Abstract
In recent years, many studies have emphasized how changes in aryl hydrocarbon receptor (AHR)-mediated gene expression result in biological effects, raising interest in this receptor as a regulator of normal biological function. This review focuses on what is known about the role of the AHR in the female reproductive system, which includes the ovaries, fallopian tubes or oviduct, uterus and vagina. This review also focuses on the role of the AHR in reproductive outcomes such as cyclicity, senescence, and fertility. Specifically, studies using potent AHR ligands, as well as transgenic mice lacking the AHR signaling pathway are discussed from a viewpoint of understanding the endogenous role of this ligand-activated transcription factor in the female reproductive lifespan. Based on findings highlighted in this paper, it is proposed that the AHR has a role in physiological functions including ovarian function, establishment of an optimum environment for fertilization, nourishing the embryo and maintaining pregnancy, as well as in regulating reproductive lifespan and fertility. The mechanisms by which the AHR regulates female reproduction are poorly understood, but it is anticipated that new models and the ability to generate specific gene deletions will provide powerful experimental tools for better understanding how alterations in AHR pathways result in functional changes in the female reproductive system.
Keywords: aryl hydrocarbon receptor, ovary, uterus, vagina, oviduct, female reproduction
1. Introduction
The aryl hydrocarbon receptor (AHR) is a ligand-activated nuclear transcription factor that transduces extracellular signals through DNA-binding dependent and independent mechanisms [1]. The AHR was first discovered in toxicological studies as a mediator of the toxicity caused by xenobiotics such as halogenated dibenzo-p-dioxins and related compounds [2,3]. Several studies on the role of the AHR in the metabolism of xenobiotics have provided a detailed understanding of its signal transduction pathway. Specifically, it has been demonstrated that the AHR is present in the cytoplasm of cells forming a complex with at least three distinct chaperone proteins such as heat shock proteins 90, immunophilin-like protein XAP2, and co-chaperone p23 [4]. When a ligand with affinity to the AHR enters the cell, it binds to the AHR and as a consequence, the AHR undergoes a conformational change facilitating its migration to the nucleus [5,6]. Once in the nucleus, the liganded AHR is released from the chaperone proteins, a step that has recently been shown to be essential for hetero-dimerization of the AHR with the aryl hydrocarbon nuclear translocator (ARNT) [5,7]. The newly formed ligand-AHR-ARNT complex binds to specific enhancer sequences termed AHR response elements (AHREs) that are localized adjacent to target promoters [8]. An assembly of coactivators and general transcription factors then interacts with gene promoters and potentiates the expression of the target gene [9]. The most well studied of these target genes include those of the cytochrome P450 subfamily such as Cyp1a1, Cyp1a2 and Cyp1b1, which encode xenobiotic-metabolizing enzymes [10,11].
Importantly, in recent years, many studies have also emphasized how changes in AHR/ARNT-mediated gene expression result in biological effects with implicit physiological roles. Current research has strengthened the idea that beyond the role of the AHR in mediating toxicity, this ligand-induced transcription factor plays a role in the functioning of normal biology [12].
Generation of recombinant mouse models by three independent research groups in which the Ahr gene has been deleted or “knocked out” (AHRKO) [13–15] has provided a versatile and powerful tool for examining the phenotypic and functional consequences of the absence of the AHR. From this perspective, the use of AHRKO mice has been critical in understanding the role of the AHR in the female reproductive system. Likewise, emerging evidence in the past several years has suggested that deletion of the AHR in various mouse models produces a wide range of phenotypes that coincide with the biological effects caused by exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), polychlorinated biphenyls (PCBs) and other potent AHR ligands [16,17]. This might be due to the fact that the AHR is activated and then down-regulated or degraded following AHR ligand exposure in some tissues, a mechanism that has been proposed to modulate AHR-dependant gene expression [17,18].
The female reproductive system includes the ovaries, fallopian tubes or oviduct, uterus and vagina, and it is hormonally regulated by the hypothalamus and pituitary. All these organs function in conjunction to fulfill the main roles of the female reproductive system, which are to produce the female sex hormones as well as to produce the female gametes and transport them to a site where they may be fertilized by sperm. Once fertilization occurs, the female reproductive system provides a favorable environment for development and delivery of the fetus. Thus, the complex functions of the female reproductive system are achieved by the proper function of every organ comprising it [19].
Both the absence of Ahr gene expression in AHRKO mice and the activation of the Ahr pathway by AHR ligand can lead to adverse phenotypes in the organs of the female reproductive system and lead to impaired reproductive function [16,20–24], indicating a potential role for the AHR in the functioning of the female reproductive system from fetal development to adulthood. Thus, studies using AHRKO mice as well as studies using exposure to AHR ligands are discussed in this paper from a viewpoint of understanding the endogenous role of the AHR in the female reproductive system. Specifically, this review focuses on the role of the AHR in the ovary, oviduct, uterus and vagina. This review also focuses on the role of the AHR in regulating reproductive functions such as cyclicity, fertility, and reproductive senescence.
2. Physiological roles of the AHR in the ovary
The ovary is a female reproductive organ that plays two intrinsic major physiological roles. First, the ovary is responsible for follicle development, follicle differentiation and release of competent oocytes for fertilization [25,26]. Second, it is responsible for synthesizing and secreting sex steroid hormones (i.e., estrogens, progesterones, and androgens) that are essential for maintaining follicle development, fertility, proper menstrual/estrous cyclicity and pregnancy [25–27].
Several studies using techniques such as immunohistochemistry, radio-labeled AHR-ligand, RT-PCR and western blot have shown that the AHR protein and transcripts are present in ovarian cell types (oocytes, granulosa cells and theca cells) in a number of species, including primates, humans, pigs, rats, mice, rabbits [28–35]. The presence of the AHR in distinct species suggests that the AHR is conserved in the ovary and also suggests that it may have a role in ovarian function. Thus, given that the AHR is present in many ovarian cell types, recent studies have examined whether this ligand-activated nuclear transcription factor regulates functions in the embryonic, neonatal, and adult ovary. These studies are described below.
2.1. The physiological role of the AHR in the embryonic and neonatal ovary
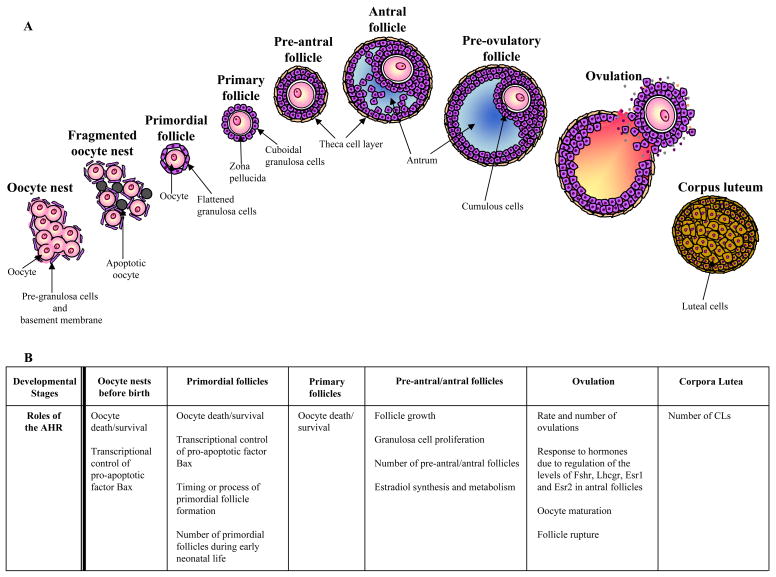
Because the role of the AHR in the embryonic and neonatal ovary has been mostly studied in the mouse, normal ovarian development is detailed below according to processes occurring in the mouse. The ovary begins to develop around embryonic day (ED) 10.5 when a founding population of primordial germ cells is allocated at the genital ridge [25,36]. The primordial germ cells or oogonia undergo extensive mitotic proliferation, remaining connected by cellular bridges due to incomplete cytokinesis and forming clusters termed oocyte nests (Figure 1) [25,37]. These nests are enveloped by a single layer of somatic cells called pre-granulosa cells (Figure 1). The oogonia cease dividing around ED13.5 and enter meiosis to form oocytes. Then, the oocytes are arrested in meiosis I at the diplotene stage of prophase by ED 17.5 [38,39]. Furthermore, in a time period ranging from ED 17.5 to postnatal day (PND) 5, approximately 70% of the oocytes in germ cell nests undergo cell death due to apoptosis, process that leads to germ cell nest breakdown and allows the formation of individual primordial follicles (Figure 1) [39]. The newly formed primordial follicles consisting of individual oocytes surrounded by a single layer of granulosa cells (Figure 1), are thought to represent a finite pool of resting oocytes available to the female during the entire reproductive lifespan [25,40].
Figure 1.
The roles of the AHR in the ovary. A) The diagram summarizes the process of ovarian follicle development and growth, from oocyte nests present in the fetal ovary through follicle growth, ovulation and CL formation during adulthood. B) The table highlights key developmental stages of follicle formation and growth through ovulation and the roles of the AHR at each of these stages.
There is evidence suggesting that the AHR plays a functional role in the ovary during embryonic and neonatal life. This evidence comes from studies supporting the hypothesis that the AHR is involved in regulating the rate of apoptosis of oocytes in germ cell nests during embryonic life and regulating survival of oocytes in the fetal and neonatal ovary. Specifically, studies have shown that ovaries obtained from AHRKO mice on ED13.5 and cultured for 72 h in absence of hormonal support with the aim of inducing apoptosis, contained higher numbers of non-apoptotic germ cells compared to wild-type (WT) ovaries cultured in same conditions [30]. These findings suggest that the AHR contributes to the induction of natural apoptosis in oocytes contained in germ cells nests present in fetal ovaries. This hypothesis although not directly tested, is supported by studies that have shown that AHRKO ovaries on PND 2–4 contain approximately twofold more primordial follicles compared to WT ovaries [16,30]. This hypothesis is also supported by studies that have demonstrated that DMBA (1 μM), an AHR ligand, and one of its metabolites, 9,10-dimethylbenz[a]anthracene-3,4-dihydrodiol (DMBA-DHD; 0.1 – 1 μM), induce apoptosis in primordial follicles from WT cultured neonatal mouse ovaries collected on PND 4, whereas no apoptosis occurs in AHRKO neonatal ovaries [41].
It is possible that the activated AHR could regulate the natural process of germ cell death/survival by transcriptionally controlling Bax and other cell death factors in the embryonic ovary (Figure 1). This possibility is supported by studies that have shown that WT mice exposed in utero to 7,12-dimethylbenz[a]anthracene (DMBA; 1 mg/kg; single IP dose) on ED 13.5 exhibit ovarian germ cell loss [42]. It has been shown that fetal germ cell loss by DMBA exposure might be attributed to transcriptional activation of a pro-apoptotic factor, Bcl2-associated X protein (Bax), via AHREs in the fetal ovary [41]. Furthermore, PCB exposure (1–100 μg/chicken egg) promotes oocyte survival in chicken neonatal ovaries [43]. It should be noted, however, that TCDD exposure (1 μg/kg; single oral dose) does not cause germ cell loss in rats [44]. Different biological effects elicited by AHR ligands might be due to differences among animal species, doses and ligand-AHR affinity [45].
Collectively, existing data suggest that the AHR plays a role in some aspects of ovarian development in embryonic and neonatal life, possibly by regulating transcription factors involved in the normal apoptosis process that leads to germ cell nest breakdown and the formation of individual primordial follicles (Figure 1). Further studies are needed to support this hypothesis. For example, it would be interesting to determine how the AHR regulates oocyte survival/death by quantifying the levels of cell death or survival markers in AHRKO and WT neonatal mice or in animals treated with AHR ligands. Additionally, in vitro studies could be conducted to identify the factors involved in primordial follicle formation in AHRKO and WT neonatal ovaries. Given the limited information on the role of the AHR in early ovarian development, future studies are needed to fully understand the role of the AHR in other processes occurring during this time period of the reproductive lifespan. For example, studies could compare the earliest stages of oogenesis and the progression of oocytes through the complex events of meiosis in AHRKO and WT animals or in animals treated with AHR ligands during development. Any errors that occur in the timing of entry into meiosis or the specific events of meiosis such as chromosomal synapsis or recombination could lead to defective oocytes [46]. Additionally, the somatic cells that become granulosa cells play an important role in follicle formation and tissue remodeling in the mouse ovary [39]. A close analysis of somatic cells in AHRKO and WT mice or in animals treated with AHR ligands would help determine if the AHR regulates somatic cells during embryonic and neonatal life.
2.2. The physiological role of the AHR in the adult ovary
During the reproductive lifespan, the primordial follicles are recruited and activated to initiate growth and differentiation at distinct times [25,26]. Growth and differentiation of a primordial follicle begins when its oocyte increases in size and its flattened shaped granulosa cells become cuboidal shaped granulosa cells. Once a primordial follicle undergoes these changes, it is called a primary follicle (Figure 1) [47,48]. The primary follicle transitions into a pre-antral follicle as the granulosa cells proliferate to form multiple layers. In addition, an outer layer of spindle shaped cells called the theca cells starts condensing around granulosa cell layers, forming part of the follicle (Figure 1) [19,49]. The pre-antral follicle becomes an antral follicle as small fluid-filled spaces form a single antral cavity or antrum (Figure 1). At this stage, the follicle is able to produce sex steroid hormones and continues growing until reaching the pre-ovulatory stage (Figure 1) [19,47]. During ovulation, the follicle ruptures and releases an oocyte for fertilization (Figure 1) [19,50]. The remaining somatic cells become the corpus luteum (CL) (Figure 1). The primary role of the CL is to produce progesterone, a critical hormone for maintenance of reproductive cyclicity and early pregnancy [51]. Several studies have suggested that the AHR plays a role in regulating follicle growth and the ability of follicles to produce sex steroid hormones. Furthermore, studies suggest that the AHR plays a role in regulating the ovulation process and CL formation. These specific roles of the AHR are described below.
2.2.1. The physiological role of the AHR in the regulation of follicle growth
Strong evidence supports a role of the AHR in regulating the ovarian follicle growth in the late stages of folliculogenesis. The first evidence comes from studies revealing that the AHR regulates the number of pre-antral and antral follicles. Specifically, histological evaluation of ovaries from adult (PND 53) mice indicates a reduced number of pre-antral and antral follicles in AHRKO ovaries compared to WT ovaries, whereas the number of primordial and primary follicles is similar in both genotypes [16]. Similarly, rats exposed in utero to PCB (2.5 mg/kg) on gestational days 7 – 13, a time when the ovary begins to develop, have reduced numbers of pre-antral and antral follicles compared to unexposed rats [52]. These findings suggest that the AHR may have a role in the growth of the pre-antral and antral follicles, but it may not have a role in the growth of the primordial and primary follicles [16].
Additional support for a role of the AHR in regulating follicle growth comes from studies evaluating follicle growth in vitro as well as from evaluating the proliferation rate of granulosa cells. Specifically, studies show that cultured AHRKO antral follicles have slower growth, evidenced by a smaller follicle diameter after 168 hours of culture, compared to WT follicles [53]. Furthermore, immunohistochemical analyses for a marker of cell proliferation such as proliferating cell nuclear antigen (PCNA), as well as quantitative RT-PCR and western blot analyses for markers of cell cycle progression such as cyclin D2 (CCND2) and cyclin dependent kinase 4 (CDK4), have indicated decreased granulosa cell proliferation in AHRKO ovaries compared to WT ovaries [53]. In addition, Bussmann et al. demonstrated that an agonist known to activate the AHR pathway increases the proliferation of cultured rat granulosa cells by amplifying the mitogenic actions of follicle stimulating hormone (FSH) and estradiol (E2) [54]. Collectively, these data suggest that the AHR may regulate follicle growth by promoting granulosa cell proliferation (Figure 1).
Atresia is a normal process in the ovary that occurs at all stages of follicular development throughout female reproductive life [55]. Atresia occurs in approximately 95% of follicles and is thought to begin with apoptosis in the granulosa cells [25,56]. Thus, the possibility that the reduced number of antral follicles observed in the AHRKO mice is due to increased atresia has been explored [57]. Specifically, Benedict et al. compared the percentage of atretic pre-antral/antral follicles in AHRKO and WT ovaries at different ages of sexual maturity including PND 32, 45, and 53, and found similar percentages of atretic follicles in AHRKO and WT ovaries [57]. In addition, Benedict et al. evaluated DNA fragmentation in ovarian follicles, a major indicator of apoptosis, and found no difference between AHRKO and WT follicles [57]. Similarly, rats exposed to TCDD (1 μg/kg; single oral dose) have reduced number of pre-antral/antral follicles with no apoptotic cell death [58]. Collectively, these studies suggest that the AHR may regulate pre-antral/antral follicle growth, but it may not play a role in regulating atresia in the adult ovary. The role of the AHR in follicular growth has been attributed to a role of the AHR in regulating E2 biosynthesis, which is discussed in the next section.
2.2.2. The physiological role of the AHR in ovarian steroidogenesis
Since the coordinated action of steroid hormones (androgens, estrogens and progestins) is necessary for follicle growth [26,27], it is reasonable to think that the AHR may play a role in follicle growth by regulating steroid hormone production in the ovary. Steroid hormone synthesis is under the control of the anterior pituitary and occurs in a sequential manner in theca and granulosa cells in follicles [59]. Briefly, luteinizing hormone (LH) secreted by the anterior pituitary binds to receptors for LH (LHCGR) in theca cells to make androgens, primarily testosterone. Steroidogenic enzymes such as cytochrome P450 cholesterol side-chain cleavage (P450scc), 3β-hydroxysteroid dehydrogenase (3β-HSD) and cytochrome P450 17α-hydroxylase (P450c17) are required for the conversion of cholesterol to testosterone in theca cells [60–62]. Then, FSH secreted by the anterior pituitary binds to the FSH receptor (FSHR) in granulosa cells and stimulates the expression of cytochrome P450c19 (aromatase), an enzyme that converts testosterone to E2 [63]. Finally, E2 stimulates growth of antral follicles to the pre-ovulatory stage via binding to estrogen receptors alpha (ESR1) and beta (ESR2) [62].
Several studies support a role of the AHR in regulating the ability of antral follicles to produce steroids. The main support comes from studies indicating that E2 production is reduced after activation or lack of the AHR signaling pathway. For example, Barnett et al. observed significantly lower circulating E2 levels, as well as lower E2 production by cultured antral follicles in prepubertal (PND 30) AHRKO mice compared to WT mice, which coincides with slow antral follicle growth in AHRKO mice [53]. Interestingly, the slow antral follicle growth in AHRKO mice was restored to WT levels with exogenous E2 treatments, suggesting that the low E2 levels in AHRKO mice contribute to slow follicle growth in AHRKO mice [53]. Similar to the phenotype of female AHRKO mice, in utero and lactational TCDD exposure (1 μg/kg; single oral dose) has been shown to reduce serum E2 concentrations in prepubertal female rats (PND 14–21) [64,65]. Further, treatment with TCDD ranging from 3.1 pM to 10 nM reduces E2 production by theca cells and granulosa cells in co-culture [66–69]. Collectively, these studies suggest that AHR deletion as well as activation of the AHR pathway affect steroidogenesis in the ovary.
The biosynthesis cascade in the follicle is dependant on proper ligand-receptor interaction [27,59] as well as on the proper action of the steroidogenic enzymes [62]. Hence, more evidence supporting the hypothesis that the AHR is necessary for ovarian steroidogenesis comes from studies that have shown alterations in some enzymes and receptors involved in follicular steroidogenesis from AHRKO mice, TCDD exposed animals and TCDD-treated ovarian cells [24,53,66,69–73]. For example, some studies have shown that Esr1, Esr2, Lhcgr and Fshr transcripts are down-regulated in ovaries from AHRKO mice compared to WT mice [53,72], as well as in ovaries from TCDD exposed (5 μg/kg; single IP dose) mice and in rat granulosa cells exposed to TCDD in concentrations ranging from 1 pM to 10 nM [70,71]. Similarly, disruptions of steroidogenic enzyme transcripts and/or activity have been observed after Ahr deletion or after TCDD exposure. Specifically, Baba et al. have shown reduced aromatase mRNA and protein expression following gonadotropin treatment in AHRKO ovaries compared to WT ovaries [24]. Similarly, Dasmahapatra et al. found that TCDD exposure reduces aromatase and P450scc mRNAs, but exerted no effect on 3β-HSD mRNA in cultured rat granulosa cells [66]. Further, Morán et al. showed that TCDD inhibits E2 production in human luteinized granulosa cells by decreasing P450c17 [69]. These findings certainly suggest that the AHR is involved in modulating follicular steroidogenesis, possibly by regulating the steroidogenic cascade at more than one site.
2.2.3. The physiological role of the AHR in ovulation
Existing evidence supports a role for the AHR in the ovulation process. Specifically, AHRKO mice have a significant reduction in the number of ovulated ova in response to gonadotropin treatment compared to WT mice [24,72]. Reduced or completely blocked ovulation has also been observed after exposure to TCDD (10 – 32 μg/kg; single oral doses) in rats [21,74–77]. Furthermore, studies indicate that an ovulatory stimulus induces expression of the Ahr in Macaque granulosa cells [78], suggesting that AHR activation is needed for the ovulation process.
Since the ovulation process is facilitated by the LH surge from the anterior pituitary [19], it is feasible to think that the reduced ovulation caused by Ahr deletion and AHR activation by ligands is due to an impaired hypothalamus-pituitary axis. However, two different studies suggest that the AHR regulates the ovulation process by directly acting on the ovary. Specifically, studies show that serum levels of both FSH and LH are similar in AHRKO and WT mice at all stages of the mouse estrous cycle [72]. In addition, studies show that gonadotrophs in the anterior pituitary of AHRKO female mice produce similar levels of gonadotropins compared to WT female mice [24].
The mechanism by which the AHR regulates ovulation is not fully understood, but a few studies suggest that the AHR may facilitate responsiveness of follicles to hormones required for ovulation. Baba et al. found that intra-ovarian E2 concentrations from superovulated mice were significantly lower in AHRKO mice compared to WT mice, suggesting a reduced responsiveness of antral follicles to gonadotropins [24]. In addition, Barnett et al. found that antral AHRKO follicles from cycling mice have reduced Fshr and Lhcgr transcripts compared to WT follicles [72]. Furthermore, Barnett et al. found a reduced ability of AHRKO ovaries to ovulate in response to exogenous gonadotropins such as human chorionic gonadotropin (hCG) and eCG compared to WT ovaries [72]. Similarly, Baba et al. were only able to partially rescue the number of ovulations in the superovulated AHRKO mice by administering E2, indicating a reduced responsiveness to E2 in AHRKO mice compared to WT mice [24].
Since oocyte maturation in pre-ovulatory follicles occurs before ovulation when the oocyte resumes meiosis and progresses from prophase I to metaphase II [79], it is possible that the AHR may also have a role in the ovulation process via regulating oocyte maturation. This idea is supported by studies that have shown an up-regulation of the Ahr in bovine oocytes after in vitro maturation in the absence of AHR ligands [35,80]. Furthermore, activation of the Ahr pathway by exposure to PCB at concentrations ranging from 0.1 to 10 μg/ml disrupts oocyte maturation [81,82]. Collectively, these findings suggest that the activation of the AHR signaling pathway may play a role in resumption of meiosis in oocytes [35,80,81]
Finally, the AHR may play a role in the ovulation process via modulating factors required for follicle rupture. Specifically, studies show that TCDD exposure (32 μg/kg; single oral dose) blocks ovulation and causes a reduction in cyclo-oxygenase-2 (COX2) [77], a factor required for follicle rupture [83].
Collectively, the AHR may play a role in the ovulation process by modulating responsiveness to hormones and/or by regulating oocyte maturation and release (Figure 1). While the studies mentioned here support a role for the AHR in ovulation, the mechanisms by which this receptor acts in this process are not fully understood. Both the AR and the PR have been shown to be important in the process of ovulation [84]. Hence, it would be interesting to compare levels of these receptors in antral follicles from AHRKO and WT animals to determine whether these receptors are regulated by the AHR and whether they have a role in the AHR-mediated ovulation.
2.2.3. The physiological role of the AHR in CL formation
After ovulation, the differentiation of the luteinized granulosa cells into luteal cells allows the formation of CLs [19]. Thus, in addition to a role for the AHR in the ovulation process, there is evidence suggesting that the AHR is involved in regulating the number of CLs. Specifically, studies show that AHRKO ovaries have significantly fewer CLs when compared to WT ovaries at PND 53 [72] and PND 63 [24]. Similarly, studies indicate that in vivo TCDD treatment (2–32 μg/kg; single oral doses) in rats reduces the numbers of CLs compared to unexposed rats [85].
Little is known about how the AHR may regulate CL numbers. Studies indicate that the LH surge during the ovulation process stimulates the synthesis of progesterone and that progesterone helps in maintaining CLs [19]. Thus, future studies should compare progesterone levels in AHRKO and WT mice and in animals exposed to AHR ligands.
3. The physiological role of the AHR in the oviduct
The oviduct or fallopian tube is a thin muscular tube that includes the fimbriae, the ampulla, the isthmus, and the utero-tubal junction regions. This structure consists of internal stroma tissue covered with ciliated and secretory epithelial cells. The secretory epithelial cells produce secretions, which form a “jelly-like” coat around the ova during their passage through oviducts and provide nutrition to the embryonic blastocyst. The main functions of the oviduct include the synchronized processes of female and male gamete transport, fertilization, development from the fertilized egg into the blastocyst, and transport of the embryonic blastocyst to the uterus for implantation [19,86].
Even though the AHR in the oviduct has not been well studied, the limited existing evidence suggests a functional role for this ligand-activated nuclear transcription factor in the function of the oviduct. AHR protein is expressed in the cytoplasm of both secretory and ciliated cells belonging to the ampulla and the isthmus [32]. Further, the AHR is present in the nucleus of stromal cells in the oviduct in the preimplantation period [32]. Since nuclear localization of the AHR has been suggested to be an indication of its recruitment to transcription sites [87], the presence of the AHR in the nucleus of stroma cells indicates active AHR signaling in the oviduct. Similarly, a study conducted by Hombach-Klonisch et al. showed the presence of transcripts for the AHR and its dimerization partner ARNT in a telomerase-immortalized oviduct porcine epithelial cell line exposed to TCDD [88]. In addition, the transcriptionally active Ahr, as indicated by the induction of the Ahr target gene Cyp1a1, has been shown to downregulate ESR1 in the oviduct [88].
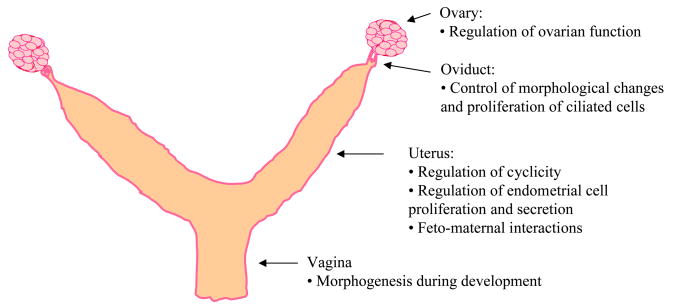
Propulsion of both female and male gametes as well as embryonic blastocysts in the oviduct is achieved by complex interactions between muscle contractions, ciliate activity and the flow of tubular secretions [86]. Coordinated regulation and interaction of ESR1 and progesterone receptors in the cilia is required for proliferation, differentiation, and motility of ciliated cells in the oviduct [86,89,90]. Furthermore, high expression of protein and mRNA ESR1 during the ovarian mid-cycle has been reported in the epithelial cells from the ampullar region of the oviduct, the main site of fertilization [91]. Thus, the observation of downregulation of the ESR1 by the AHR-liganded transduction pathway may suggest that the AHR is involved in controlling the morphological changes and proliferative activity of the ciliated cells (Figure 2), possibly by moduling the estrogen-ESR1 signaling pathway. This possibility is supported by studies suggesting that modulation of the ESR1 signaling pathway is an intrinsic function of the AHR in other organs of the female reproductive system [64,92–94]. Given the limited information on the role of the AHR in the oviduct, future studies are needed to evaluate the specific function of the AHR in this organ. For example, future investigations are needed to elucidate whether induction or deletion of the AHR signaling pathway modifies the oviductal secretions that provide an optimum environment for fertilization to occur and whether deletion/activation of the AHR in the oviduct has repercussions for female fertility.
Figure 2.
The roles of the AHR in the female reproductive organs. The diagram shows the female mouse reproductive organs and indicates the known physiological roles of the AHR in each organ.
4. The physiological role of the AHR in the uterus
The adult uterus is comprised of two layers, a thick external layer termed the myometrium and a thinner internal layer termed the endometrium. The myometrium consists of smooth muscle, whereas the endometrium consists of luminal epithelium and a fibromuscular stroma penetrated by extensions of associated glands [19].
The uterus undergoes cyclic changes that depend on the steroids produced by the ovary. The rise in estrogens produced by the ovary causes a thickening of the endometrium due to both stromal and surface epithelium cell proliferation. This stage in the uterus is known as the proliferative phase. The proliferative phase is followed by the secretory phase or pre-implantation period after ovulation. In the secretory phase or pre-implantation period, a rise in progesterone produced by the ovary stimulates the synthesis of secretory material by the glands of the endometrium so that they become distended with a thick secretion rich in glycoproteins, sugars and amino acids. If fertilization occurs, the stroma undergoes major remodeling and differentiative processes that prepare the uterus for receiving and nourishing the embryonic blastocyst. Then, the embryonic blastocyst implants in the uterus establishing contact with the luminal epithelium and maternal blood vessels, a process known as decidualization [19].
There are convincing reasons to think that the AHR is involved in the normal function of the uterus. First, studies conducted in different species have demonstrated that protein and gene expression of the AHR as well as its dimerization partner ARNT are present in the uterus, and that the AHR-ARNT signaling pathway may be activated in the presence or absence of exogenous AHR ligands [95–101]. Some of the first evidence for the presence of the AHR in the uterus, as well as for the activation of the AHR signaling pathway was shown by Bhattacharyya et al. using hypophysectomized rats treated with β-naphthoflavone (70 mg/kg; single IP dose), an AHR agonist [95]. The study showed that β-naphthoflavone induced mRNA and protein of both CYP1B1 and CYP1A1 in the whole rat uterus [95]. In the mouse uterus, mRNA and protein expression of the AHR, accompanied by ARNT, were detected in both epithelium and stroma using western blot analysis [96]. Constitutive expression of Ahr and Arnt mRNA, as well as their corresponding proteins, has been detected in endometrium and myometrium from reproductive age and post-menopausal women [97–99]. The activation of the AHR signaling pathway in the human uterus has been shown by the induction of both Cyp1a1 and Cyp1b1 mRNA after TCDD exposure (1 pM - 10 nM) [98,100], which coincides with downregulation of Ahr mRNA in cultured human endometrial cells [101]. The fact that the AHR is expressed ubiquitously in mammalian uteri and that it responds to exogenous ligands provides evidence for a role of a functional AHR in uterine physiological function.
The second line of evidence supporting a functional role of the AHR in uterine tissues is obtained from studies that have shown that changes in both gene and protein expression of the AHR are correlated with menstrual cyclicity. Western blot and immunohistochemistry analyses conducted by Küchenhoff et al. on endometrial tissue of healthy women with regular menstrual cycles revealed changes in the expression of the AHR over the cycle [97]. These changes consisted of an increase in the expression of AHR protein in the proliferative phase of the endometrium, reaching the maximum expression around the time of the ovulation, and decreasing in the secretory phase. The expression of the AHR was limited to the cytoplasm in the epithelial cells of the endometrial glands, and it was not detected in the stroma [97]. A further study also conducted in healthy women, however, showed that stroma cells cultured from endometrium in the proliferative phase express AHR protein in both the cytoplasm and nucleus, as evidenced by immunohistochemistry analysis [99]. These findings suggest that the AHR may translocate to the nucleus where it triggers its signaling pathway [99]. In addition, Pitt et al. observed that endometrial explants from women during the uterine proliferative phase manifested the highest activation of the AHR after TCDD treatment (10 nM) [98]. While most studies indicate that AHR expression in the uterus changes during the menstrual cycle, one study reported no change of Ahr and Arnt mRNA during the menstrual cycle in healthy women [31].
Even though different results have been reported regarding the level and pattern of expression of the AHR during the menstrual cycle, one cannot exclude the possibility that the AHR-signaling pathway is related to changes in the menstrual cycle (Figure 2). Differences observed in the distinct studies may be attributed to several circumstances that were not controlled or that inevitably occurred such as: 1) the limited number of individual tissues evaluated; 2) possible exposure of participating women to toxic compounds that may alter the AHR signaling pathway (i.e., compounds found in cigarette smoke); 3) disturbances in the functioning of the ovary, which are known to influence uterine activity; and 4) possible genetic variation among participating women such as genetic polymorphisms that may contribute to altered AHR-mediated signaling pathway, as have been reported for differences in susceptibility to malignant gynecological conditions [31,102].
The third line of evidence supporting a functional role of the AHR in the uterus is provided by studies that have shown that the AHR is involved in the secretory function of the uterus (Figure 2). For example, protein secretion and gene transcription of glycodelin, a predominant glycoprotein produced by secretory-phase endometrial cells, are up-regulated by TCDD treatment (10 nM) in an AHRE-dependent manner in cultured AHR-expressing human endometrial adrenocarcinoma cells [103]. Further, luminal epithelium from ovariectomized AHRKO and WT mice has secretory activity in response to E2 treatment (1.5 μg/kg; single IP dose), as evidenced by uterine epithelial proliferation and mRNA expression of lactoferrin, epithelial secretory protein [96]. However, TCDD (5 μg/kg; single IP dose) and E2 (1.5 μg/kg; single IP dose) treatment results in partial inhibition of both uterine epithelial proliferation and mRNA expression of lactoferrin in WT mice, but not in AHRKO mice [96]. These results suggest that activation of the AHR in the endometrium may be necessary to control uterine epithelial proliferation as well as secretory function, possibly via regulating the estrogen receptor as recently shown in luminal epithelium [104].
The fourth reason for supporting the hypothesis that the AHR is involved in uterine function comes from studies indicating that the AHR may have a functional role in the establishment and maintenance of normal pregnancy. For example, pregnant mice at midgestational ages have significantly higher levels of AHR in the placenta compared with nonpregnant mice, suggesting that the AHR is regulated by pregnancy-specific hormones [105]. Further evidence supporting activation of the AHR in the uterus in response to hormonal changes promoted by pregnancy comes from immunohistochemical comparisons between endometria from nonpregnant and pregnant rabbits, which showed a distinct pattern of AHR expression by pregnancy state [106]. Specifically, the AHR was localized in the cytoplasm of glandular epithelial endometrium in nonpregnant rabbits during the pre-implantation period, whereas the AHR was localized in the nucleus in pregnant rabbits, indicating control of AHR expression by maternal steroids [106]. These results suggest a functional role of the AHR pathway in the endometrium during the pre-implantation period, which may be due to higher activity of the AHR pathway during feto-maternal interactions (Figure 2). This concept is supported by studies of Tscheudschilsuren et al. in which the investigators evaluated the co-expression of AHR and ARNT within the uterine and uroplacental tissues in rabbits using western blot, non radioactive in situ hybridization, and immunohistochemistry [107]. They found that shortly prior to implantation of the embryo, the AHR and ARNT proteins were restricted to the epithelium in the uterine glands, whereas after implantation, AHR and ARNT were present in the differentiated maternal stroma [107]. Another study supporting AHR activity in feto-maternal interactions was conducted by Kitajima et al. who examined AHR expression in the peri-implantation period of the mouse uterus using immunohistochemistry [108]. The investigators found that during the implantation period, the AHR was localized in the blood vessels of the stroma and smooth muscle cells [108]. Once the embryo was implanted, the AHR was localized in the nucleus of the luminal epithelium and differentiated stroma cells. Finally, as the pregnancy was established in the uterus, the AHR was more evident in the walls of blood vessels in the decidualizing zone [108]. Thus, changes in the pattern of AHR expression during pregnancy are possibly needed to maintain pregnancy. This idea is supported by studies indicating that AHRKO mice have an impaired ability to support embryo-fetal development [23]. The mechanisms by which the expression of AHR is regulated by pregnancy are unknown, but may be related to pregnancy-specific hormones since the AHR has been shown to be regulated by progesterone and 17β-E2 [94,105,106,109].
Taken in sum, evidence from several studies suggests that the AHR is involved in the normal function of the endometrium, possibly by modulating cellular proliferation in response to hormones, by regulating the expression of upstream genes involved in the function of the endometrium, and/or by controlling uterine secretory function. Importantly, the AHR may be essential for successful pregnancy in mammals, but more studies are needed to elucidate whether the AHR is regulated by pregnancy-specific hormones and corresponding receptors, and/or whether the AHR has a role in the placentation.
5. The physiological role of the AHR in the vagina
The vagina is a tube composed of four layers: i) a superficial layer of noncornified stratified squamous epithelium; ii) a subepithelial layer of dense connective tissue; iii) a layer of smooth muscle (tunica muscularis); and iv) a layer of loose connective tissue. In terms of physiology, the vagina plays a critical role in providing support to the pelvic organs. In terms of pregnancy, the vagina participates in maintaining, holding, and delivering a pregnancy [19,110].
The AHR may play a role in the development of the vagina. This suggestion is supported by studies that have shown that embryonic exposure to TCDD (1 μg/kg; single oral dose) during a period that includes major organogenesis, induces malformations in the external genitalia such as the presence of a vaginal thread that leads to delayed vaginal opening in rats [20,44,111,112] as well as in hamsters [113]. Furthermore, it has been shown that exposure to TCDD during embryonic life interferes with vaginal development by impairing regression of the Wolffian ducts, and by preventing fusion of the Müllerian ducts [111,112], events required for normal development of the vagina and other organs such as the uterus and cervix [19]. Collectively, these studies suggest that the AHR signaling pathway may be involved in the timing of morphogenetic events in the developing vagina (Figure 2), possibly via crosstalk with critical regulators of cell proliferation, cell movement, as well as activity of hormones and corresponding receptors that control fusion of the Müllerian tube [111].
The role of the AHR in the vagina has been not been well studied in the adult. One published study in this field focused on localizing the AHR in the vagina from pregnant and nonpregnant rabbits [32]. In the study, Hasan and Fischer found that in nonpregnant rabbits, the AHR was localized in the cytoplasm and some nuclei of the epithelium, whereas no AHR expression was present in the tunica muscularis [32]. In the preimplantation period of pregnant rabbits, however, no AHR was localized in the epithelium, whereas the tunica muscularis showed both cytoplasmic and nuclear AHR expression [32]. These results suggest that the AHR in the vagina may respond and/or is involved in structural changes promoted by pregnancy. Collectively, these data suggest a functional role of the AHR in both developing and adult vagina. However, more studies are needed to confirm whether this is the case. For example, studies should compare the morphology and function of the adult vagina in WT and AHRKO mice.
6. The physiological role of the AHR in cyclicity, fertility, and reproductive senescence
The data described in the previous sections suggest that the AHR plays a role in regulating the normal functions of the organs comprising the female reproductive system (Figure 2). Proper function of the female reproductive organs is needed for estrous/menstrual cyclicity, fertility and normal reproductive senescence [19,114]. Thus, it is not surprising that the AHR plays a role in regulating these reproductive functions. For example, some studies support a role of the AHR in regulating estrous cyclicity. Specifically, TCDD exposure (10 μg/kg; single oral dose) causes irregular estrous cycles in rats, which are characterized by the loss of proestrous and estrous phases [74] or by persistent estrus or diestrus at earlier age compared to unexposed rats [115,116]. In addition, alterations in cyclicity have been observed in a follow-up study conducted in women accidentally exposed to high concentrations of TCDD after an industrial accident [117]. The study showed an association between irregular menstrual cycles in women who were premenarcheal at the time of the accidental exposure and high concentrations of TCDD in serum (10 – 100 ppt) [117]. While studies using TCDD support a role of the AHR in regulating estrous cyclicity, studies using distinct recombinant AHRKO mouse models have provided contrasting results. For example, Baba et al. observed abnormal estrous cycles in AHRKO mice compared to WT mice [24], whereas Barnett et al. observed no difference between AHRKO and WT mice regarding the amount of time spent in each stage of the cycle over a 20 day period [72]. The reasons contributing to the differences observed in studies on cyclicity in AHRKO mice may be due to genetic background effects and differences in environmental factors [118].
Studies also show that the AHR plays a role in regulating fertility [23,24,82,119,120]. Since the AHR peptide sequence is not well conserved across species [45], the first studies on the role of the AHR in fertility were conducted in mouse lines that had been developed from progenitors possessing a high-affinity AHR receptor (AHR-responsive strain), as well as from progenitors possessing a low-affinity AHR receptor (AHR-non responsive strain) [119]. These studies showed that mice possessing the high-affinity AHR receptor exhibit enhanced breeding efficiency and give birth to more pups compared to mice possessing the low-affinity AHR receptor [119], suggesting that the activity of the AHR plays a role in successful fertility. Later studies using AHRKO mice support this role of the AHR in regulating fertility [23,24]. Specifically, Abbott et al. found that AHRKO females had difficulty maintaining pregnancy, surviving pregnancy and lactation, and rearing pups to weaning [23]. Baba et al. reported that the AHRKO females had fewer and smaller litters when compared to WT females [24]. Additionally, some studies using modulation of the AHR-dependant gene expression by different AHR ligands report reduced conception rates and increased abortion [82,121]. Thus, the AHR may play a role in regulating fertility, possibly by regulating the functioning of the female reproductive organs, as discussed above in each section.
Reproductive senescence refers to the loss of reproductive function with age. It is characterized by depletion of the follicle reserves, high FSH levels, and low E2 production [122,123]. Although Ahr deletion does not result in depletion of follicle reserves or alteration in FSH levels as mentioned in previous sections [16,72], the AHR plays a role in ovarian function by modulating E2 production, follicle growth and ovulation [53,57]. The role of the AHR in regulating ovarian function and differences observed in ovarian function at different ages of sexual maturity [16,124] suggest that the AHR may play a role in regulating reproductive senescence. The molecular mechanisms by which the AHR regulates this physiological process are unknown. Hence, a thorough understanding of either the activation or the lack of the AHR transduction signaling pathway during the reproductive life span is needed to understand the role of the AHR in regulating reproductive senescence. For example, it would be interesting to elucidate whether critical genes involved in survival and longevity are altered as a consequence of the activation or lack of the AHR pathway.
7. Conclusions
Transgenic mouse models and the use of AHR potent ligands have been useful for understanding whether alterations of AHR function impact the female reproductive system. Although the intrinsic role of the AHR in the female reproductive system is of recent and ongoing investigation, existing evidence suggests that the AHR may be involved in the following functions: i) regulation of ovarian function by controlling the number and growth of antral follicles, as well the ability to produce steroids and to reach ovulation; ii) providing a optimum environment for fertilization, nourishing the embryo and maintaining pregnancy; iii) regulating fertility; and iv) controlling reproductive senescence.
Future studies should examine whether the AHR participates in other functions of the female reproductive system. For example, it is unknown whether the AHR participates in embryonic development of the uterus and oviducts. It is also unknown whether the participation of the AHR regulating reproductive senescence is directly caused by the AHR pathway or whether the AHR pathway modifies genes involving longevity. Furthermore, it is unknown whether a combination of activation and deletion of the AHR signaling pathway are needed to regulate functions of the female reproductive system. Finally, the endogenous ligands that activate the AHR signaling pathway in the female reproductive system are unknown. Hence, additional studies are required for elucidating the complex mechanisms by which the AHR regulates female reproductive function. This will facilitate establishment of preventive/treatment measures for reproductive disorders such as infertility, abortions and reduction or lack of reproductive lifespan.
Acknowledgments
This work was supported by R01HD 047275 and NIEHS ES07326.
NON-STANDARD ABBREVIATIONS
- 3β-HSD
3β-hydroxysteroid dehydrogenase
- AHR
Aryl hydrocarbon receptor
- AHREs
AHR response elements
- AHRKO
Ahr deficient mice
- ARNT
Aryl hydrocarbon nuclear translocator
- Aromatase
Cytochrome P450c19
- Bax
Bcl2-associated X protein
- Bcl2
B-cell leukemia/lymphoma 2
- CCND2
Cyclin D2
- CDK4
Cyclin dependent kinase 4
- CL
Corpus luteum
- CLs
Corpora lutea
- COCs
Cumulus oocyte-complexes
- COX2
Cyclo-oxygenase-2
- DMBA
7,12-dimethylbenz[a]anthracene
- DMBA-DHD
9,10-dimethylbenz[a]anthracene-3,4-dihydrodiol
- E2
Estradiol
- eCG
Equine chorionic gonadotropin
- ED
Embryonic day
- ESR1
Estrogen receptor 1 (alpha)
- ESR2
Estrogen receptor 2 (beta)
- FSH
Follicle stimulating hormone
- FSHR
Follicle stimulating hormone receptor
- GnRH
Gonadotropin releasing hormone
- hCG
Human chorionic gonadotropin
- IP
Intraperitoneal
- LH
Luteinizing hormone
- LHCGR
Luteinizing hormone receptor
- P450c17
Cytochrome P450 17α-hydroxylase
- P450c19
Cytochrome P450 19
- P450scc
Cytochrome P450 cholesterol side-chain cleavage
- PCB
Polychlorinated biphenyl
- PCNA
Proliferating cell nuclear antigen
- PND
Postnatal days
- PR
Progesterone receptor
- RT-PCR
Real-time polymerase chain reaction
- TCDD
2,3,7,8-tetrachlorodibenzo-p-dioxin
- Tgfb1
Transforming growth factor beta
- WT
Wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rowlands JC, Gustafsson JA. Aryl hydrocarbon receptor-mediated signal transduction. Crit Rev Toxicol. 1997;27:109–134. doi: 10.3109/10408449709021615. [DOI] [PubMed] [Google Scholar]
- 2.Poland A, Glover E. Chlorinated dibenzo-p-dioxins: potent inducers of delta-aminolevulinic acid synthetase and aryl hydrocarbon hydroxylase. II. A study of the structure-activity relationship. Mol Pharmacol. 1973;9:736–747. [PubMed] [Google Scholar]
- 3.Poland A, Glover E, Kende AS. Stereospecific, high affinity binding of 2,3,7,8-tetrachlorodibenzo-p-dioxin by hepatic cytosol. Evidence that the binding species is receptor for induction of aryl hydrocarbon hydroxylase. J Biol Chem. 1976;251:4936–4946. [PubMed] [Google Scholar]
- 4.Petrulis JR, Perdew GH. The role of chaperone proteins in the aryl hydrocarbon receptor core complex. Chem Biol Interact. 2002;141:25–40. doi: 10.1016/s0009-2797(02)00064-9. [DOI] [PubMed] [Google Scholar]
- 5.Heid SE, Pollenz RS, Swanson HI. Role of heat shock protein 90 dissociation in mediating agonist-induced activation of the aryl hydrocarbon receptor. Mol Pharmacol. 2000;57:82–92. [PubMed] [Google Scholar]
- 6.Hankinson O. The aryl hydrocarbon receptor complex. Ann Rev Pharm Toxicol. 1995;35:307–340. doi: 10.1146/annurev.pa.35.040195.001515. [DOI] [PubMed] [Google Scholar]
- 7.Reyes H, Reisz-Porszasz S, Hankinson O. Identification of the Ah receptor nuclear translocator protein (Arnt) as a component of the DNA binding form of the Ah receptor. Science. 1992;256:1193–1195. doi: 10.1126/science.256.5060.1193. [DOI] [PubMed] [Google Scholar]
- 8.Hoffman EC, Reyes H, Chu FF, Sander F, Conley LH, Brooks BA, et al. Cloning of a factor required for activity of the Ah (dioxin) receptor. Science. 1991;252:954–958. doi: 10.1126/science.1852076. [DOI] [PubMed] [Google Scholar]
- 9.Hankinson O. Role of coactivators in transcriptional activation by the aryl hydrocarbon receptor. Arch Biochem Biophys. 2005;433:379–386. doi: 10.1016/j.abb.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 10.Safe SH. Modulation of gene expression and endocrine response pathways by 2,3,7,8-tetrachlorodibenzo-p-dioxin and related compounds. Pharmacol Ther. 1995;67:247–281. doi: 10.1016/0163-7258(95)00017-b. [DOI] [PubMed] [Google Scholar]
- 11.Whitlock JJ. Induction of cytochrome P4501A1. Ann Rev Pharmacol Toxicol. 1999;39:103–125. doi: 10.1146/annurev.pharmtox.39.1.103. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen LP, Bradfield CA. The search for endogenous activators of the aryl hydrocarbon receptor. Chem Res Toxicol. 2008;21:102–116. doi: 10.1021/tx7001965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernandez-Salguero P, Pineau T, Hilbert DM, McPhail T, Lee SS, Kimura S, et al. Immune system impairment and hepatic fibrosis in mice lacking the dioxin-binding Ah receptor. Science. 1995;268:722–726. doi: 10.1126/science.7732381. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt JV, Su GH, Reddy JK, Simon MC, Bradfield CA. Characterization of a murine Ahr null allele: involvement of the Ah receptor in hepatic growth and development. Proc Natl Acad Sci U S A. 1996;93:6731–6736. doi: 10.1073/pnas.93.13.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mimura J, Yamashita K, Nakamura K, Morita M, Takagi TN, Nakao K, et al. Loss of teratogenic response to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in mice lacking the Ah (dioxin) receptor. Genes Cells. 1997;2:645–654. doi: 10.1046/j.1365-2443.1997.1490345.x. [DOI] [PubMed] [Google Scholar]
- 16.Benedict JC, Lin TM, Loeffler IK, Peterson RE, Flaws JA. Physiological role of the aryl hydrocarbon receptor in mouse ovary development. Toxicol Sci. 2000;56:382–388. doi: 10.1093/toxsci/56.2.382. [DOI] [PubMed] [Google Scholar]
- 17.Pollenz RS. The mechanism of AH receptor protein down-regulation (degradation) and its impact on AH receptor-mediated gene regulation. Chem Biol Interact. 2002;141:41–61. doi: 10.1016/s0009-2797(02)00065-0. [DOI] [PubMed] [Google Scholar]
- 18.Chaffin CL, Hutz RJ. Regulation of the aromatic hydrocarbon receptor (AHR) by in-utero and lactational exposure to 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin (TCDD) J Reprod Dev. 1997;43:47–51. [Google Scholar]
- 19.Johnson MH, Everitt BJ. Essential Reproduction. 5. Cambridge, MA: Blackwell Science; 1995. [Google Scholar]
- 20.Gray LE, Jr, Ostby JS. In utero 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) alters reproductive morphology and function in female rat offspring. Toxicol Appl Pharmacol. 1995;133:285–294. doi: 10.1006/taap.1995.1153. [DOI] [PubMed] [Google Scholar]
- 21.Li X, Johnson DC, Rozman KK. Reproductive effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in female rats: ovulation, hormonal regulation, and possible mechanism(s) Toxicol Appl Pharmacol. 1995;133:321–327. doi: 10.1006/taap.1995.1157. [DOI] [PubMed] [Google Scholar]
- 22.Fernandez-Salguero PM, Ward JM, Sundberg JP, Gonzalez FJ. Lesions of aryl-hydrocarbon receptor-deficient mice. Vet Pathol. 1997;34:605–614. doi: 10.1177/030098589703400609. [DOI] [PubMed] [Google Scholar]
- 23.Abbott BD, Schmid JE, Pitt JA, Buckalew AR, Wood CR, Held GA, et al. Adverse Reproductive Outcomes in the Transgenic Ah Receptor-Deficient Mouse. Toxicol Appl Pharmacol. 1999;155:62–70. doi: 10.1006/taap.1998.8601. [DOI] [PubMed] [Google Scholar]
- 24.Baba T, Mimura J, Nakamura N, Harada N, Yamamoto M, Morohashi K-I, et al. Intrinsic function of the aryl hydrocarbon (dioxin) receptor as a key factor in female reproduction. Mol Cell Biol. 2005;25:10040–10051. doi: 10.1128/MCB.25.22.10040-10051.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirshfield AN. Development of follicles in the mammalian ovary. Int Rev Cytol. 1991;124:43–101. doi: 10.1016/s0074-7696(08)61524-7. [DOI] [PubMed] [Google Scholar]
- 26.McGee EA, Hsueh AJ. Initial and cyclic recruitment of ovarian follicles. Endocr Rev. 2000;21:200–214. doi: 10.1210/edrv.21.2.0394. [DOI] [PubMed] [Google Scholar]
- 27.Richards JS. Hormonal control of gene expression in the ovary. Endocr Rev. 1994;15:725–751. doi: 10.1210/edrv-15-6-725. [DOI] [PubMed] [Google Scholar]
- 28.Karchner SI, Powell WH, Hahn ME. Identification and functional characterization of two highly divergent aryl hydrocarbon receptors (AHR1 and AHR2) in the teleost fundulus heteroclitus. Evidence for a novel subfamily of ligand-binding basic helix loop helix-per-arnt-sim (bHLH-PAS) factors. J Biol Chem. 1999;274:33814–33824. doi: 10.1074/jbc.274.47.33814. [DOI] [PubMed] [Google Scholar]
- 29.Davis BJ, McCurdy EA, Miller BD, Lucier GW, Tritscher AM. Ovarian tumors in rats induced by chronic 2,3,7,8-tetrachlorodibenzo-p-dioxin treatment. Cancer Res. 2000;60:5414–5419. [PubMed] [Google Scholar]
- 30.Robles R, Morita Y, Mann KK, Perez GI, Yang S, Matikainen T, et al. The aryl hydrocarbon receptor, a basic helix-loop-helix transcription factor of the PAS gene family, is required for normal ovarian germ cell dynamics in the mouse. Endocrinology. 2000;141:450–453. doi: 10.1210/endo.141.1.7374. [DOI] [PubMed] [Google Scholar]
- 31.Khorram O, Garthwaite M, Golos T. Uterine and ovarian aryl hydrocarbon receptor (AHR) and aryl hydrocarbon receptor nuclear translocator (ARNT) mRNA expression in benign and malignant gynaecological conditions. Mol Hum Reprod. 2002;8:75–80. doi: 10.1093/molehr/8.1.75. [DOI] [PubMed] [Google Scholar]
- 32.Hasan A, Fischer B. Epithelial cells in the oviduct and vagina and steroid-synthesizing cells in the rabbit ovary express AhR and ARNT. Anat Embryol (Berl) 2003;207:9–18. doi: 10.1007/s00429-003-0318-5. [DOI] [PubMed] [Google Scholar]
- 33.Bussmann UA, Barañao JL. Regulation of aryl hydrocarbon receptor expression in rat granulosa cells. Biol Reprod. 2006;75:360–369. doi: 10.1095/biolreprod.106.053017. [DOI] [PubMed] [Google Scholar]
- 34.Baldridge MG, Hutz RJ. Autoradiographic localization of aromatic hydrocarbon receptor (AHR) in rhesus monkey ovary. Am J Primatol. 2007;69:681–691. doi: 10.1002/ajp.20381. [DOI] [PubMed] [Google Scholar]
- 35.Nestler D, Risch M, Fischer B, Pocar P. Regulation of aryl hydrocarbon receptor activity in porcine cumulus-oocyte complexes in physiological and toxicological conditions: the role of follicular fluid. Reproduction. 2007;133:887–897. doi: 10.1530/REP-06-0246. [DOI] [PubMed] [Google Scholar]
- 36.McClellan KA, Gosden R, Taketo T. Continuous loss of oocytes throughout meiotic prophase in the normal mouse ovary. Dev Biol. 2003;258:334–348. doi: 10.1016/s0012-1606(03)00132-5. [DOI] [PubMed] [Google Scholar]
- 37.Pepling ME, Spradling AC. Female mouse germ cells form synchronously dividing cysts. Development. 1998;125:3323–3328. doi: 10.1242/dev.125.17.3323. [DOI] [PubMed] [Google Scholar]
- 38.Evans CW, Robb DI, Tuckett F, Challoner S. Regulation of meiosis in the foetal mouse gonad. J Embryol Exp Morphol. 1982;68:59–67. [PubMed] [Google Scholar]
- 39.Pepling ME, Spradling AC. Mouse ovarian germ cell cysts undergo programmed breakdown to form primordial follicles. Dev Biol. 2001;234:339–351. doi: 10.1006/dbio.2001.0269. [DOI] [PubMed] [Google Scholar]
- 40.Zuckerman S. The number of oocytes in the mature ovary. Recent Prog Horm Res. 1951;6:63–109. [Google Scholar]
- 41.Matikainen T, Perez GI, Jurisicova A, Pru JK, Schlezinger JJ, Ryu HY, et al. Aromatic hydrocarbon receptor-driven Bax gene expression is required for premature ovarian failure caused by biohazardous environmental chemicals. Nat Genet. 2001;28:355–360. doi: 10.1038/ng575. [DOI] [PubMed] [Google Scholar]
- 42.Matikainen T, Moriyama T, Morita Y, Perez GI, Korsmeyer SJ, Sherr DH, et al. Ligand Activation of the Aromatic Hydrocarbon Receptor Transcription Factor Drives Bax-Dependent Apoptosis in Developing Fetal Ovarian Germ Cells. Endocrinology. 2002;143:615–620. doi: 10.1210/endo.143.2.8624. [DOI] [PubMed] [Google Scholar]
- 43.Changge F, Caiqiao Z, Huili Q, Guoliang X, Yaoxing C. Sexual difference in gonadal development of embryonic chickens after treatment of polychlorinated biphenyls. Chinese Science Bulletin. 2001;46:1900–1903. [Google Scholar]
- 44.Flaws JA, Sommer RJ, Silbergeld EK, Peterson RE, Hirshfield AN. In utero and lactational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) induces genital dysmorphogenesis in the female rat. Toxicol Appl Pharmacol. 1997;147:351–362. doi: 10.1006/taap.1997.8295. [DOI] [PubMed] [Google Scholar]
- 45.Reichard JF, Dalton TP, Shertzer HG, Puga A. Induction of Oxidative Stress Responses by Dioxin and other Ligands of the Aryl Hydrocarbon Receptor. Dose Response. 2005;3:306–331. doi: 10.2203/dose-response.003.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hunt PA, Hassold TJ. Human female meiosis: what makes a good egg go bad? Trends Genet. 2008;24:86–93. doi: 10.1016/j.tig.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 47.Griffin J, Emery BR, Huang I, Peterson CM, Carrell DT. Comparative analysis of follicle morphology and oocyte diameter in four mammalian species (mouse, hamster, pig, and human) J Exp Clin Assist Reprod. 2006;3:2. doi: 10.1186/1743-1050-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Makabe S, Naguro T, Stallone T. Oocyte-follicle cell interactions during ovarian follicle development, as seen by high resolution scanning and transmission electron microscopy in humans. Microsc Res Tech. 2006;69:436–449. doi: 10.1002/jemt.20303. [DOI] [PubMed] [Google Scholar]
- 49.Magoffin DA. Ovarian theca cell. Int J Biochem Cell Biol. 2005;37:1344–1349. doi: 10.1016/j.biocel.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 50.Richards JS. Perspective: the ovarian follicle--A perspective in 2001. Endocrinology. 2001;142:2184–2193. doi: 10.1210/endo.142.6.8223. [DOI] [PubMed] [Google Scholar]
- 51.Stocco C, Telleria C, Gibori G. The molecular control of corpus luteum formation, function, and regression. Endocr Rev. 2007;28:117–149. doi: 10.1210/er.2006-0022. [DOI] [PubMed] [Google Scholar]
- 52.Baldridge MG, Stahl RL, Gerstenberger SL, Tripoli V, Hutz RJ. Modulation of ovarian follicle maturation in Long-Evans rats exposed to polychlorinated biphenyls (PCBs) in-utero and lactationally. Reprod Toxicol. 2003;17:567–573. doi: 10.1016/s0890-6238(03)00095-9. [DOI] [PubMed] [Google Scholar]
- 53.Barnett KR, Tomic D, Gupta RK, Miller KP, Meachum S, Paulose T, et al. The Aryl Hydrocarbon Receptor Affects Mouse Ovarian Follicle Growth via Mechanisms Involving Estradiol Regulation and Responsiveness. Biol Reprod. 2007;76:1062–1070. doi: 10.1095/biolreprod.106.057687. [DOI] [PubMed] [Google Scholar]
- 54.Bussmann UA, Bussmann LE, Barañao JL. An aryl hydrocarbon receptor agonist amplifies the mitogenic actions of estradiol in granulosa cells: evidence of involvement of the cognate receptors. Biol Reprod. 2006;74:417–426. doi: 10.1095/biolreprod.105.043901. [DOI] [PubMed] [Google Scholar]
- 55.Gosden R, Spears N. Programmed cell death in the reproductive system. Br Med Bull. 1997;53:644–661. doi: 10.1093/oxfordjournals.bmb.a011636. [DOI] [PubMed] [Google Scholar]
- 56.Matsuda-Minehata F, Inoue N, Goto Y, Manabe N. The regulation of ovarian granulosa cell death by pro- and anti-apoptotic molecules. J Reprod Dev. 2006;52:695–705. doi: 10.1262/jrd.18069. [DOI] [PubMed] [Google Scholar]
- 57.Benedict JC, Miller KP, Lin TM, Greenfeld C, Babus JK, Peterson RE, et al. Aryl hydrocarbon receptor regulates growth, but not atresia, of mouse preantral and antral follicles. Biol Reprod. 2003;68:1511–1517. doi: 10.1095/biolreprod.102.007492. [DOI] [PubMed] [Google Scholar]
- 58.Heimler I, Trewin AL, Chaffin CL, Rawlins RG, Hutz RJ. Modulation of ovarian follicle maturation and effects on apoptotic cell death in Holtzman rats exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in utero and lactationally. Reprod Toxicol. 1998;12:69–73. doi: 10.1016/s0890-6238(97)00101-9. [DOI] [PubMed] [Google Scholar]
- 59.Drummond AE, Britt KL, Dyson M, Jones ME, Kerr JB, O’Donnell L, et al. Ovarian steroid receptors and their role in ovarian function. Mol Cell Endocrinol. 2002;191:27–33. doi: 10.1016/s0303-7207(02)00052-7. [DOI] [PubMed] [Google Scholar]
- 60.Findlay JK, Britt K, Kerr JB, O’Donnell L, Jones ME, Drummond AE, Simpson ER. The road to ovulation: the role of oestrogens. Reprod Fertil Dev. 2001;13:543–547. doi: 10.1071/rd01071. [DOI] [PubMed] [Google Scholar]
- 61.Huhtaniemi I. The Parkes lecture: Mutations of gonadotropin and gonadotropin receptor genes: what do they teach us about reproductive physiology? J Reprod Fertil. 2001;119:173–186. doi: 10.1530/jrf.0.1190173. [DOI] [PubMed] [Google Scholar]
- 62.Drummond AE. The role of steroids in follicular growth. Reprod Biol Endocrinol. 2006;10:1–11. doi: 10.1186/1477-7827-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Donadeu FX, Ascoli M. The differential effects of the gonadotropin receptors on aromatase expression in primary cultures of immature rat granulosa cells are highly dependent on the density of receptors expressed and the activation of the inositol phosphate cascade. Endocrinology. 2005;146:3907–3916. doi: 10.1210/en.2005-0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chaffin CL, Peterson RE, Hutz RJ. In utero and lactational exposure of female Holtzman rats to 2,3,7,8-tetrachlorodibenzo-p-dioxin: modulation of the estrogen signal. Biol Reprod. 1996;55:62–67. doi: 10.1095/biolreprod55.1.62. [DOI] [PubMed] [Google Scholar]
- 65.Myllymäki SA, Haavisto TE, Brokken LJ, Viluksela M, Toppari J, Paranko J. In utero and lactational exposure to TCDD; steroidogenic outcomes differ in male and female rat pups. Toxicol Sci. 2005;88:534–544. doi: 10.1093/toxsci/kfi308. [DOI] [PubMed] [Google Scholar]
- 66.Dasmahapatra AK, Wimpee BA, Trewin AL, Wimpee CF, Ghorai JK, Hutz RJ. Demonstration of 2,3,7,8-tetrachlorodibenzo-p-dioxin attenuation of P450 steroidogenic enzyme mRNAs in rat granulosa cell in vitro by competitive reverse transcriptase-polymerase chain reaction assay. Mol Cell Endocrinol. 2000;164:5–18. doi: 10.1016/s0303-7207(00)00245-8. [DOI] [PubMed] [Google Scholar]
- 67.Grochowalski A, Chrzaszcz R, Pieklo R, Gregoraszczuk EL. Estrogenic and antiestrogenic effect of in vitro treatment of follicular cells with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Chemosphere. 2001;43:823–824. doi: 10.1016/s0045-6535(00)00440-9. [DOI] [PubMed] [Google Scholar]
- 68.Heimler I, Rawlins RG, Owen H, Hutz RJ. Dioxin perturbs, in a dose- and time-dependent fashion, steroid secretion, and induces apoptosis of human luteinized granulosa cells. Endocrinology. 1998;139:4373–4379. doi: 10.1210/endo.139.10.6264. [DOI] [PubMed] [Google Scholar]
- 69.Morán FM, VandeVoort CA, Overstreet JW, Lasley BL, Conley AJ. Molecular target of endocrine disruption in human luteinizing granulosa cells by 2,3,7,8-tetrachlorodibenzo-p-dioxin: inhibition of estradiol secretion due to decreased 17alpha-hydroxylase/17,20-lyase cytochrome P450 expression. Endocrinology. 2003;144:467–473. doi: 10.1210/en.2002-220813. [DOI] [PubMed] [Google Scholar]
- 70.Hirakawa T, Minegishi T, Abe K, Kishi H, Inoue K, Ibuki Y, et al. Effect of 2,3,7,8-tetrachlorodibenzo-p-dioxin on the expression of follicle-stimulating hormone receptors during cell differentiation in cultured granulosa cells. Endocrinology. 2000;141:1470–1476. doi: 10.1210/endo.141.4.7424. [DOI] [PubMed] [Google Scholar]
- 71.Hirakawa T, Minegishi T, Abe K, Kishi H, Ibuki Y, Miyamoto K. Effect of 2,3,7,8-tetrachlorodibenzo-p-dioxin on the expression of luteinizing hormone receptors during cell differentiation in cultured granulosa cells. Arch Biochem Biophys. 2000;375:371–376. doi: 10.1006/abbi.1999.1678. [DOI] [PubMed] [Google Scholar]
- 72.Barnett KR, Tomic D, Gupta RK, Babus JK, Roby KF, Terranova PF, et al. The aryl hydrocarbon receptor is required for normal gonadotropin responsiveness in the mouse ovary. Toxicol Appl Pharmacol. 2007;223:66–72. doi: 10.1016/j.taap.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tian Y, Ke S, Thomas T, Meeker RJ, Gallo MA. Transcriptional suppression of estrogen receptor gene expression by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) J Steroid Biochem Mol Biol. 1998;67:17–24. doi: 10.1016/s0960-0760(98)00067-3. [DOI] [PubMed] [Google Scholar]
- 74.Li X, Johnson DC, Rozman KK. Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on estrous cyclicity and ovulation in female Sprague-Dawley rats. Toxicol Lett. 1995;78:219–222. doi: 10.1016/0378-4274(95)03252-g. [DOI] [PubMed] [Google Scholar]
- 75.Son DS, Ushinohama K, Gao X, Taylor CC, Roby KF, Rozman KK, et al. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) blocks ovulation by a direct action on the ovary without alteration of ovarian steroidogenesis: lack of a direct effect on ovarian granulosa and thecal-interstitial cell steroidogenesis in vitro. Reprod Toxicol. 1999;13:521–530. doi: 10.1016/s0890-6238(99)00048-9. [DOI] [PubMed] [Google Scholar]
- 76.Petroff BK, Roby KF, Gao X, Son D, Williams S, Johnson D, Rozman KK, et al. A review of mechanisms controlling ovulation with implications for the anovulatory effects of polychlorinated dibenzo-p-dioxins in rodents. Toxicology. 2001;158:91–107. doi: 10.1016/s0300-483x(00)00367-x. [DOI] [PubMed] [Google Scholar]
- 77.Mizuyachi K, Son DS, Rozman KK, Terranova PF. Alteration in ovarian gene expression in response to 2,3,7,8-tetrachlorodibenzo-p-dioxin: reduction of cyclooxygenase-2 in the blockage of ovulation. Reprod Toxicol. 2002;16:299–307. doi: 10.1016/s0890-6238(02)00024-2. [DOI] [PubMed] [Google Scholar]
- 78.Chaffin CL, Stouffer RL, Duffy DM. Gonadotropin and steroid regulation of steroid receptor and aryl hydrocarbon receptor messenger ribonucleic acid in macaque granulosa cells during the periovulatory interval. Endocrinology. 1999;140:4753–4760. doi: 10.1210/endo.140.10.7056. [DOI] [PubMed] [Google Scholar]
- 79.Jamnongjit M, Hammes SR. Oocyte maturation: the coming of age of a germ cell. Semin Reprod Med. 2005;23:234–241. doi: 10.1055/s-2005-872451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pocar P, Augustin R, Fischer B. Constitutive expression of CYP1A1 in bovine cumulus oocyte-complexes in vitro: mechanisms and biological implications. Endocrinology. 2004;145:1594–1601. doi: 10.1210/en.2003-1254. [DOI] [PubMed] [Google Scholar]
- 81.Pocar P, Brevini TA, Antonini S, Gandolfi F. Cellular and molecular mechanisms mediating the effect of polychlorinated biphenyls on oocyte in vitro maturation. Reprod Toxicol. 2006;22:242–249. doi: 10.1016/j.reprotox.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 82.Hombach-Klonisch S, Pocar P, Kietz S, Klonisch T. Molecular actions of polyhalogenated arylhydrocarbons (PAHs) in female reproduction. Curr Med Chem. 2005;12:599–616. doi: 10.2174/0929867310504050599. [DOI] [PubMed] [Google Scholar]
- 83.Lim H, Paria BC, Das SK, Dinchuk JE, Langenbach R, Trzaskos JM, Dey SK. Multiple female reproductive failures in cyclooxygenase 2-deficient mice. Cell. 1997;91:197–208. doi: 10.1016/s0092-8674(00)80402-x. [DOI] [PubMed] [Google Scholar]
- 84.Jamnongjit M, Hammes SR. Ovarian steroids: the good, the bad, and the signals that raise them. Cell Cycle. 2006;5:1178–1183. doi: 10.4161/cc.5.11.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Petroff BK, Roby KF, Gao X, Son DS, Williamsn S, Johnson D, et al. A review of mechanisms controlling ovulation with implications for the anovulatory effects of polychlorinated dibenzo-p-dioxins in rodents. Toxicology. 2001;158:91–107. doi: 10.1016/s0300-483x(00)00367-x. [DOI] [PubMed] [Google Scholar]
- 86.Lyons RA, Saridogan E, Djahanbakhch O. The reproductive significance of human Fallopian tube cilia. Hum Reprod Update. 2006;12:363–372. doi: 10.1093/humupd/dml012. [DOI] [PubMed] [Google Scholar]
- 87.Elbi C, Misteli T, Hager GL. Recruitment of dioxin receptor to active transcription sites. Mol Biol Cell. 2002;13:2001–2015. doi: 10.1091/mboc.13.6.mk0602002001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hombach-Klonisch S, Pocar P, Kauffold J, Klonisch T. Dioxin exerts anti-estrogenic actions in a novel dioxin-responsive telomerase-immortalized epithelial cell line of the porcine oviduct (TERT-OPEC) Toxicol Sci. 2006;90:519–528. doi: 10.1093/toxsci/kfj102. [DOI] [PubMed] [Google Scholar]
- 89.Steinhauer N, Boos A, Günzel-Apel AR. Morphological changes and proliferative activity in the oviductal epithelium during hormonally defined stages of the oestrous cycle in the bitch. Reprod Domest Anim. 2004;39(2):110–119. doi: 10.1111/j.1439-0531.2004.00490.x. [DOI] [PubMed] [Google Scholar]
- 90.Shao R, Weijdegård B, Fernandez-Rodriguez J, Egecioglu E, Zhu C, Andersson N, et al. Ciliated epithelial-specific and regional-specific expression and regulation of the estrogen receptor-beta2 in the fallopian tubes of immature rats: a possible mechanism for estrogen-mediated transport process in vivo. Am J Physiol Endocrinol Metab. 2007;293:E147–E158. doi: 10.1152/ajpendo.00101.2007. [DOI] [PubMed] [Google Scholar]
- 91.Amso NN, Crow J, Lewin J, Shaw RW. A comparative morphological and ultrastructural study of endometrial gland and fallopian tube epithelia at different stages of the menstrual cycle and the menopause. Hum Reprod. 1994;9:2234–2241. doi: 10.1093/oxfordjournals.humrep.a138429. [DOI] [PubMed] [Google Scholar]
- 92.Tian Y, Ke S, Thomas T, Meeker RJ, Gallo MA. Transcriptional suppression of estrogen receptor gene expression by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) J Steroid Biochem Mol Biol. 1998;67:17–24. doi: 10.1016/s0960-0760(98)00067-3. [DOI] [PubMed] [Google Scholar]
- 93.Boverhof DR, Burgoon LD, Williams KJ, Zacharewski TR. Inhibition of estrogen-mediated uterine gene expression responses by dioxin. Mol Pharmacol. 2008;73:82–93. doi: 10.1124/mol.107.040451. [DOI] [PubMed] [Google Scholar]
- 94.Ohtake F, Baba A, Fujii-Kuriyama Y, Kato S. Intrinsic AhR function underlies cross-talk of dioxins with sex hormone signalings. Biochem Biophys Res Commun. 2008;370:541–546. doi: 10.1016/j.bbrc.2008.03.054. [DOI] [PubMed] [Google Scholar]
- 95.Bhattacharyya KK, Brake PB, Eltom SE, Otto SA, Jefcoate CR. Identification of a rat adrenal cytochrome P450 active in polycyclic hydrocarbon metabolism as rat CYP1B1. Demonstration of a unique tissue-specific pattern of hormonal and aryl hydrocarbon receptor-linked regulation. J Biol Chem. 1995;270:11595–11602. doi: 10.1074/jbc.270.19.11595. [DOI] [PubMed] [Google Scholar]
- 96.Buchanan DL, Sato T, Peterson RE, Cooke PS. Antiestrogenic effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin in mouse uterus: critical role of the aryl hydrocarbon receptor in stromal tissue. Toxicol Sci. 2000;57:302–311. doi: 10.1093/toxsci/57.2.302. [DOI] [PubMed] [Google Scholar]
- 97.Küchenhoff A, Seliger G, Klonisch T, Tscheudschilsuren G, Kaltwasser P, Seliger E, et al. Arylhydrocarbon receptor expression in the human endometrium. Fertil Steril. 1999;71:354–360. doi: 10.1016/s0015-0282(98)00437-3. [DOI] [PubMed] [Google Scholar]
- 98.Pitt JA, Feng L, Abbott BD, Schmid J, Batt RE, Costich TG, et al. Expression of AhR and ARNT mRNA in cultured human endometrial explants exposed to TCDD. Toxicol Sci. 2001;62:289–298. doi: 10.1093/toxsci/62.2.289. [DOI] [PubMed] [Google Scholar]
- 99.Zhao D, Pritts EA, Chao VA, Savouret JF, Taylor RN. Dioxin stimulates RANTES expression in an in-vitro model of endometriosis. Mol Hum Reprod. 2002;8:849–854. doi: 10.1093/molehr/8.9.849. [DOI] [PubMed] [Google Scholar]
- 100.Bofinger DP, Feng L, Chi LH, Love J, Stephen FD, Sutter TR, et al. Effect of TCDD exposure on CYP1A1 and CYP1B1 expression in explant cultures of human endometrium. Toxicol Sci. 2001;62:299–314. doi: 10.1093/toxsci/62.2.299. [DOI] [PubMed] [Google Scholar]
- 101.Yang JH. Expression of dioxin-responsive genes in human endometrial cells in culture. Biochem Biophys Res Commun. 1999;257:259–263. doi: 10.1006/bbrc.1999.0451. [DOI] [PubMed] [Google Scholar]
- 102.Tsuchiya M, Katoh T, Motoyama H, Sasaki H, Tsugane S, Ikenoue T. Analysis of the AhR, ARNT, and AhRR gene polymorphisms: genetic contribution to endometriosis susceptibility and severity. Fertil Steril. 2005;84:454–458. doi: 10.1016/j.fertnstert.2005.01.130. [DOI] [PubMed] [Google Scholar]
- 103.Mueller MD, Vigne JL, Streich M, Tee MK, Raio L, Dreher E, et al. 2,3,7,8-Tetrachlorodibenzo-p-dioxin increases glycodelin gene and protein expression in human endometrium. J Clin Endocrinol Metab. 2005;90:4809–4815. doi: 10.1210/jc.2004-2064. [DOI] [PubMed] [Google Scholar]
- 104.Kitajima M, Khan KN, Fujishita A, Masuzaki H, Ishimaru T. Histomorphometric alteration and cell-type specific modulation of arylhydrocarbon receptor and estrogen receptor expression by 2,3,7,8-tetrachlorodibenzo-p-dioxin and 17beta-estradiol in mouse experimental model of endometriosis. Reprod Toxicol. 2004;18:793–801. doi: 10.1016/j.reprotox.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 105.Wang H, Wu X, Hudkins K, Mikheev A, Zhang H, Gupta A, et al. Expression of the breast cancer resistance protein (Bcrp1/Abcg2) in tissues from pregnant mice: effects of pregnancy and correlations with nuclear receptors. Am J Physiol Endocrinol Metab. 2006;291:E1295–E1304. doi: 10.1152/ajpendo.00193.2006. [DOI] [PubMed] [Google Scholar]
- 106.Hasan A, Fischer B. Hormonal control of arylhydrocarbon receptor (AhR) expression in the preimplantation rabbit uterus. Anat Embryol (Berl) 2001;204:189–196. doi: 10.1007/s004290100209. [DOI] [PubMed] [Google Scholar]
- 107.Tscheudschilsuren G, Hombach-Klonisch S, Küchenhoff A, Fischer B, Klonisch T. Expression of the arylhydrocarbon receptor and the arylhydrocarbon receptor nuclear translocator during early gestation in the rabbit uterus. Toxicol Appl Pharmacol. 1999;160:231–237. doi: 10.1006/taap.1999.8773. [DOI] [PubMed] [Google Scholar]
- 108.Kitajima M, Khan KN, Fujishita A, Masuzaki H, Koji T, Ishimaru T. Expression of the arylhydrocarbon receptor in the peri-implantation period of the mouse uterus and the impact of dioxin on mouse implantation. Arch Histol Cytol. 2004;67:465–474. doi: 10.1679/aohc.67.465. [DOI] [PubMed] [Google Scholar]
- 109.Shanker YG, Rao AJ. Progesterone receptor expression in the human placenta. Mol Hum Reprod. 1999;5:481–486. doi: 10.1093/molehr/5.5.481. [DOI] [PubMed] [Google Scholar]
- 110.Daucher JA, Clark KA, Stolz DB, Meyn LA, Moalli PA. Adaptations of the rat vagina in pregnancy to accommodate delivery. Obstet Gynecol. 2007;109:128–135. doi: 10.1097/01.AOG.0000246798.78839.62. [DOI] [PubMed] [Google Scholar]
- 111.Dienhart MK, Sommer RJ, Peterson RE, Hirshfield AN, Silbergeld EK. Gestational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin induces developmental defects in the rat vagina. Toxicol Sci. 2000;56:141–149. doi: 10.1093/toxsci/56.1.141. [DOI] [PubMed] [Google Scholar]
- 112.Hurst CH, Abbott B, Schmid JE, Birnbaum LS. 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) disrupts early morphogenetic events that form the lower reproductive tract in female rat fetuses. Toxicol Sci. 2002;65:87–98. doi: 10.1093/toxsci/65.1.87. [DOI] [PubMed] [Google Scholar]
- 113.Wolf CJ, Ostby JS, Gray LE., Jr Gestational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) severely alters reproductive function of female hamster offspring. Toxicol Sci. 1999;51:259–264. doi: 10.1093/toxsci/51.2.259. [DOI] [PubMed] [Google Scholar]
- 114.al-Azzawi F. Endocrinological aspects of the menopause. Br Med Bull. 1992;48:262–275. doi: 10.1093/oxfordjournals.bmb.a072547. [DOI] [PubMed] [Google Scholar]
- 115.Franczak A, Nynca A, Valdez KE, Mizinga KM, Petroff BK. Effects of acute and chronic exposure to the aryl hydrocarbon receptor agonist 2,3,7,8-tetrachlorodibenzo-p-dioxin on the transition to reproductive senescence in female Sprague-Dawley rats. Biol Reprod. 2006;74:125–130. doi: 10.1095/biolreprod.105.044396. [DOI] [PubMed] [Google Scholar]
- 116.Shi Z, Valdez KE, Ting AY, Franczak A, Gum SL, Petroff BK. Ovarian endocrine disruption underlies premature reproductive senescence following environmentally relevant chronic exposure to the aryl hydrocarbon receptor agonist 2,3,7,8-tetrachlorodibenzo-p-dioxin. Biol Reprod. 2007;76:198–202. doi: 10.1095/biolreprod.106.053991. [DOI] [PubMed] [Google Scholar]
- 117.Eskenazi B, Warner M, Mocarelli P, Samuels S, Needham LL, Patterson DG, Jr, et al. Serum dioxin concentrations and menstrual cycle characteristics. Am J Epidemiol. 2002;156:383–392. doi: 10.1093/aje/kwf046. [DOI] [PubMed] [Google Scholar]
- 118.Schmidt JV, Bradfield CA. Ah receptor signaling pathways. Annu Rev Cell Dev Biol. 1996;12:55–89. doi: 10.1146/annurev.cellbio.12.1.55. [DOI] [PubMed] [Google Scholar]
- 119.Nebert DW, Brown DD, Towne DW, Eisen HJ. Association of fertility, fitness and longevity with the murine Ah locus among (C57BL/6N) (C3H/HeN) recombinant inbred lines. Biol Reprod. 1984;30:363–373. doi: 10.1095/biolreprod30.2.363. [DOI] [PubMed] [Google Scholar]
- 120.Naves LA, Daly AF, Vanbellinghen JF, Casulari LA, Spilioti C, Magalhaes AV, et al. Variable pathological and clinical features of a large Brazilian family harboring a mutation in the aryl hydrocarbon receptor-interacting protein gene. Eur J Endocrinol. 2007;157:383–391. doi: 10.1530/EJE-07-0533. [DOI] [PubMed] [Google Scholar]
- 121.McNulty WP. Toxicity and fetotoxicity of TCDD, TCDF and PCB isomers in rhesus macaques (Macaca mulatta) Environ Health Perspect. 1985;60:77–88. doi: 10.1289/ehp.856077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Meredith S, Butcher RL. Role of decreased numbers of follicles on reproductive performance in young and aged rats. Biol Reprod. 1985;32:788–794. doi: 10.1095/biolreprod32.4.788. [DOI] [PubMed] [Google Scholar]
- 123.Wise PM, Smith MJ, Dubal DB, Wilson ME, Rau SW, Cashion AB, et al. Neuroendocrine modulation and repercussions of female reproductive aging. Recent Prog Horm Res. 2002;57:235–256. doi: 10.1210/rp.57.1.235. [DOI] [PubMed] [Google Scholar]
- 124.Valdez KE, Petroff BK. Potential roles of the aryl hydrocarbon receptor in female reproductive senescence. Reprod Biol. 2004;4:243–258. [PubMed] [Google Scholar]