/infdis/160.1.145. [DOI] [PubMed] [Google Scholar]
- Barrett T. J., Blake P. A. Epidemiological usefulness of changes in hemolytic activity of Vibrio cholerae biotype El Tor during the seventh pandemic. J Clin Microbiol. 1981 Jan;13(1):126–129. doi: 10.1128/jcm.13.1.126-129.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake P. A., Allegra D. T., Snyder J. D., Barrett T. J., McFarland L., Caraway C. T., Feeley J. C., Craig J. P., Lee J. V., Puhr N. D. Cholera--a possible endemic focus in the United States. N Engl J Med. 1980 Feb 7;302(6):305–309. doi: 10.1056/NEJM198002073020601. [DOI] [PubMed] [Google Scholar]
- Blumberg H. M., Kiehlbauch J. A., Wachsmuth I. K. Molecular epidemiology of Yersinia enterocolitica O:3 infections: use of chromosomal DNA restriction fragment length polymorphisms of rRNA genes. J Clin Microbiol. 1991 Nov;29(11):2368–2374. doi: 10.1128/jcm.29.11.2368-2374.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourke A. T., Cossins Y. N., Gray B. R., Lunney T. J., Rostron N. A., Holmes R. V., Griggs E. R., Larsen D. J., Kelk V. R. Investigation of cholera acquired from the riverine environment in Queensland. Med J Aust. 1986 Mar 3;144(5):229–234. doi: 10.5694/j.1326-5377.1986.tb115883.x. [DOI] [PubMed] [Google Scholar]
- Chen F., Evins G. M., Cook W. L., Almeida R., Hargrett-Bean N., Wachsmuth K. Genetic diversity among toxigenic and nontoxigenic Vibrio cholerae O1 isolated from the Western Hemisphere. Epidemiol Infect. 1991 Aug;107(1):225–233. doi: 10.1017/s0950268800048846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook W. L., Wachsmuth K., Johnson S. R., Birkness K. A., Samadi A. R. Persistence of plasmids, cholera toxin genes, and prophage DNA in classical Vibrio cholerae O1. Infect Immun. 1984 Jul;45(1):222–226. doi: 10.1128/iai.45.1.222-226.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Buyser M. L., Morvan A., Grimont F., el Solh N. Characterization of Staphylococcus species by ribosomal RNA gene restriction patterns. J Gen Microbiol. 1989 Apr;135(4):989–999. doi: 10.1099/00221287-135-4-989. [DOI] [PubMed] [Google Scholar]
- DePaola A., Capers G. M., Motes M. L., Olsvik O., Fields P. I., Wells J., Wachsmuth I. K., Cebula T. A., Koch W. H., Khambaty F. Isolation of Latin American epidemic strain of Vibrio cholerae O1 from US Gulf Coast. Lancet. 1992 Mar 7;339(8793):624–624. doi: 10.1016/0140-6736(92)90917-r. [DOI] [PubMed] [Google Scholar]
- Desmarchelier P. M., Senn C. R. A molecular epidemiological study of Vibrio cholerae in Australia. Med J Aust. 1989 Jun 5;150(11):631–634. doi: 10.5694/j.1326-5377.1989.tb136726.x. [DOI] [PubMed] [Google Scholar]
- Faruque S. M., Albert J. Genetic relation between Vibrio cholerae O1 strains in Ecuador and Bangladesh. Lancet. 1992 Mar 21;339(8795):740–741. doi: 10.1016/0140-6736(92)90636-h. [DOI] [PubMed] [Google Scholar]
- Fields P. I., Popovic T., Wachsmuth K., Olsvik O. Use of polymerase chain reaction for detection of toxigenic Vibrio cholerae O1 strains from the Latin American cholera epidemic. J Clin Microbiol. 1992 Aug;30(8):2118–2121. doi: 10.1128/jcm.30.8.2118-2121.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangarosa E. J., Sanati A., Saghari H., Feeley J. C. Multiple serotypes of vibrio cholerae isolated from a case of cholera. Evidence suggesting in-vivo mutation. Lancet. 1967 Mar 25;1(7491):646–648. doi: 10.1016/s0140-6736(67)92542-1. [DOI] [PubMed] [Google Scholar]
- Grimont F., Grimont P. A. Ribosomal ribonucleic acid gene restriction patterns as potential taxonomic tools. Ann Inst Pasteur Microbiol. 1986 Sep-Oct;137B(2):165–175. doi: 10.1016/s0769-2609(86)80105-3. [DOI] [PubMed] [Google Scholar]
- Hinojosa-Ahumada M., Swaminathan B., Hunter S. B., Cameron D. N., Kiehlbauch J. A., Wachsmuth I. K., Strockbine N. A. Restriction fragment length polymorphisms in rRNA operons for subtyping Shigella sonnei. J Clin Microbiol. 1991 Nov;29(11):2380–2384. doi: 10.1128/jcm.29.11.2380-2384.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston J. M., Martin D. L., Perdue J., McFarland L. M., Caraway C. T., Lippy E. C., Blake P. A. Cholera on a Gulf Coast oil rig. N Engl J Med. 1983 Sep 1;309(9):523–526. doi: 10.1056/NEJM198309013090903. [DOI] [PubMed] [Google Scholar]
- Kaper J. B., Bradford H. B., Roberts N. C., Falkow S. Molecular epidemiology of Vibrio cholerae in the U.S. Gulf Coast. J Clin Microbiol. 1982 Jul;16(1):129–134. doi: 10.1128/jcm.16.1.129-134.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehlbauch J. A., Plikaytis B. D., Swaminathan B., Cameron D. N., Wachsmuth I. K. Restriction fragment length polymorphisms in the ribosomal genes for species identification and subtyping of aerotolerant Campylobacter species. J Clin Microbiol. 1991 Aug;29(8):1670–1676. doi: 10.1128/jcm.29.8.1670-1676.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klontz K. C., Tauxe R. V., Cook W. L., Riley W. H., Wachsmuth I. K. Cholera after the consumption of raw oysters. A case report. Ann Intern Med. 1987 Dec;107(6):846–848. doi: 10.7326/0003-4819-107-6-846. [DOI] [PubMed] [Google Scholar]
- Koblavi S., Grimont F., Grimont P. A. Clonal diversity of Vibrio cholerae O1 evidenced by rRNA gene restriction patterns. Res Microbiol. 1990 Jul-Aug;141(6):645–657. doi: 10.1016/0923-2508(90)90059-y. [DOI] [PubMed] [Google Scholar]
- Lin F. Y., Morris J. G., Jr, Kaper J. B., Gross T., Michalski J., Morrison C., Libonati J. P., Israel E. Persistence of cholera in the United States: isolation of Vibrio cholerae O1 from a patient with diarrhea in Maryland. J Clin Microbiol. 1986 Mar;23(3):624–626. doi: 10.1128/jcm.23.3.624-626.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry P. W., Pavia A. T., McFarland L. M., Peltier B. H., Barrett T. J., Bradford H. B., Quan J. M., Lynch J., Mathison J. B., Gunn R. A. Cholera in Louisiana. Widening spectrum of seafood vehicles. Arch Intern Med. 1989 Sep;149(9):2079–2084. doi: 10.1001/archinte.149.9.2079. [DOI] [PubMed] [Google Scholar]
- McCarthy S. A., McPhearson R. M., Guarino A. M., Gaines J. L. Toxigenic Vibrio cholerae O1 and cargo ships entering Gulf of Mexico. Lancet. 1992 Mar 7;339(8793):624–625. doi: 10.1016/0140-6736(92)90918-s. [DOI] [PubMed] [Google Scholar]
- Miller C. J., Feachem R. G., Drasar B. S. Cholera epidemiology in developed and developing countries: new thoughts on transmission, seasonality, and control. Lancet. 1985 Feb 2;1(8423):261–262. doi: 10.1016/s0140-6736(85)91036-0. [DOI] [PubMed] [Google Scholar]
- Morris J. G., Jr Non-O group 1 Vibrio cholerae: a look at the epidemiology of an occasional pathogen. Epidemiol Rev. 1990;12:179–191. doi: 10.1093/oxfordjournals.epirev.a036052. [DOI] [PubMed] [Google Scholar]
- Olsvik O., Wahlberg J., Petterson B., Uhlén M., Popovic T., Wachsmuth I. K., Fields P. I. Use of automated sequencing of polymerase chain reaction-generated amplicons to identify three types of cholera toxin subunit B in Vibrio cholerae O1 strains. J Clin Microbiol. 1993 Jan;31(1):22–25. doi: 10.1128/jcm.31.1.22-25.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace N. R., Olsen G. J., Woese C. R. Ribosomal RNA phylogeny and the primary lines of evolutionary descent. Cell. 1986 May 9;45(3):325–326. doi: 10.1016/0092-8674(86)90315-6. [DOI] [PubMed] [Google Scholar]
- Rao A., Stockwell B. A. The Queensland cholera incident of 1977. 1. The index case. Bull World Health Organ. 1980;58(4):663–664. [PMC free article] [PubMed] [Google Scholar]
- Sack R. B., Miller C. E. Progressive changes of Vibrio serotypes in germ-free mice infected with Vibrio cholerae. J Bacteriol. 1969 Sep;99(3):688–695. doi: 10.1128/jb.99.3.688-695.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi R. J., Ahroon W., Saunders S. S., Arnold S. A. Evoked potentials: computer-automated threshold-tracking procedure using an objective detection criterion. Ear Hear. 1987 Jun;8(3):151–156. [PubMed] [Google Scholar]
- Sarafian S. K., Woods T. C., Knapp J. S., Swaminathan B., Morse S. A. Molecular characterization of Haemophilus ducreyi by ribosomal DNA fingerprinting. J Clin Microbiol. 1991 Sep;29(9):1949–1954. doi: 10.1128/jcm.29.9.1949-1954.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroeher U. H., Karageorgos L. E., Morona R., Manning P. A. Serotype conversion in Vibrio cholerae O1. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2566–2570. doi: 10.1073/pnas.89.7.2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stull T. L., LiPuma J. J., Edlind T. D. A broad-spectrum probe for molecular epidemiology of bacteria: ribosomal RNA. J Infect Dis. 1988 Feb;157(2):280–286. doi: 10.1093/infdis/157.2.280. [DOI] [PubMed] [Google Scholar]
- Tauxe R. V., Blake P. A. Epidemic cholera in Latin America. JAMA. 1992 Mar 11;267(10):1388–1390. [PubMed] [Google Scholar]
- Wachsmuth I. K., Bopp C. A., Fields P. I., Carrillo C. Difference between toxigenic Vibrio cholerae O1 from South America and US gulf coast. Lancet. 1991 May 4;337(8749):1097–1098. doi: 10.1016/0140-6736(91)91744-f. [DOI] [PubMed] [Google Scholar]
- Wachsmuth I. K., Evins G. M., Fields P. I., Olsvik O., Popovic T., Bopp C. A., Wells J. G., Carrillo C., Blake P. A. The molecular epidemiology of cholera in Latin America. J Infect Dis. 1993 Mar;167(3):621–626. doi: 10.1093/infdis/167.3.621. [DOI] [PubMed] [Google Scholar]
- Wachsmuth K. Molecular epidemiology of bacterial infections: examples of methodology and of investigations of outbreaks. Rev Infect Dis. 1986 Sep-Oct;8(5):682–692. doi: 10.1093/clinids/8.5.682. [DOI] [PubMed] [Google Scholar]
- Weissman J. B., DeWitt W. E., Thompson J., Muchnick C. N., Portnoy B. L., Feeley J. C., Gangarosa E. J. A case of cholera in Texas, 1973. Am J Epidemiol. 1974 Dec;100(6):487–498. doi: 10.1093/oxfordjournals.aje.a112061. [DOI] [PubMed] [Google Scholar]
- Woods T. C., Helsel L. O., Swaminathan B., Bibb W. F., Pinner R. W., Gellin B. G., Collin S. F., Waterman S. H., Reeves M. W., Brenner D. J. Characterization of Neisseria meningitidis serogroup C by multilocus enzyme electrophoresis and ribosomal DNA restriction profiles (ribotyping). J Clin Microbiol. 1992 Jan;30(1):132–137. doi: 10.1128/jcm.30.1.132-137.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]