Abstract
To improve behavior, one must detect errors and initiate subsequent corrective adaptations. This action monitoring process has been widely studied, but little is known about how one may improve this aspect of cognition. To examine the relationship between cardiorespiratory fitness and action monitoring, we recorded the error-related negativity (ERN), an event-related brain potential believed to index action monitoring, as well as post-error behavioral indices of action monitoring from healthy young adults (18–25 years) who varied in cardiorespiratory fitness. These measures were collected during the execution of flanker tasks emphasizing response accuracy or speed to better assess the specificity of any potential relationships between fitness and action monitoring. Higher fitness was associated with greater post-error accuracy and ERN amplitude during task conditions emphasizing accuracy, as well as greater modulation of these indices across task instruction conditions. These findings suggest that higher fitness is associated with increased cognitive flexibility, evidenced through greater change in action monitoring indices as a function of task parameters. Thus, fitness may benefit action monitoring by selectively increasing cognitive control under conditions where error detection and performance adjustments are more salient.
Keywords: Cognitive Control, Action Monitoring, Error-Related Negativity (ERN), Event-Related Brain Potentials (ERPs), Fitness
During the completion of difficult cognitive tasks, an additional ongoing cognitive process is utilized to monitor performance. This process, labeled action monitoring (Gehring and Knight, 2000), is used extensively to identify instances of behavioral conflict or mistakes and direct subsequent behavior to correct these problems. Action monitoring is one example of the ability to guide thoughts and actions in a way consistent with internal intentions, termed cognitive control (Cohen et al., 2000), and has been linked to the field of cognitive neuroscience through the assessment of the error-related negativity (ERN; Gehring et al., 1993; or Ne; Falkenstein et al., 1991).
The ERN is a component of the response-locked event-related brain potential (ERP), typically identified as either a reinforcement learning index of error detection (Holroyd and Coles, 2002) or an early indicator of response conflict in association with erroneous task performance (Yeung et al., 2004). Electrophysiological dipole modeling has localized neural generators of the ERN to the anterior cingulate cortex (ACC; Dehaene et al., 1994; Herrmann et al., 2004; Holroyd et al., 1998; Miltner et al., 2003, van Veen and Carter, 2002), with hemodynamic neuroimaging research providing additional support (Carter et al., 1998; Kerns et al., 2004). Specifically, the ERN is believed to reflect the detection of conflict in the ACC (Botvinick et al., 2001; Carter et al., 1998; Yeung et al., 2004) or the transmission of a negative reinforcement learning signal to the ACC, so that the ACC may select the appropriate motor controller (Holroyd & Coles, 2002).
Investigation of the ERN has uncovered a variety of factors that influence its amplitude. Task instructions stressing accuracy over speed (Gehring et al., 1993) and psychological factors, including obsessive-compulsive disorder (Gehring et al., 2000), worry (Hajcak et al., 2003), and self-efficacy (Themanson et al., 2008) have been associated with enhanced ERN amplitudes. Researchers have suggested that these findings are due to motivational factors associated with an increased importance or salience of errors (Gehring et al., 1993; Hajcak et al., 2005). However, with regard to the manipulation of task instruction (i.e., emphasis on response speed vs. response accuracy), the conflict theory of the ERN (Yeung et al., 2004) suggests an alternative explanation, which bypasses the assumption of a motivational influence on ERN amplitude. In short, when accuracy is stressed, an increase in attentional focus on the target stimulus leads to a more rapid upsurge in post-error activation of the correct response, resulting in enhanced ERN amplitude (Yeung et al., 2004).
Although the debate about motivational influences on ERN persists, it is clear that researchers have endeavored to uncover specific individual and task characteristics that not only affect action monitoring, but also improve its function in relation to behavioral adjustments following errors. The detection of errors and the subsequent refinement of behavioral problems are important for improving one’s performance to successfully meet intentions and goals. Thus, the identification of any factors through which these action monitoring processes may be improved has implications for enhancing the quality of one’s actions in demanding cognitive situations.
One individual characteristic that has been associated with enhanced performance on tasks requiring variable amounts of cognitive control is cardiorespiratory fitness (Themanson and Hillman, 2006; Themanson et al., 2006). The examination of fitness is significant because it provides insight into a readily available, low-cost, and widely applicable means through which people may substantially improve their cognitive function (Kramer and Erickson, 2007). The cognitive benefits of fitness are apparent in both behavioral (Castelli et al., 2007; Kramer et al., 1999) and neural (Colcombe et al., 2004; Hillman et al., 2005, 2006) indices of psychomotor performance, suggesting that a physically active lifestyle resulting in higher cardiorespiratory fitness may protect or enhance cognitive health and well-being across the lifespan (see Hillman et al., 2008 for a review). Further, fitness has been associated with variations in both prefrontal and ACC activation during speeded-response task execution (Colcombe et al., 2004). Specifically, the beneficial impact of fitness appears to be strongest for aspects of cognitive function that involve extensive cognitive control (Colcombe and Kramer, 2003) and are supported by frontal and prefrontal brain regions (Colcombe et al., 2004). Notably, during the completion of three cognitive tasks, aerobically fit individuals exhibited improved performance on task conditions requiring mental flexibility, response inhibition, and working memory, but no differences were present in less challenging task conditions (Kramer et al., 1999). Thus, the beneficial effect of cardiorespiratory fitness on cognitive function was selective to cognitive control processes (Kramer et al., 1999), which supports the notion that higher levels of fitness may be related to an enhanced ability to meet task demands through greater flexibility in the application of cognitive control. Determining the extent to which fitness relates to improved flexibility in the application of cognitive control, and the nature of that improvement, will help clarify what specific aspect(s) of cognitive control may be most affected by fitness. This remains unaddressed in the literature (Kramer and Erickson, 2007), and has important implications for the use of fitness as a “treatment” to restore or enhance cognitive function.
In this study, we investigated the relationships between fitness and ERN amplitude, post-error accuracy, and post-error response time (RT) in conjunction with task instructions emphasizing either speed or accuracy to determine whether fitness was related to the ability to more flexibly implement cognitive control in response to specific task demands. A strengthening of the relationships between ERN amplitude and post-error behavior (accuracy, RT) with fitness when accuracy was stressed (compared to when speed was stressed) would indicate that higher fitness increases one’s ability to flexibly modulate the recruitment and implementation of cognitive control in response to salient task parameters. This would be evidenced through a magnification of the early detection signal indexed by the ERN and a concomitant improvement in post-error behavioral performance (i.e., response accuracy following errors) when accuracy was stressed. In contrast, similar relationships between ERN amplitude and post-error behavior with fitness across task instruction conditions would suggest that fitness may be generally related to cognitive control, but does not facilitate greater flexibility in the implementation of cognitive control in support of error detection and post-error behavioral adjustments across variable task parameters.
Experimental Procedures
Participants
Table 1 lists participants’ demographic and fitness information. Seventy-two healthy younger adults (between 18–25 years of age) were recruited from undergraduate kinesiology courses at the University of Illinois at Urbana-Champaign. Participants received extra course credit in exchange for their participation. Participants (n = 4) with fewer than five errors in each task condition (i.e., accuracy, speed) were discarded from the analyses (Hajcak et al., 2005; Vidal et al., 2000) as were participants (n = 4) who did not perform above 50% accuracy in each task condition and participants (n = 2) who were outliers (i.e., more than 3 s.d. from sample mean; 23.0 ± 11.4) on the body mass index (BMI) as evidence suggests a relationship exists between BMI and cognitive function (Cournot et al., 2006). The study was approved by the Institutional Review Board of the University of Illinois at Urbana-Champaign.
Table 1.
Mean (Standard Deviation) Demographic Information for All Participants and for All Participants Categorized by Sex
| All Participants | Males | Females | |
|---|---|---|---|
| Variable | M (SD) | M (SD) | M (SD) |
| Sample size (n) | 62 | 23 | 39 |
| Age (years) | 19.7 (1.6) | 20.0 (1.9) | 19.6 (1.4) |
| BMI | 22.5 (2.7) | 22.9 (2.7) | 22.3 (2.7) |
| IQ (K-BIT composite) | 108.2 (6.6) | 108.6 (6.8) | 107.9 (6.6) |
| VO2max (mL/kg/min) | 43.0 (8.4) | 51.2 (7.4) | 38.2 (4.1) |
| Max HR (bpm) during GXT | 192.4 (7.1) | 192.5 (7.9) | 192.4 (6.8) |
| Max RER during GXT | 1.19 (.05) | 1.19 (.05) | 1.19 (.05) |
Behavioral Task
Participants completed a modified version of the Eriksen flanker task (Eriksen and Eriksen, 1974) utilizing symbols that were either congruent (<<<<< or >>>>>) or incongruent (>><>> or <<><<) to the central target stimulus. The central target symbol “>”required a right-handed response and the central target symbol “<” required a left handed response. Thus, on congruent trials, the flanking symbols were identical to the target and did not require inhibition of the incorrect response mapping. Alternatively, on incongruent trials, the flanking symbols pointed in the opposite direction of the target, which was designed to elicit activation of the incorrect response mapping and lead to an increased amount of performance errors and response delays. Participants viewed a series of white stimuli on a black background presented focally on a computer monitor at a distance of 1 m. Symbols were 4 cm in height and were presented for 80 ms with an inter-stimulus interval (ISI) varying between 1000, 1200, and 1400 ms. The symbols were grouped into two task blocks, with a brief rest period between each block. One block was conducted under the instruction to respond as accurately as possible (i.e., accuracy instruction) and the other was conducted under the instruction to respond as quickly as possible (i.e., speed instruction). Each block contained 300 trials. Congruent and incongruent conditions were equiprobable and randomly ordered within each task block. Finally, the two blocks were counterbalanced and randomized across participants.
Cardiorespiratory Assessment
A maximal graded exercise test involving the measurement of maximal oxygen uptake (VO2max) was used to assess cardiorespiratory fitness. Specifically, aerobic endurance capacity was assessed on a motor-driven treadmill by employing a modified Balke protocol (ACSM, 2005), which involved walking/running on a treadmill at a constant speed with increasing grade increments of 2% every two minutes. The test continued until the participants were unable to continue due to volitional exhaustion. Oxygen uptake was measured continuously using open-circuit spirometry (Parvomedics True Max 2400, Sandy, UT). VO2max is conventionally determined by the highest oxygen uptake that corresponds to at least two of the following criteria: a) a plateau in VO2 values despite an increase in exercise intensity; b) maximal heart rate within 10 beats per min of the age-predicted maximum (220 bpm minus age in years); and c) a respiratory exchange ratio (RER) greater than 1.10. Heart rate was monitored using a wireless Polar heart rate monitoring system. Maximal oxygen uptake (VO2max) was measured from expired air samples taken at 30-sec intervals until the highest VO2 was attained at the point of test termination.
Neuroelectric Assessment
The electroencephalogram (EEG) was recorded from 64 sintered Ag-AgCl electrodes embedded in an elastic cap, arranged in an extended 10–20 system montage with a ground electrode (AFz) on the forehead. The sites were referenced online to a midline electrode placed at the midpoint between Cz and CPz. Vertical and horizontal bipolar electrooculographic activity (EOG) was recorded to monitor eye movements using sintered Ag-AgCl electrodes placed above and below the right orbit and near the outer canthus of each eye. Impedances were kept below 10 kΩ for all electrodes. A Neuroscan Synamps2 bioamplifier (Neuro Inc., El Paso, TX), with a 24 bit A/D converter and +/− 200 millivolt (mV) input range, was used to continuously digitize (500 Hz sampling rate; Mathalon et al., 2003), amplify (gain of 10), and filter (70 Hz low-pass filter, including a 60 Hz notch filter) the raw EEG signal in DC mode (763 µV/bit resolution). EEG activity was recorded using Neuroscan Scan software (v 4.3.1). Stimulus presentation, timing, and measurement of behavioral response time and accuracy were controlled by Neuroscan Stim (v 2.0) software.
Offline processing of the ERN component included: eye blink correction using a spatial filter (Compumedics Neuroscan, 2003), re-referencing to average mastoids, creation of response-locked epochs (−400 to 1000 ms relative to behavioral response), baseline correction (100 ms time window that runs from −100 ms to 0 ms prior to the response; Yeung et al., 2004), low-pass filtering (15 Hz; 24dB/octave), and artifact rejection (epochs with signal that exceeded ± 75µV were rejected). Average ERP waveforms for correct trials were matched to error trial waveforms on response time and number of trials to protect against differential artifacts from any stimulus-related activity (Coles et al., 2001). Matching involved selecting individual correct trials for each participant, without replacement, that matched the response time for each of the error trials for that individual. This procedure removes any differences that may exist in the timing of processing due to differences in response latency for correct and incorrect trials. Specifically, error trials tend to be associated with faster RT than correct trials (Falkenstein et al., 2001; Mathewson et al., 2005; Ridderinkhof et al., 2004; Yeung et al., 2004). Thus, any artifacts associated with that RT difference are removed by selecting a subset of correct trials that match the RT of the error trials, and results in an equal number of matched-correct trials and error trials for each individual to compare differences across accuracy conditions. ERN was quantified as the maximum negative deflection between 0–200 ms post-response in each of these two average waveforms at FCz due to evidence that localizes the ERN at or near the ACC (Carter et al., 1998; Dehaene et al., 1994; Miltner et al., 2003). Amplitude was measured as a change from the pre-stimulus baseline.
Task Performance and Assessment
Behavioral data were collected on reaction time (i.e., time in ms from the presentation of the stimulus) and response accuracy (i.e., number of correct and error responses) for all trials across task blocks. Errors of omission (non-responses to task stimuli) were categorized as incorrect responses for calculations of response accuracy, but these trials were not included in the creation of ERP waveforms due to the lack of a behavioral response. Multiple average response latencies were calculated for each participant within each instruction condition. Specifically, these latencies were calculated for: 1) correct trials, 2) error trials, 3) correct trials following an error trial (post-error RT), 4) matched-correct trials (the subset of correct trials matched to specific error trials based on RT described in the previous paragraph), and 5) correct trials following a matched-correct trial (post-matched-correct RT). Each participant’s average post-error RT (#3) was compared to his or her average post-matched-correct RT (#5) due to the consistent finding that average RT on error trials is faster than average RT on correct trials. Thus, this comparison accounts for any effects of post-error slowing that are present simply because responses on error trials generally tend to be faster than responses on correct trials.
General Procedure
For each participant, testing occurred on two separate days. Participants were recruited for the study to ensure a normal distribution of cardiorespiratory fitness levels as measured by maximal oxygen uptake (VO2max). This distribution utilized age- and sex-specific normative values of VO2 (ACSM, 2005). Session one was used to complete the paperwork and graded maximal exercise test (GXT) utilized in this study. Following the completion of the informed consent form, the participants complete a handedness inventory (Chapman and Chapman, 1987), the Kaufman Brief Intelligence Test (K-BIT; Kaufman and Kaufman, 1990) to provide an estimate of their intelligence quotient (IQ), a health history and demographics questionnaire, and had their height and weight measured to compute their Body Mass Index (BMI). After completing all of the questionnaires described above, participants then completed the GXT. This session lasted approximately 90 minutes, with the informed consent and paperwork accounting for 45 minutes, the GXT preparation and orientation accounting for 15 minutes, and the GXT, cool-down, and debriefing accounting for 30 minutes.
Session two consisted of the cognitive testing. Participants were prepared for neuroelectric assessment in accordance with the guidelines of the Society for Psychophysiological Research (Picton et al., 2000). After acceptable EEG signals were observed, the lights were dimmed and participants were given task instructions. Participants were given the opportunity to ask questions and practice trials were administered prior to each task block. Following the completion of the last task block, the electrode cap was removed and participants were briefed on the purpose of the study. This session lasted approximately 120 minutes, with the EEG preparation taking approximately 40 minutes, task orientation, instruction, and practice taking 20 minutes, cognitive testing (with rest periods) accounting for 45 minutes, and participant clean-up and debriefing lasting approximately 15 minutes.
Data Analyses
Primary analyses were conducted using multiple linear hierarchical regression analyses. The alpha level was set at p ≤ .05 for each individual analysis and all analyses included every participant in the final sample (n = 62). Prior to hypothesis testing, bivariate Pearson Product Moment correlations were calculated between the dependent variables, fitness (VO2max), and all demographic factors (i.e., age, IQ, BMI). Any variable exhibiting a significant correlation with the dependent variable was included as a covariate in the first step of the multiple linear regression analyses (Miller and Chapman, 2001). Sex was included in the first step of all regression analyses due to its established relationship with VO2max (ACSM, 2005). Further, dummy coding was utilized for sex (0 = male, 1 = female) to allow for the examination of sex differences in the dependent measures. Table 2 provides correlations among fitness, behavior, and dependent measures for each instruction condition.
Table 2.
Zero-Order Correlations Between Fitness, Behavior, and Dependent Measures for Each Instruction Condition.
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. VO2max | — | ||||||||||
| 2. Sex | −.76 | — | |||||||||
| 3. Age | .13 | −.15 | — | ||||||||
| 4. IQ (K-BIT) | .20 | −.05 | −.09 | — | |||||||
| 5. BMI | −.09 | −.11 | .16 | −.17 | — | ||||||
| 6. Accy. ERN | −.17 | −.03 | −.04 | −.11 | .13 | — | |||||
| 7. Spd. ERN | .13 | −.25 | .05 | −.08 | .04 | .75 | — | ||||
| 8. Accy. P-E PC | .22 | −.07 | .10 | .08 | .12 | −.32 | −.30 | — | |||
| 9. Spd. P-E PC | −.11 | .12 | .20 | −.04 | .08 | −.03 | −.21 | .42 | — | ||
| 10. Accy. P-E RT | −.21 | .36 | .10 | .03 | .03 | .08 | .03 | −.01 | .23 | — | |
| 11. Spd. P-E RT | −.24 | .41 | −.06 | .04 | −.03 | .02 | −.10 | −.02 | .15 | .62 | — |
Note. VO2max = maximal oxygen uptake (mL/kg/min); Sex, 0 = male, 1 = female; Accy. = accuracy instruction; Spd. = speed instruction; ERN = error-related negativity; P-E = post-error; PC = percentage correct (response accuracy); RT = response time.
All correlation coefficients ≥ .246 were significant at p ≤ .05.
Separate multiple hierarchical linear regression analyses were conducted for each dependent measure. Sex and any demographic factors (age, IQ, BMI) significantly correlated with the dependent measure were entered in the first step of the analysis and cardiorespiratory fitness (VO2max) was added in the second step of the analysis. Goodness-of-fit of the models was considered in terms of variance explained by the variables in the equation, expressed as R2. The increase in variance explained by the models was tested for significance after each step to establish whether fitness (VO2max) accounted for a significant proportion of the variance in the dependent measure.
Results
Task Performance
The mean (± s.d.) VO2 score (mL/kg/min) for males was 51.2 (± 7.4) with scores ranging from 41.7 to 65.7, which spans from the 30th to over the 90th percentile in relation to age- and sex-specific normative values of VO2 (ACSM, 2005). For females, the mean (± s.d.) VO2 score was 38.2 (± 4.1) with scores ranging from 27.1 to 49.1, which spans from below the 10th to over the 90th percentile in relation to age- and sex-specific normative values (ACSM, 2005). Finally, all participants achieved a respiratory exchange ratio (RER) greater than 1.1 and maximum heart rate (HR) greater than 90% of their age-predicted maximum HR during the treadmill test used to measure their maximal oxygen consumption (VO2max) values, verifying that maximal effort was given in order to obtain a true and accurate measure of VO2max.
In relation to overall task performance, individuals performed significantly more accurately (F(1, 61) = 61.4, p < .001; repeated-measures MANOVA) and more slowly (F(1, 61) = 131.4, p < .001) under instructions stressing accuracy (mean ± s.d. = 86% correct ± 8.7; 391 ms ± 41.4) compared to speed (mean ± s.d. = 79% correct ± 9.3; 353 ms ± 40.3). Additionally, participants were less accurate (F(1, 61) = 183.7, p < .001) and slower (F(1, 61) = 681.7, p < .001) during the completion of incongruent trials (mean ± s.d. = 77% correct ± 9.5; 390 ms ± 43.0) compared to congruent trials (mean ± s.d. = 87% correct ± 7.3; 354 ms ± 35.7). Fitness did not significantly add to the prediction of overall response accuracy or response time for either trial type in either instruction condition (F’s(1, 57) ≤ 2.0, p’s ≥ .17; multiple hierarchical regression). Finally, Bonferroni-corrected paired-samples t-tests conducted in both the speed and accuracy conditions verified the expected differences in error and correct response time (Falkenstein et al., 2001; Mathewson et al., 2005; Rabbitt, 1966; Yeung et al., 2004), with errors being significantly faster than correct trials in both the accuracy condition (t(61) = 18.7, p < .001; error RT mean ± s.d. = 323 ms ± 44.9; correct RT mean ± s.d. = 391 ms ± 42.2) and the speed condition (t(61) = 20.5, p < .001; error RT mean ± s.d. = 294 ms ± 36.7; correct RT mean ± s.d. = 352 ms ± 40.1).
ERN
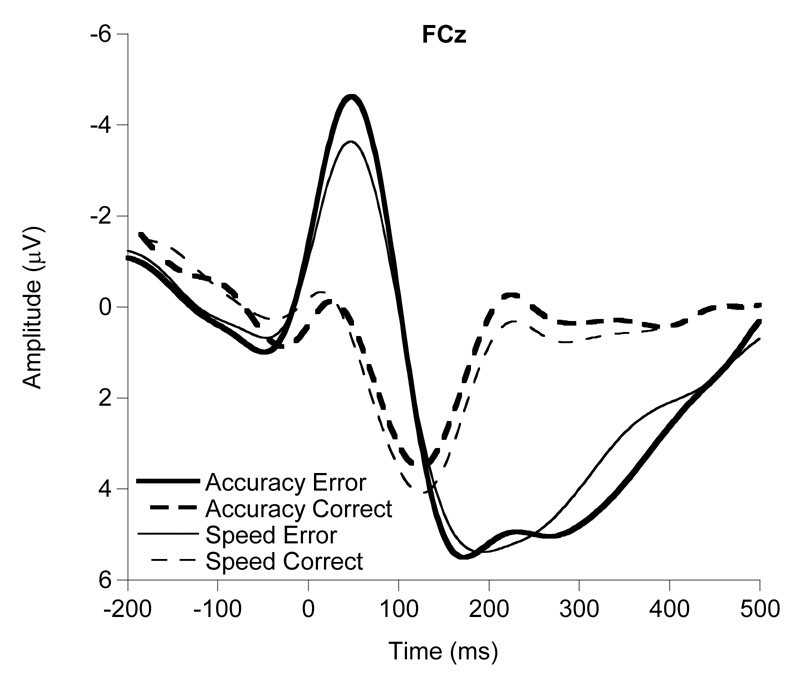
A multivariate repeated-measures ANOVA (Rodríquez-Fornells et al., 2002) was conducted on ERN amplitude to verify that these data conformed to the expected topography and trial effects. Analyses revealed the expected significant and largest trial effect (ERN amplitude larger on error vs. correct trials) at FCz, (F(1, 61) = 78.6, p < .001), with smaller but significant effects at surrounding sites, Fz (F(1, 61) = 33.7, p = .001), Cz (F(1, 61) = 22.4, p = .001), and Pz (F(1, 61) = 7.1, p = .01). Accordingly, all subsequent analyses involving ERN use amplitude scores from the error trial waveforms at FCz (Falkenstein et al., 2001; Themanson and Hillman, 2006; Themanson et al., 2006, 2008). Figure 1 provides grand-averaged waveforms by instruction condition (accuracy, speed) and response accuracy (error, correct) at FCz.
Figure 1.
Grand averaged response-locked waveforms for the accuracy and speed conditions on error and correct trials at the FCz electrode site.
To verify the established relationship (Falkenstein et al., 1990, 2000; Gehring et al., 1993, Yeung et al., 2004) between ERN amplitude and instruction condition (accuracy, speed), a paired-samples t-test was conducted that compared ERN amplitude at FCz in the accuracy condition to ERN amplitude at FCz in the speed condition for all participants. Results indicated the expected significant effect for instruction condition (t(61) = 4.1, p < .001), with larger ERN amplitude in the accuracy condition (mean ± s.d. = −6.2 µV ± 3.8) than in the speed condition (mean ± s.d. = −4.9 µV ± 3.7).
To assess the relationship between fitness and ERN amplitude, multiple hierarchical regression analyses were conducted regressing ERN amplitude in each instruction condition on sex, entered in the first step, and fitness, entered in the second step. Under accuracy instructions, the overall regression model was marginally significant (R2 = .09, F(2, 59) = 2.89, p = .06), with no significant effect for sex in the first step, but a significant fitness influence in the second step, ΔR2 = .09, F(1, 59) = 5.71, p = .02, suggesting that higher fitness was associated with larger ERN amplitude independent of sex. Under speed instructions, the overall regression model was not significant (R2 = .07, F(2, 59) = 2.22, p = .12), with no significant relationship present for sex in the first step or fitness in the second step, ΔR2 = .01, F(1, 59) = .53, p = .47, suggesting fitness was not related with ERN amplitude in the speed condition. Table 3 provides a summary of these regression analyses.
Table 3.
Summary of Regression Analyses for Variables Predicting ERN Amplitude in the Accuracy Instruction Condition (top) and Speed Instruction Condition (bottom).
| Accuracy | |||
|---|---|---|---|
| ERN | B | SE B | β |
| Step 1 | |||
| Sex | −.26 | 1.00 | −.03 |
| Step 2 | |||
| Sex | −2.94 | 1.45 | −.38* |
| Fitness | −.20 | .09 | −.46* |
| Speed | |||
| ERN | B | SE B | β |
| Step 1 | |||
| Sex | −1.87 | .94 | −.25* |
| Step 2 | |||
| Sex | −2.67 | 1.45 | −.35 |
| Fitness | −.06 | .08 | −.14 |
Note. p < .05.
An additional multiple hierarchical regression analysis was conducted to examine the difference in ERN amplitude across instruction conditions, with ERN amplitude in the speed condition subtracted from ERN amplitude in the accuracy condition for each participant due to the finding that ERN amplitude is greater in the accuracy condition. This analysis tested the possible relationship between fitness and one’s ability to flexibly modulate the cognitive control of action monitoring in accord with specific internal intentions. A larger value indicates a greater difference in ERN amplitude across the accuracy and speed instruction conditions. The overall regression model was significant (R2 = .18, F(2, 59) = 6.26, p < .01), with both a significant effect for sex in the first step (adjusted R2 = .07, F(1, 60) = 5.70, p =.02) and a significant fitness influence in the second step, ΔR2 = .09, F(1, 59) = 6.32, p = .01, indicating that being male and possessing higher fitness were both associated with greater modulation of ERN amplitude across task conditions. Table 4 provides a summary of this regression analysis.
Table 4.
Summary of Regression Analysis for Variables Predicting the Modulation of ERN Amplitude Across Task Instruction Conditions.
Note. p < .05.
Corrective Behavioral Actions
To verify the hypothesized response slowing on trials following an error, Bonferroni-corrected paired-samples t-tests were conducted for each instruction condition comparing error RT to post-error RT. In the accuracy condition, results indicated significantly longer post-error RT (t(61) = 17.5, p < .001; mean ± s.d. = 392 ms ± 48.8) compared to error RT (mean ± s.d. = 323 ms ± 44.9). Further, a significant effect was present comparing post-error RT to post-matched-correct RT (t(61) = 7.2, p < .001). In particular, post-error RT (detailed above) was significantly longer than post-matched-correct RT (mean ± s.d. = 367 ms ± 41.3).
In the speed condition, a similar pattern of findings was revealed when comparing error RT to post-error RT. Specifically, results indicated (t(61) = 20.6, p < .001) significantly longer post-error RT (mean ± s.d. = 368 ms ± 45.7) compared to error RT (mean ± s.d. = 294 ms ± 36.7). Analyses comparing post-error RT to post-matched-correct RT revealed a significant Accuracy effect (t(61) = 10.1, p < .001), with longer post-error RT compared to post-matched-correct RT (mean ± s.d. = 333 ms ± 40.4).
To assess the relationship between fitness and corrective post-error behavior, multiple hierarchical regression analyses were conducted regressing indices of post-error behavior (post-error accuracy, post-error RT) in each instruction condition on sex and overall task performance (accuracy, RT) entered as covariates in the first step, with fitness entered in the second step.
The overall regression models for accuracy instruction (R2 = .49, F(3, 58) = 18.29, p < .001) and speed instruction (R2 = .65, F(2, 63) = 61.21, p < .001) conditions were significant as both analyses revealed the expected significant effect for overall accuracy in the first step of the regression. In the accuracy instruction analysis, a significant fitness influence was present in the second step, ΔR2 = .06, F(1, 58) = 6.30, p = .01, suggesting that higher fitness was associated with greater post-error accuracy in the accuracy condition. Under speed instructions, no significant fitness influence was present (ΔR2 = .01, F(1, 58) = .65, p = .42) in the second step. Table 5 provides a summary of these regression analyses.
Table 5.
Summary of Regression Analyses for Variables Predicting Post-Error Accuracy in the Accuracy Instruction Condition (top) and Speed Instruction Condition (bottom).
| Accuracy Condition | |||
|---|---|---|---|
| Post-error | |||
| Accuracy | B | SE B | β |
| Step 1 | |||
| Overall Accy. | .70 | .10 | .65* |
| Sex | −1.46 | 1.86 | −.08 |
| Step 2 | |||
| Overall Accy. | .69 | .10 | .65* |
| Sex | 3.74 | 2.74 | .20 |
| Fitness | .40 | .16 | .36* |
| Speed Condition | |||
| Post-error | |||
| Post-error Accuracy | B | SE B | β |
| Step 1 | |||
| Overall Accy. | .87 | .09 | .80* |
| Sex | −.70 | 1.66 | −.03 |
| Step 2 | |||
| Overall Accy. | .87 | .09 | .81* |
| Sex | −2.24 | 2.53 | −.11 |
| Fitness | −.12 | .14 | −.10 |
Note. Accy. = response accuracy.
p < .05.
In analyses of post-error RT, the overall regression models for accuracy instruction (R2 = .72, F(3, 58) = 49.21, p < .001) and speed instruction (R2 = .79, F(3, 58) = 72.88, p < .001) conditions were significant as both analyses revealed the expected significant effect for overall RT in the first step of the regression. However, no significant influences were present for fitness in the second step in either the accuracy, ΔR2 = .01, F(1, 58) = .51, p = .48, or speed, ΔR2 = .01, F(1, 58) = .95, p = .33, instruction conditions, suggesting that fitness was not associated with post-error RT in either instruction condition. Table 6 provides a summary of these regression analyses.
Table 6.
Summary of Regression Analyses for Variables Predicting Post-Error RT in the Accuracy Instruction Condition (top) and Speed Instruction Condition (bottom).
| Accuracy Condition | |||
|---|---|---|---|
| Post-error RT | B | SE B | β |
| Step 1 | |||
| Overall RT | 1.00 | .09 | .87* |
| Sex | −4.89 | 7.92 | −.05 |
| Step 2 | |||
| Overall RT | 1.00 | .09 | .86* |
| Sex | 1.13 | 11.55 | .01 |
| Fitness | .45 | .62 | .08 |
| Speed Condition | |||
| Post-error RT | B | SE B | β |
| Step 1 | |||
| Overall RT | 1.02 | .08 | .89* |
| Sex | −.89 | 6.38 | −.01 |
| Step 2 | |||
| Overall RT | 1.01 | .08 | .89* |
| Sex | 5.69 | 9.28 | .06 |
| Fitness | .49 | .50 | .09 |
Note. RT = reaction time.
p < .05.
As with ERN amplitude, an additional hierarchical regression analysis was conducted to test whether fitness is related to the ability to flexibly modulate levels of cognitive control associated with task goals and parameters. In this analysis, post-error accuracy in the speed instruction condition was subtracted from post-error accuracy in the accuracy instruction condition for each participant due to the finding that post-error accuracy is greater in the accuracy condition compared to the speed condition. A larger value indicates a greater difference in post-error response accuracy across the accuracy and speed instruction conditions The overall regression model was significant (R2 = .09, F(2, 59) = 3.03, p = .05), with no significant effect for sex in the first step (adjusted R2 = .02, F(1, 60) = 2.13, p =.15), but a significant fitness effect in the second step, ΔR2 = .06, F(1, 59) = 3.84, p = .05, indicating that higher fitness was associated with greater modulation of post-error accuracy across task conditions. Table 7 provides a summary of this regression analysis.
Table 7.
Summary of Regression Analysis for Variables Predicting the Modulation of Post-Error Accuracy Across Task Instruction Conditions.
| B | SE B | β | |
|---|---|---|---|
| Step 1 | |||
| Sex | −3.94 | 2.70 | −.18 |
| Step 2 | |||
| Sex | 2.06 | 4.05 | .10 |
| Fitness | .46 | .23 | .37* |
Note. p < .05.
Discussion
Our data suggest that fitness is beneficially related to action monitoring processes, and more specifically, to an increased flexibility in the implementation of cognitive control. In particular, we found a differential relationship between fitness and both neural and behavioral indices of action monitoring across task parameters as more fit individuals displayed larger ERN amplitudes and greater post-error accuracy under instructions stressing accuracy, but not under instructions stressing speed. These data suggest that higher fitness may be related to increased flexibility in the modulation of cognitive control to meet specific task demands and correct behavior, especially when performance accuracy is most salient. Further, higher levels of cardiorespiratory fitness were related to a greater difference in ERN amplitude and post-error accuracy across task conditions. These relationships speak not only to the differences inherent in tasks emphasizing speed or accuracy, but also to the increased cognitive flexibility associated with higher cardiorespiratory fitness as evidenced by neural and behavioral indices of self-regulatory action monitoring.
Fitness and Post-error Behavior
Beyond general behavioral performance, researchers have endeavored to understand what happens to behavior after an error takes place (Gehring et al., 1993; Kerns et al., 2004; Themanson and Hillman, 2006; Themanson et al., 2006, 2008). The fundamental goal of post-error adjustments is to improve subsequent performance, and the degree to which individuals are accurate following errors is a direct index of their ability to interact with the environment. This measure has been studied since the initial examinations of post-error behavior in cognitive psychology, and research has shown that accuracy improves following error commission (Rabbitt, 1966).
In the present investigation, fitness was related to increased post-error accuracy in the accuracy condition. This is consistent with the concept that fitness may facilitate the recruitment of cognitive control to meet desired outcomes as directed by task parameters. The current task placed importance upon different aspects of performance through modifications in task instructions, without any changes to the structure or difficulty of the task. In the accuracy condition, the most salient feature was response correctness. In the speed condition, the speed of responding was stressed. However, participants still performed the task accurately and were aware of their errors, as evidenced by post-error slowing in the speed condition. Given this context, when accuracy was stressed, cognitive control was amplified following an error to improve accuracy during subsequent performance. Thus, the main goal associated with task completion and the resultant fitness-related increase in cognitive control were consistent, which led to improved response accuracy. Conversely, when speed was emphasized, being correct was no longer the single highest priority associated with task completion. Another salient feature of the task was to respond quickly. However, the recruitment of cognitive control following error commission has been associated with a slowing of performance (Gehring and Knight, 2000; Kerns et al., 2004; Rabbitt, 1966). Thus, the effects were inconsistent, as the faster responses associated with task instructions were countered by the slowing related to increases in cognitive control due to fitness.
Further, higher fitness was associated with an increased difference in post-error accuracy across task instruction conditions, with increased post-error accuracy in the accuracy condition than in the speed condition. This pattern of findings suggests that fitness may be related to the efficiency and effectiveness with which cognitive control is adapted to make requisite or desired adjustments in behavior following errors. Altering one’s lifestyle to increase fitness appears to be one means through which individuals may improve their ability to adaptively recruit and implement cognitive control.
Fitness and ERN
In addition to post-error accuracy, our data show a relationship between fitness and ERN amplitude in the accuracy condition, with more fit individuals exhibiting greater ERN amplitudes. This finding extends the post-error accuracy finding described above to include neural measures of the increased ability of fit individuals to more effectively modulate cognitive control. The ERN component is believed to reflect the transmission of a negative reinforcement learning signal to the ACC (Holyroyd and Coles, 2002) or the detection of conflict in the ACC (Botvinick et al., 2001; Carter et al., 1998; Yeung et al., 2004). Thus, the ERN provides an index of the evaluative component of cognitive control (Botvinick et al., 2004; MacDonald et al., 2000). Analysis of the ERN component provides an indication of the extent to which these evaluative processes are implemented following the commission of an error. The present findings substantiate previous research that details a relationship between task parameters and ERN amplitude, with larger ERN amplitude for tasks or task components emphasizing accurate responses. This heightened response to errors is believed to reflect either the increased salience of an error (Gehring et al., 1993) or increased attentional focus (Yeung et al., 2004) when accuracy is stressed during task completion. Thus, the present findings suggest that higher levels of cardiorespiratory fitness are related to an augmentation of the detection signal indexed by the ERN above and beyond the influence of task parameters in situations where errors are most meaningful.
Beyond the relationship between fitness and ERN amplitude in the accuracy condition, higher cardiorespiratory fitness was also associated with an increased difference in ERN amplitude across task instruction conditions of the flanker task. Although previous research has investigated task-related influences on ERN amplitude (Gehring et al., 1993; Yeung et al., 2004), no studies have examined factors that may influence the degree of ERN modulation across task instruction conditions. Cardiorespiratory fitness appears to be one factor that magnifies these task-related differences. Specifically, higher fitness was associated with greater cognitive flexibility inherent in the different instruction conditions, as indicated by a larger difference in ERN amplitudes during accuracy and speed instruction conditions. Thus, higher fit individuals may implement cognitive control with greater specificity depending on task instructions. As a result of this increased adaptability of cognitive control, the difference in ERN amplitude across task conditions is heightened for these individuals. Previous research corroborates the present findings with evidence indicating decreased ERN amplitudes during tasks emphasizing the speed of behavioral responses for more physically active individuals (Themanson et al., 2006) as well as for individuals exhibiting higher levels of cardiorespiratory fitness compared to less-fit individuals (Themanson and Hillman, 2006). These findings are consistent with the interpretation that individuals with higher levels of fitness are more adept at flexibly exerting cognitive control during task execution in accord with task demands and intended behavioral outcomes.
Potential Mechanisms
Since motivational and attentional aspects of cognitive processing were not assessed separately, the present study is unable to disentangle whether fitness influences either of these mechanisms or their relation to the modulation of ERN amplitude as a function of task instruction conditions. However, assessment of motivation and attention in collaboration with knowledge regarding potential mechanisms underlying the influence of fitness on cognitive function should provide a basis to explicitly test the differential ERN amplitude associated with task instruction conditions in future research.
At present, there appear to be several plausible mechanisms for the effect of fitness on cognitive function. In humans, aerobic fitness training and cross-sectional differences in fitness have been related to larger volumes of anterior white matter and prefrontal and temporal grey matter (Colcombe et al., 2004, 2006) as well as increased cerebral blood volume (CBV) in the dentate gyrus of the hippocampus (Pereira et al., 2007). Further, a large body of animal research has demonstrated relationships between aerobic exercise and increased levels of neurochemicals associated with neuronal survival and brain plasticity. These chemicals include dopamine (Spirduso and Farrar, 1981), brain-derived neurotrophic factor (BDNF; Neeper et al., 1995) and Insulin-like growth factor 1 (IGF-1; Carro et al., 2001) as well as others (Dishman et al., 2006). Finally, additional evidence has related increased cell proliferation and survival in the hippocampus with exercise (Trejo et al., 2001; van Praag et al., 1999), which has implications for enhanced learning and memory.
Additional explanations for the influence of fitness on cognitive function are less focused on changes in the structure and function of neuronal systems, but rather relate to other influences of fitness on performance and motor competency (Castelli and Valley, 2007). For instance, individuals who are more fit or more experienced with physical activity may have developed greater expertise in movement domains, as a result of automaticity or improved attentional flexibility. Classical research supports this notion as the relation of fitness to both central and peripheral components of movement time have been observed (Baylor and Spirduso, 1988; Clarkson, 1978; Clarkson and Kroll, 1978). Thus, fitness may be related to an increased ability to control one’s body to move and perform as intended. Still, other alternative explanations suggest that emotion, and anxiety in particular, may have an adverse relationship with cognitive performance (Eysenck et al., 2007). Given the well-established association between aerobic activity and reduced anxiety (Petruzzello et al., 1991), fitness effects on cognitive function may be related to these anxiolytic effects. Clearly, future efforts should aim to disentangle the various mechanisms to better understand how fitness influences cognition.
Limitations
Although we report on interesting relationships among fitness and behavioral and neural indices of action monitoring, there are a number of limitations to the present study. For example, while our analyses were able to determine the extent to which fitness was independently associated with post-error behavior and ERN amplitude, it is important to clarify that no causal relationships are being proposed. The cross-sectional nature of the study, as well as the lack of random assignment to levels of fitness, limits the strength of the findings because the effects may be attributable to other factors, including differences in personality, motivation, or anxiety. However, multiple demographic factors were assessed (sex, age, BMI, and IQ), which helps to reduce these variables as potential influences on the fitness findings. Future examinations employing experimental designs to manipulate fitness levels are warranted as are studies examining key variables that may moderate or mediate fitness effects on neural and behavioral indices of action monitoring. Finally, future research should implement a broader array of cognitive measures to more completely assess the relationships between fitness and indices of cognitive control and specifically test potential mechanisms for their impact on the relationship between fitness and cognitive function.
Conclusions
In summary, our results suggest that cardiorespiratory fitness may be one modifiable lifestyle factor that leads to increased cognitive health in relation to action monitoring and post-error adjustments in behavior. Notably, higher levels of cardiorespiratory fitness may be related to an individual’s improved ability to flexibly modulate and implement cognitive control to meet specific task parameters and internal intentions. Evidence for this benefit exists both in relation to post-error behavior and neural indices of cognitive processes associated with behavioral missteps. These data not only suggest a specific and powerful relationship between fitness and cognitive control processes, but they also reveal improvements in a healthy young adult population, suggesting that fitness can benefit those who are at their cognitive peak (Hillman et al., 2008; Salthouse and Davis, 2006) and may provide a readily available means for improving cognitive flexibility and behavioral adjustments to more appropriately meet task demands or intended goals.
Acknowledgements
This research was supported by grants from the National Institute of Mental Health (F31 MH076463) to Jason Themanson and the National Institute on Aging (RO1 AG021188) to Charles Hillman.
Comprehensive List of Abbreviations
- ACC
anterior cingulate cortex
- BDNF
brain-derived neurotrophic factor
- BMI
body mass index
- bpm
beats per minute
- CBV
cerebral blood volume
- cm
centimeter
- EEG
electroencephalogram
- EOG
electrooculogram
- ERN
error-related negativity
- ERP
event-related brain potential
- GXT
graded exercise test
- HR
heart rate
- Hz
Hertz
- IGF-1
insulin-like growth factor 1
- ISI
inter-stimulus interval
- kg
kilogram
- µV
microvolt
- mL
milliliter
- ms
millisecond
- min
minute
- Ne
error negativity
- RER
Respiratory exchange ratio
- RPE
ratings of perceived exertion
- RT
reaction time
- VO2max
maximal oxygen uptake
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interest Statement
The authors declare no competing or conflicting interests.
References
- American College of Sports Medicine. ACSM’s guidelines for exercise testing and prescription. 7th ed. New York: Lippincott Williams & Wilkins; 2005. [DOI] [PubMed] [Google Scholar]
- Baylor A, Spirduso W. Systematic aerobic exercise and components of reaction time in older women. J Gerontol: Psychol Sci. 1988;43:121–126. doi: 10.1093/geronj/43.5.p121. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulated cortex: an update. Trends Cogn Sci. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Carro E, Trejo LJ, Busiguina S, Torres-Aleman I. Circulating insulin-like growth factor 1 mediates the protective effects of physical exercise against brain insults of different etiology and anatomy. J Neurosci. 2001;21:5678–5684. doi: 10.1523/JNEUROSCI.21-15-05678.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulated cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Castelli DM, Hillman CH, Buck SM, Erwin HE. Physical fitness and academic achievement in third- and fifth-grade students. J Sport Exerc Psychol. 2007;29:239–252. doi: 10.1123/jsep.29.2.239. [DOI] [PubMed] [Google Scholar]
- Castelli DM, Valley JA. Chapter 3: The relationship of physical fitness and motor competence to physical activity. J Teach Phys Educ. 2007;26:358–374. [Google Scholar]
- Chapman LJ, Chapman JP. The measurement of handedness. Brain Cogn. 1987;6:175–183. doi: 10.1016/0278-2626(87)90118-7. [DOI] [PubMed] [Google Scholar]
- Clarkson PM. The effect of age and activity level on simple and choice fractionated response time. Eur J Appl Physiol. 1978;40:17–25. doi: 10.1007/BF00420985. [DOI] [PubMed] [Google Scholar]
- Clarkson P, Kroll W. Practice effect on fractionated response time related to age and activity level. J Mot Behav. 1978;10:275–286. doi: 10.1080/00222895.1978.10735161. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Botvinick M, Carter CS. Anterior cingulate and prefrontal cortex: who’s in control? Nat Neurosci. 2000;3:421–423. doi: 10.1038/74783. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Erickson KI, Scalf P, Kim JS, Prakash R, McAuley E, Elavsky S, Marquez DX, Hu L, Kramer AF. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci. 2006;61:1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychol Sci. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, Webb A, Jerome GJ, Marquez DX, Elavsky S. Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci U S A. 2004;101:3316–3321. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles MGH, Scheffers MK, Holroyd CB. Why is there an ERN/Ne on correct trials? Response representations, stimulus-related components, and the theory of error-processing. Biol Psychol. 2001;56:173–189. doi: 10.1016/s0301-0511(01)00076-x. [DOI] [PubMed] [Google Scholar]
- Compumedics Neuroscan. Offline analysis of acquired data (SCAN 4.3 - Vol. II, EDIT 4.3) El Paso, TX: Author; 2003. [Software Manual] [Google Scholar]
- Cournot M, Marquié JC, Ansiau D, Martinaud C, Fonds H, Ferrières J, Ruidavets JB. Relation between body mass index and cognitive function in healthy middle-aged men and women. Neurology. 2006;67:1208–1214. doi: 10.1212/01.wnl.0000238082.13860.50. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Posner MI, Tucker DM. Localization of a neural system for error detection and compensation. Psychol Sci. 1994;5:303–305. [Google Scholar]
- Dishman RK, Berthoud HR, Booth FW, Cotman CW, Edgerton VR, Fleshner MR, Gandevia SC, Gomez-Pinilla F, Greenwood BN, Hillman CH, Kramer AF, Levin BE, Moran TH, Russo-Neustadt AA, Salamone JD, Van Hoomissen JD, Wade CE, York DA, Zigmond MJ. The neurobiology of exercise. Obes Res. 2006;14:345–356. doi: 10.1038/oby.2006.46. [DOI] [PubMed] [Google Scholar]
- Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of a target letter in a nonresearch task. Percept Psychophys. 1974;16:143–149. [Google Scholar]
- Eysenck MW, Derakshan N, Santos R, Calvo MG. Anxiety and cognitive performance: Attentional control theory. Emotion. 2007;7:336–353. doi: 10.1037/1528-3542.7.2.336. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J, Blanke L. Effects of errors in choice reaction tasks on the ERP under focused and divided attention. In: Brunia CHM, Gaillard AWK, Kok A, editors. Psychophysiological brain research. Tilberg, the Netherlands: Tilberg University Press; 1990. pp. 192–195. [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J, Blanke L. Effects of crossmodal divided attention on late ERP components: II. Error processing in choice reaction tasks. Electroencephalogr Clin Neurophysiol. 1991;78:447–455. doi: 10.1016/0013-4694(91)90062-9. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, Christ S, Hohnsbein J. ERP components on reaction errors and their functional significance: A tutorial. Biol Psychol. 2000;51:87–107. doi: 10.1016/s0301-0511(99)00031-9. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, Hohnsbein J. Changes of error-related ERPs with age. Exp Brain Res. 2001;138:258–262. doi: 10.1007/s002210100712. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E. A neural system for error detection and compensation. Psychol Sci. 1993;4:385–390. [Google Scholar]
- Gehring WJ, Himle J, Nisenson LG. Action-monitoring dysfunction in obsessive-compulsive disorder. Psychol Sci. 2000;11:1–6. doi: 10.1111/1467-9280.00206. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Knight RT. Prefrontal-cingulate interactions in action monitoring. Nat Neurosci. 2000;3:516–520. doi: 10.1038/74899. [DOI] [PubMed] [Google Scholar]
- Hajcak G, McDonald N, Simons RF. Anxiety and error-related brain activity. Biol Psychol. 2003;64:77–90. doi: 10.1016/s0301-0511(03)00103-0. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Yeung N, Simons RF. On the ERN and the significance of errors. Psychophysiology. 2005;42:151–160. doi: 10.1111/j.1469-8986.2005.00270.x. [DOI] [PubMed] [Google Scholar]
- Herrmann MJ, Römmler J, Ehlis A, Heidrich A, Fallgatter AJ. Source localization (LORETA) of the error-related-negativity (ERN/Ne) and positivity (Pe) Cogn Brain Res. 2004;20:294–299. doi: 10.1016/j.cogbrainres.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Hillman CH, Castelli DM, Buck SM. Aerobic fitness and neurocognitive function in healthy preadolescent children. Med Sci Sports Exerc. 2005;37:1967–1974. doi: 10.1249/01.mss.0000176680.79702.ce. [DOI] [PubMed] [Google Scholar]
- Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nat Rev Neurosci. 2008;9:58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- Hillman CH, Kramer AF, Belopolsky AV, Smith DP. A cross-sectional examination of age and physical activity on performance and event-related brain potentials in a task switching paradigm. Int J Psychophysiol. 2006;59:30–39. doi: 10.1016/j.ijpsycho.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Coles MGH. The neural basis of human error processing: Reinforcement learning, dopamine, and the error-related negativity. Psychol Rev. 2002;109:679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Dien J, Coles MGH. Error-related scalp potentials elicited by hand and foot movements: Evidence for an output-independent error-processing system in humans. Neurosci Lett. 1998;242:65–68. doi: 10.1016/s0304-3940(98)00035-4. [DOI] [PubMed] [Google Scholar]
- Kaufman AS, Kaufman NL. Kaufman Brief Intelligence Test manual. Circle Pines, MN: American Guidance Service; 1990. [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, III, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Erickson KI. Capitalization on cortical plasticity: influence of physical activity on cognition and brain function. Trends Cogn Sci. 2007;11:342–348. doi: 10.1016/j.tics.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Sowon H, Cohen NJ, Banich MT, McAuley E, Harrison CR, Chason J, Vakil E, Bardell L, Boileau RA, Colcombe A. Ageing, fitness, and neurocognitive function. Nature. 1999;400:418–419. doi: 10.1038/22682. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, III, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Mathalon DH, Whitfield SL, Ford JM. Anatomy of an error: ERP and fMRI. Biol Psychol. 2003;64:119–141. doi: 10.1016/s0301-0511(03)00105-4. [DOI] [PubMed] [Google Scholar]
- Mathewson KJ, Dywan J, Segalowitz SJ. Brain bases of error-related ERPs as influenced by age and task. Biol Psychol. 2005;70:88–104. doi: 10.1016/j.biopsycho.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Miller GA, Chapman JP. Misunderstanding analysis of covariance. J Abnorm Psychol. 2001;110:40–48. doi: 10.1037//0021-843x.110.1.40. [DOI] [PubMed] [Google Scholar]
- Miltner WHR, Lemke U, Weiss T, Holroyd C, Scheffers MK, Coles MGH. Implementation of error-processing in the human anterior cingulated cortex: A source analysis of the magnetic equivalent of the error-related negativity. Biol Psychol. 2003;64:157–166. doi: 10.1016/s0301-0511(03)00107-8. [DOI] [PubMed] [Google Scholar]
- Neeper S, Gomez-Pinilla F, Choi J, Cottman C. Exercise and brain neurotrophins. Nature. 1995;373:109. doi: 10.1038/373109a0. [DOI] [PubMed] [Google Scholar]
- Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, Sloan R, Gage FH, Brown TR, Small SA. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci U S A. 2007;104 doi: 10.1073/pnas.0611721104. 5638-3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petruzzello SJ, Landers DM, Hatfield BD, Kubitz KA, Salazar W. A meta-analysis on the anxiety-reducing effects of acute and chronic exercise: Outcomes and mechanisms. Sports Med. 1991;11:143–182. doi: 10.2165/00007256-199111030-00002. [DOI] [PubMed] [Google Scholar]
- Picton TW, Bentin S, Berg P, Donchin E, Hillyard SA, Johnson R, Jr, Miller GA, Ritter W, Ruchkin DS, Rugg MD, Taylor MJ. Guidelines for using human event-related-potentials to study cognition: Recording standards and publication criteria. Psychophysiology. 2000;37:127–152. [PubMed] [Google Scholar]
- Rabbitt PMA. Errors and error correction in choice-response tasks. J Exp Psychol. 1966;71:264–272. doi: 10.1037/h0022853. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Rodríquez-Fornells A, Kurzbuch AR, Münte TF. Time course of error detection and correction in humans: Neurophysiological evidence. J Neurosci. 2002;22:9990–9996. doi: 10.1523/JNEUROSCI.22-22-09990.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA, Davis HP. Organization of cognitive abilities and neuropsychological variables across the lifespan. Dev Rev. 2006;26:31–54. [Google Scholar]
- Spirduso WW, Farrar RP. Effects of aerobic training on reactive capacity: An animal model. J Gerontol. 1981;36:654–662. doi: 10.1093/geronj/36.6.654. [DOI] [PubMed] [Google Scholar]
- Themanson JR, Hillman CH. Cardiorespiratory fitness and acute aerobic exercise effects on neuroelectric and behavioral measures of action monitoring. Neuroscience. 2006;141:757–767. doi: 10.1016/j.neuroscience.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Themanson JR, Hillman CH, Curtin JJ. Age and physical activity influences on neuroelectric indices of action monitoring during task switching. Neurobiol Aging. 2006;27:1335–1345. doi: 10.1016/j.neurobiolaging.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Themanson JR, Hillman CH, McAuley E, Buck SM, Doerksen SE, Morris KS, Pontifex MB. Self-efficacy effects on neuroelectric and behavioral indices of action monitoring in older adults. Neurobiol Aging. 2008;29:1111–1122. doi: 10.1016/j.neurobiolaging.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trejo JL, Carro E, Torres-Aleman I. Circulating insulin-like growth factor mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J Neurosci. 2001;21:1628–1634. doi: 10.1523/JNEUROSCI.21-05-01628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage GH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- van Veen V, Carter CS. The timing of action-monitoring processes in the anterior cingulated cortex. J Cogn Neurosci. 2002;14:593–602. doi: 10.1162/08989290260045837. [DOI] [PubMed] [Google Scholar]
- Vidal F, Hasbroucq T, Grapperon J, Bonnet M. Is the ‘error negativity’ specific to errors? Biol Psychol. 2000;51:109–128. doi: 10.1016/s0301-0511(99)00032-0. [DOI] [PubMed] [Google Scholar]
- Yeung N, Cohen JD, Botvinick MM. The neural basis of error detection: conflict monitoring and the error-related negativity. Psychol Rev. 2004;111:931–959. doi: 10.1037/0033-295x.111.4.939. [DOI] [PubMed] [Google Scholar]