Abstract
We previously investigated the potential of targeted radiotherapy using a bismuth-213-labeled anti-CD45 antibody to replace total body irradiation as conditioning for hematopoietic cell transplantation in a canine model. While this approach allowed sustained marrow engraftment, limited availability, high cost and short half-life of bismuth-213 induced us to investigate an alternative α-emitting radionuclide, astatine-211, for the same application. Biodistribution and toxicity studies were conducted with conjugates of the anti-murine CD45 antibody 30F11 with either bismuth-213 or astatine-211. Mice were injected with 2−50 μCi on 10 μg or 20 μCi on 2 or 40 μg 30F11 conjugate. Biodistribution studies showed that the spleen contained the highest concentration of radioactivity, ranging from 167±23 to 417±109 % injected dose/gram (%ID/g) after injection of the astatine-211 conjugate and 45±9 to 166±11 %ID/g after injection of the bismuth-213 conjugate. The higher concentrations observed for astatine-211-labeled 30F11 were due to its longer half-life, which permitted better localization of isotope to the spleen before decay. Astatine-211 was more effective at producing myelosuppression for the same quantity of injected radioactivity. All mice injected with 20 or 50 μCi astatine-211 but none with the same quantities of bismuth-213 had lethal myeloablation. Severe reversible acute hepatic toxicity occurred with 50 μCi bismuth-213, but not with lower doses of bismuth-213 or with any dose of astatine-211. No renal toxicity occurred with either radionuclide. The data suggest that smaller quantities of astatine-211-labeled anti-CD45 antibody are sufficient to achieve myelosuppression and myeloablation with less non-hematological toxicity compared with bismuth-213-labeled antibody.
Keywords: allogeneic hematopoietic cell transplantation (HCT), transplant conditioning, radioimmunotherapy, astatine-211 (211At), bismuth-213 (213Bi)
Introduction
Allogeneic hematopoietic cell transplantation (HCT) is a curative modality for patients with various malignant and non-malignant hematopoietic diseases. Recently, to reduce late toxicities from total body γ-irradiation (TBI) while increasing specificity and efficacy, monoclonal antibodies (MAb) labeled with β-emitting radionuclides, such as 131 I-labeled anti-CD45 MAb, have been investigated (1-4). However, β-emitting radionuclides are not optimal for killing the targeted hematopoietic cells due to their long path length and low dose rates (5-8). Owing to the long β-particle path (i.e. mean range 0.4 to 5 mm (9)) the majority of the emitted energy is deposited outside of the targeted hematopoietic cells. Thus, while specific targeting of hematopoietic cells may be achieved with the MAb, the β-particles may deliver non-lethal doses to the targeted cells while causing non-specific toxicity to surrounding normal tissues.
In contrast to β-emissions, α-particles are characterized by very high linear energy transfer, with most of the particles’ energy being deposited over only a few cell diameters (i.e. 40−90 μm). Given this favorable feature, we investigated bismuth-213 (213Bi)-labeled anti-CD45 MAb as replacement for TBI in a nonmyeloablative conditioning regimen for HCT in a canine model (10-12). Although the treatment was effective in allowing successful engraftment of marrow, several pragmatic obstacles precluded translating 213Bi-labeled MAbs into clinical studies including the very high cost of the parent radionuclide to 213Bi, actinium-225 (225Ac). Furthermore, adequate quantities of 225Ac were not available for clinical studies.
Astatine-211 (211At) is an alternative α-particle-emitting radionuclide for radioimmunotherapy (13). 211At has a longer half-life than 213Bi (7.21 h vs. 45.6 min), potentially making an 211At-labeled anti-CD45 MAb more effective for targeting and killing hematopoietic cells. Based on our success with 213Bi-labeled anti-CD45 MAb in conditioning for HCT, we compared biodistributions, myelosuppression and non-hematopoietic toxicities in mice with a MAb targeting hematopoietic tissues after radiolabeling it with either 211At or 213Bi. The antibody, a rat anti-murine CD45 MAb, 30F11 (2, 14) used in the mouse provided a model for understanding differences between the two radionuclides.
Materials and Methods
Antibody and Chemicals
The rat anti-murine CD45 MAb, 30F11, is an IgG2b MAb that recognizes all murine CD45 isoforms (2). The 30F11 hybridoma cell line was a gift from Dr. Irv Bernstein (Fred Hutchinson Cancer Research Center). The 30F11 MAb was produced by injecting the hybridoma into pristane-primed mice to generate ascites. The 30F11 MAb was purified from ascitic fluid by protein G immunoabsorption column chromatography. The protein-reactive 213Bi-chelation reagent, isothiocyanatobenzyl-CHX-A″-DTPA (referred to as IB-CHX-A″) used to modify 30F11 was purchased from Macrocyclics (Dallas, TX). The 211At-reactive protein modification reagent, N-(15-(aminoacyldecaborate)-4,7,10-trioxatridecanyl)-3-maleimidopropionamide (referred to as ADTM) was prepared as previously described (15).
Radionuclides
213Bi was obtained by elution from an 225Ac generator purchased from the US Department of Energy (Oak Ridge, TN) as previously described (10). 211At was obtained by irradiating bismuth metal with a 28 MeV α-beam in a Scandatronix MC50 cyclotron housed in the Department of Radiation Oncology at the University of Washington. The 211At was removed from irradiated bismuth targets by dry distillation and isolated in 0.05 N NaOH as previously described (16).
Modification of MAb 30F11 for Radiolabeling
Modification of 30F11 for labeling with 213Bi was achieved by conjugation of IB-CHX-A″ with 30F11 in 50 mM HEPES buffer, pH 8.5, at room temperature for 18 h as previously described (10). Rigorous demetallation was conducted before conjugation with the MAb and again after the MAb-conjugate was purified. Modification of 30F11 for labeling with 211At was achieved by treatment with 10 mM dithiothreitol (DTT) for 1 hour at room temperature, followed by buffer exchange into 20 mM sodium phosphate at pH 6.5, containing 1 mM EDTA, then addition of 10 equivalents of ADTM in DMSO to the DTT-treated 30F11 with ADTM. After the conjugation reaction proceeded for 1 hour at room temperature, the reaction mixture was eluted on a PD-10 column (Sephadex G-25) pre-equilibrated in PBS, pH 7.2. The fractions containing protein were pooled and concentrated in a Centricon-30 to provide the 30F11-ADTM. The 30F11-ADTM conjugates were analyzed by size-exclusion HPLC and IEF to assess modification to the MAb.
Radiolabeling Methods
The 30F11-CHX-A″ conjugate was radiolabeled with 213Bi in 0.3 M NH4OAc, pH 4.2−4.5, for 2−5 min as described (10). The 30F11-ADTM conjugate was labeled with 211At as follows. To 100−200 μL of 1−5 mg/mL solution of 30F11-ADTM conjugate in PBS was added 2−100 μL of Na[211At]At, then 20−40 μL of chloramine-T (1 mg/mL) in H2O. The reaction was allowed to proceed for 30 s to 2 min; then, 20−40 μL of a 1 mg/mL solution of Na2S2O5 in H2O was added to quench the reaction. The reaction mixture was then passed over a G-25 Sephadex column (PD-10, Pharmacia) eluting with 0.9% saline (15). Fractions were collected and those containing protein were combined. Radiochemical yield was determined by the amount of radioactivity associated with the protein relative to the amount of radioactivity placed on the column. Radiochemical purity of the radiolabeled proteins was determined by SE-HPLC.
Animal Studies
All mouse experiments were conducted under a protocol approved by the Fred Hutchison Cancer Research Center Institutional Animal Care and Use Committee. Female BALB/c mice were obtained from the Jackson Laboratory (Bar Harbor, Maine, USA). All reagents were administered to the BALB/c mice in a total volume of approximately 200 μL via the lateral tail vein. Sets of 30 mice were injected with either 211At- or 213Bi-labeled MAb. Of those mice, 20 were sacrificed at predetermined times to obtain tissue distribution data, and the remaining 10 were evaluated over 8 weeks for myelosuppression and toxicities. All mice were weighed weekly to assess for toxicity.
A total of 13 biodistribution studies were conducted. Seven experiments were conducted with [211At]30F11-ADTM (211At-MAb) and six with [213Bi]30F11-CHX-A″ (213Bi-MAb). In the experiments, tissue distributions of conjugates containing varying quantities of both radioactivity and MAb were evaluated (i.e. 2 μCi/10 μg, 10 μCi/10 μg, 50 μCi/10 μg, 6 μCi/2 μg, 20 μCi/2 μg, 20 μCi/10 μg or 20 μCi/40 μg for labeled MAbs, and an additional experiment that had 20 μCi/10 μg for the 211At-MAb). In 213Bi (t1/2 = 45.6 min) experiments, groups of 5 mice were sacrificed at 15, 45, 90 and 180 min after injection, when 20%, 50%, 75%, and 94% of the radionuclide had decayed, whereas for the 211At (t1/2 = 7.21 h) experimental groups mice were sacrificed 1, 3, 7 and 24 h after injection, when 9%, 25%, 49% and 90% of the radionuclide had decayed.
For evaluation of tissue concentrations of radioactivity, eight tissues were examined, including muscle, lung, kidney, spleen, liver, intestine, neck and stomach. The spleen was used as a surrogate tissue for hematopoiesis as total organ weight could be serially followed in addition to tissue concentrations of radioactivity. Bone marrows were sent for pathological examination only. Blood samples were obtained by heart puncture immediately after sacrificing the mice. Excised tissues were blotted free of blood and weighed. The total blood volume was estimated to be 6% of body weight (17). The radioactivity in each tissue was measured with a γ-counter (PACKARD® COBRA™ GMI, Inc., Minnesota, USA), and counts per minute were corrected for decay of each sample from the initiation of counting. Tissue concentrations of radioactivity were expressed as percentages of the injected dose per gram (%ID/g). The calculation of %ID/g was based on standards containing 1μL of the injected dose.
For pathological examination, selected tissues (spleen, liver, and bone marrow) were analyzed in untreated mice and those sacrificed at 24 h, 48 h, 1 week, 2 weeks and 1 month after injection of 10 μCi 211At on 10 μg of MAb. Necropsies were also performed to investigate the causes of death in five mice given 50 μCi 211At on 10 μg MAb (n=4) or 20 μCi 211At on 40 μg of MAb (n=1) in which lethal toxicity occurred. Each tissue was fixed in 10% neutral buffered formaldehyde and then embedded in paraffin. Tissue sections were cut (4 μm) and stained with haematoxylin and eosin (HE staining) by an automatic staining system (Tissue-Tek® DRS™ 2000, Sakura Finetek U.S.A., Inc., CA).
Tissue Radiation Dose Estimates
Radiation absorbed doses were calculated for 213Bi and 211At in mouse tissues using standard methods for α-particle and beta (electron) dosimetry. Using the mathematical formalism established by the Medical Internal Radiation Dose (MIRD) Committee of the Society of Nuclear Medicine (18), the absorbed doses (cGy) to target tissues for α-particles and electrons or β-particles were calculated from the available nuclear decay data (19) and biodistribution data (see Supplemental Information). The time-integrated activity (or total number of disintegrations) in each tissue was determined for each radionuclide by plotting the activity-time curve, identifying an appropriate function to represent the plotted data (by least-squares linear regression analysis), and by integrating the best-fit regression curve from time 0 to infinity. The radiation absorbed doses were then calculated using the MIRD formalism. The total absorbed dose to each tissue was calculated as the sum of the alpha plus electron contributions.
Myelosuppression and Toxicities
Myelosuppression and non-hematological toxicities were evaluated in 10 surviving mice from each experimental group remaining after biodistribution studies. After injection of the labeled MAb, blood samples were obtained by retro-orbital bleeding at 3 hr for 213Bi or 24 hr for 211At, and weekly (alternating between two groups of 5 mice each) for a total of 8 weeks. At each time point, the blood from the 5 mice within the group was pooled and peripheral blood counts, liver enzymes, and kidney function were monitored in collected blood. Blood was also obtained from 5 control mice for peripheral blood counts or for chemistry and pooled to allow sufficient blood volume. Five percent ethylenediaminetetraacetic acid (EDTA) was used as anticoagulant for peripheral blood samples. White blood cell (WBC) counts, hemoglobin (Hb) level and platelet (Plt) counts were automatically measured by a quantitative automated hematology analyzer (the Sysmex XT2000i, Sysmex America, Mundelein, IL, USA). The analyses were conducted at the Seattle Cancer Care Alliance hematology laboratory. Serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin (Bil), blood urea nitrogen (BUN) and creatinine (Cr) were measured to evaluate liver and renal toxicities. Additionally, to determine normal ranges [mean ± 2 standard deviations (SD)] of WBC counts, Hb level and Plt counts in peripheral blood, and AST, ALT, total Bil, BUN and Cr, as a baseline for comparison, blood counts and chemistry data were also analyzed from 42 individual untreated female BALB/c mice. Blood chemistry analyses were conducted by the Department of Laboratory Medicine Research Testing Services at the University of Washington.
Results
Tissue Distributions
Tissue distributions were obtained for three different quantities (2, 10 and 40 μg) of 211At- MAb or 213Bi-MAb to determine which provided the most favorable biodistribution for therapy (i.e. had highest concentrations in spleen). Tissue concentrations were analyzed at four time points after injection to determine tissue radiation dose estimates. Concentrations (%ID/g) of radioactivity in selected tissues at the chosen time points after injection are shown in Figure 1. The spleen, which contains large numbers of CD45-containing hematopoietic cells, had the highest concentration of radioactivity in all experiments. Spleen concentrations were higher in the 211At studies than in the 213Bi studies, with the % injected dose/gram (mean ± standard deviation; %ID/g) ranging from 167±23 (1 hr) to 417±109 (24 hr) after injection of the 211At conjugate and ranging from 45±9 (15 min) to 166±11 (3 h) after injection of the 213Bi conjugate. Interestingly, spleen weights obtained at euthanasia at each time point decreased dramatically from 73.9 ± 13.0 to 33.1 ± 3.2 mg at 24 h after injection of 211At (P<.0001, unpaired t test), but no weight changes in spleens were noted after injection of 213Bi (data not shown). The spleen weight changes were reversible as mice euthanized at later time points had normal spleens weight 2 weeks after injection of 211At. The liver contained the second highest concentrations, with study group averages ranging from 18−50 %ID/g for 211At and 19−33 %ID/g for 213Bi. The weights of the livers decreased slightly from 1.10 ± 0.09 to 0.80 ± 0.12 g at 1 h after injection in the 211At studies, but the changes were reversible. Kidney concentrations were low, with group averages ranging from 8−10 %ID/g after 211At and 7−8 %ID/g after 213Bi. Blood concentrations were similar between 213Bi and 211At groups, and reflected the quantity of MAb injected. Of note, free 211At would be expected to accumulate in the thyroid (as measured in the neck which contains the thyroid), lung and stomach.
Figure 1. 213Bi- and 211At-labeled rat anti-mouse anti-CD45 antibody 30F11 conjugate (213Bi- and 211At-MAb) biodistributions.
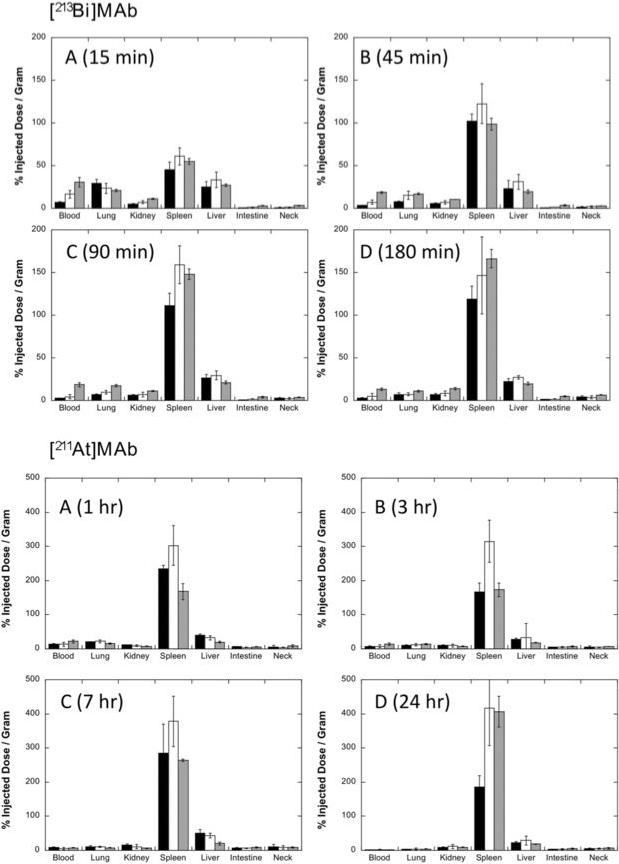
Tissue biodistributions were obtained in mice to assess MAb targeting to spleen, which has high concentrations of CD45-containing cells, and to determine the effect of varying the quantity of MAb on tissue concentrations. The graphs of the tissue concentrations, expressed as percent injected dose / gram (%ID/g), for studies that employed 2 μg (black bars), 10 μg (white bars) or 40 μg (gray bars) of MAb are shown. Data were obtained at 15, 45, 90 and 180 min after injection of 213Bi-MAb and at 1, 3, 7 and 24 h after injection of 211At-MAb. Data were obtained from groups of 5 mice per time point and were plotted as average values ± one standard deviation. Values plotted for injections of 2 and 40 μg quantities of MAb were obtained from single experiments (5 mice per time point), whereas values plotted for 10 μg were averaged from 3 (213Bi) or 4 (211At) separate experiments (total of 15−20 mice per time point) because the biodistributions of MAb labeled with a specific radionuclide differed only when the quantities of MAb were different. Note that the y-axis maximum is 200 %ID/g for 213Bi-MAb and 500 %ID/g for 211At-MAb.
Tissue Radiation Dose Estimates
The biodistribution data were used to estimate the tissue radiation doses when 2, 10 or 40 μg of 211At-MAb or 213Bi-MAb were administered (Table 1A). For comparison, the absorbed doses of 213Bi were multiplied by 9.49 (difference in half-lives) to equate the total number of 213Bi atoms to that of 211At. As a further comparison, in Table 1B, the tissue doses obtained using 50 μCi or, hypothetically, 500 μCi 213Bi (administered on the three different quantities of MAb) were compared to those obtained if 50 μCi of 211At were administered. The 500 μCi 213Bi value was an arbitrary value chosen as it would provide about the same radioactive dose as 50 μCi of 211At taking into account the 9.49× half-life factor between the two isotopes.
Table 1.
Calculated tissue dose estimates for [213Bi]30F11 and [211At]30F11.
|
A. Radiation absorbed doses per unit administered activity (cGy / μCi) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Nuclide | Bi-213 | Bi × 9.49* | At-211 | Bi-213 | Bi × 9.49* | At-211 | Bi-213 | Bi × 9.49* | At-211 |
| MAb wt | 2 μg | 2 μg | 10 μg | 10 μg | 40 μg | 40 μg | |||
| Blood | 0.78 | 7.38 | 9.00 | 1.28 | 12.15 | 8.01 | 3.42 | 32.46 | 12.60 |
| Muscle | 0.19 | 1.84 | 1.11 | 0.19 | 1.84 | 1.96 | 0.31 | 2.95 | 1.98 |
| Lung | 2.68 | 25.43 | 13.30 | 2.35 | 22.30 | 14.67 | 2.82 | 26.76 | 12.30 |
| Kidney | 1.13 | 10.72 | 15.70 | 1.42 | 13.48 | 17.30 | 2.24 | 21.26 | 12.10 |
| Spleen | 17.80 | 168.92 | 327.00 | 23.30 | 221.12 | 589.00 | 21.40 | 203.09 | 419.00 |
| Liver | 4.12 | 39.10 | 52.10 | 5.13 | 48.68 | 47.80 | 3.71 | 35.21 | 27.00 |
| Intestine | 0.16 | 1.47 | 7.02 | 0.27 | 2.58 | 7.32 | 0.74 | 7.01 | 9.13 |
| Neck | 0.37 | 3.50 | 11.10 | 0.43 | 4.06 | 8.82 | 0.72 | 6.82 | 10.60 |
| Stomach | 0.19 | 1.84 | 3.35 | 0.21 | 2.03 | 7.77 | 0.62 | 5.90 | 4.49 |
|
B. Organ or tissue absorbed dose (cGy) through complete decay of the activities administered. | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Nuclide | Bi-213 | Bi-213 | At-211 | Bi-213 | Bi-213 | At-211 | Bi-213 | Bi-213 | At-211 |
| MAb wt | 2 μg | 2 μg | 2 μg | 10 μg | 10 μg | 10 μg | 40 μg | 40 μg | 40 μg |
| Activity | 50 μCi | 500 μCi** | 50 μCi | 50 μCi | 500 μCi** | 50 μCi | 50 μCi | 500 μCi** | 50 μCi |
| Blood | 39 | 389 | 450 | 64 | 640 | 401 | 171 | 1710 | 630 |
| Muscle | 10 | 97 | 56 | 10 | 97 | 98 | 16 | 156 | 99 |
| Lung | 134 | 1340 | 665 | 118 | 1175 | 734 | 141 | 1410 | 615 |
| Kidney | 57 | 565 | 785 | 71 | 710 | 865 | 112 | 1120 | 605 |
| Spleen | 890 | 8900 | 16350 | 1165 | 11650 | 29450 | 1070 | 10700 | 20950 |
| Liver | 206 | 2060 | 2605 | 257 | 2565 | 2390 | 186 | 1855 | 1350 |
| Intestine | 8 | 78 | 351 | 14 | 136 | 366 | 37 | 370 | 457 |
| Neck | 18 | 185 | 555 | 21 | 214 | 441 | 36 | 360 | 530 |
| Stomach | 10 | 97 | 168 | 11 | 107 | 389 | 31 | 311 | 225 |
This value represents an equivalent number of 213Bi atoms to 211At atoms obtained by multiplying by a half-life difference factor (9.49×)
The 500 μCi is an arbitrary value chosen to provide about the same dose to most tissues; 10× is similar to 9.49× half-life factor.
Myelosuppression
Normal ranges determined for the WBC count, Hb, and Plt count in an untreated cohort of mice were 4.8 ± 3.4 (× 103 mm3), 16.0 ± 2.0 (g/dl) and 92.3 ± 28.4 (× 104 mm3), respectively. No significant cytopenias were observed in the 213Bi groups (Figure 2). On the other hand, lethal myelosuppression was observed in all 5 mice receiving 20 μCi 211At on 40 μg of MAb (data not shown in the figure) or 50 μCi 211At on 10 μg MAb (minimal WBC count 0.21 × 103 and 0.12 × 103/mm3, Plt counts 1.2 × 104 and 0.3 × 104/mm3, Hb level 1.5 and 4.2 g/dl, respectively). Pancytopenias started to appear 1 week after injection and were irreversible. In the mice receiving 20 μCi 211At on 10 μg of MAb, significant pancytopenias were observed, with nadirs at 2 weeks after injection (minimal WBC count 0.45 × 103/mm3). However, the pancytopenias resolved at 3 weeks after injection. In mice receiving 211At-MAb, leukopenia appeared at 24 h after injection, except in mice receiving the lowest quantity (2 μg) of MAb. In contrast, leukopenia was not observed in mice administered 213Bi-MAb.
Figure 2. Myelosupression with varying amounts (2, 10, 20, 50 μCi) of 213Bi-MAb or 211At-MAb.
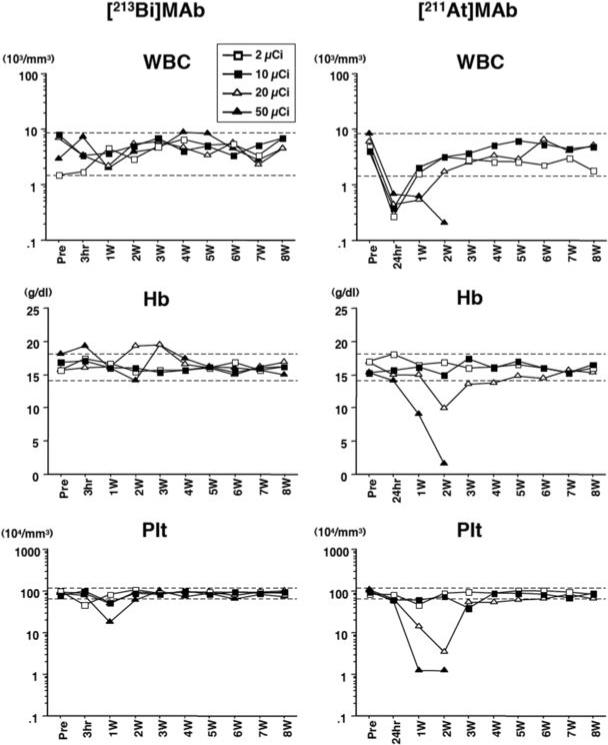
Peripheral blood counts were monitored in collected blood at 180 min and then weekly for 213Bi and at 24 h and then weekly for 211At. White blood cell (WBC) counts, hemoglobin (Hb) levels and platelet (Plt) counts were monitored up to 8 weeks after injection. The displayed data were obtained from mice treated with 2, 10, 20 and 50 μCi of 213Bi or 211At on 10 μg of MAb. The dashed lines indicate normal ranges (mean ± 2 standard deviations) which were calculated with data obtained from 42 untreated female BALB/c mice.
Hepatic toxicity
The normal ranges [mean ± 2 SD] for AST, ALT, and total Bil were 129 ± 96 (U/L), 52 ± 31 (U/L) and 0.48 ± 0.27 (mg/dl), respectively, in an untreated cohort of mice. Severe but non-lethal hepatic toxicity was observed at 3 hr after injection of 50 μCi 213Bi on 10 μg of MAb (maximal AST 1329 U /L and ALT 928 U /L) (Figure 3A). This hepatic toxicity resolved by day 15. In all 213Bi groups, except for mice given 2 μCi 213Bi on 2 μg of MAb, mild temporary hepatic enzyme elevations were detected at 3 h. However, the values recovered to near normal levels at 1 week. On the other hand, no significant hepatic toxicity was observed in any 211At-treated group.
Figure 3. Hepatic and renal toxicity with varying amounts (2, 10, 20, 50 μCi) of 213Bi-MAb 211At-MAb.
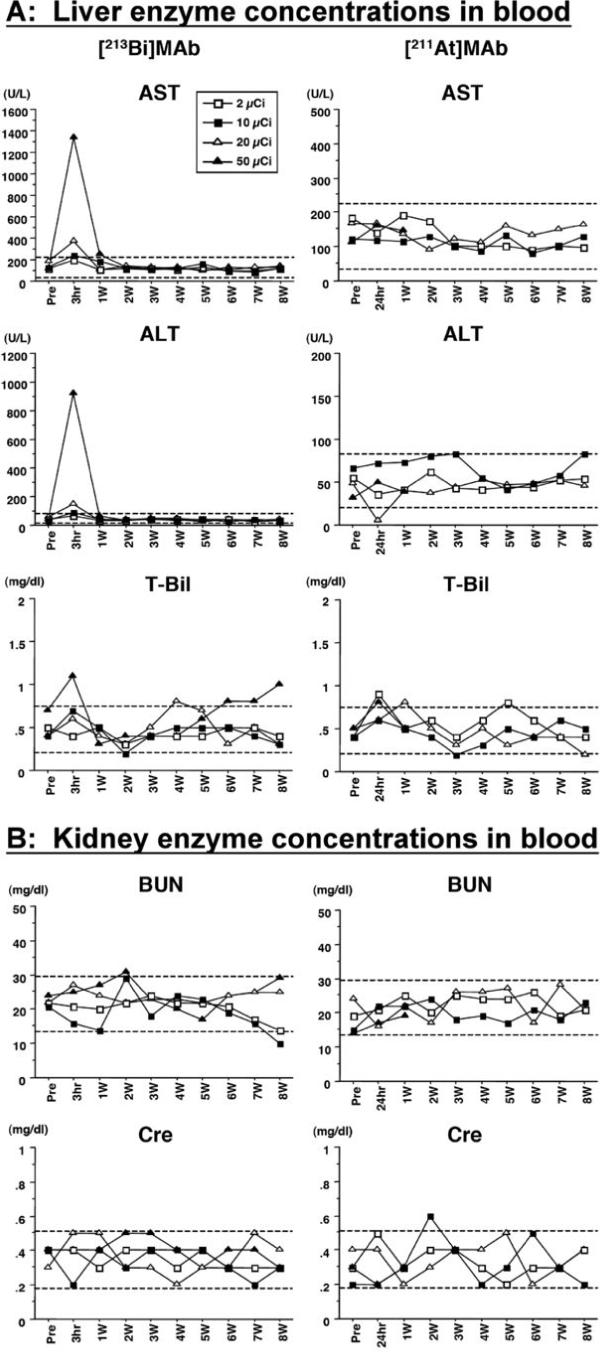
Hepatic and renal toxicities were monitored in sera from peripheral blood collected at 180 min (213Bi) or 24 h (211At) after injection, and then weekly. Liver toxicity was assessed by monitoring the enzymes aspartate aminotransferase (AST) and alanine aminotransferase (ALT), and total bilirubin (T-Bil). Renal toxicity was assessed by monitoring the blood urea nitrogen (BUN) and creatinine (Cre). The monitoring was performed up to 8 weeks after injection. The displayed data were obtained from mice treated with 2, 10, 20 and 50 μCi of 213Bi or 211At on 10 μg of MAb. The dashed lines indicate normal ranges (mean ± 2 standard deviations) which were calculated with data obtained from 42 untreated female BALB/c mice.
Renal toxicity
The normal ranges (mean ± 2 SD) determined for BUN and Cr were 21.5 ± 7.8 (mg/dl) and 0.35 ± 0.17 (mg/dl), respectively, in an untreated cohort of mice. No significant renal toxicity was observed in mice administered either 213Bi or 211At (Figure 3B).
Tissue pathology (211At)
Mice in all experimental study groups gained weight over the study period, except the group receiving 20 μCi 211At on 40 μg of MAb. In the group receiving 10 μCi 211At, the bone marrow demonstrated progressive hypocellularity, and hematopoiesis significantly decreased at 24 h and 48 h after injection (Figure 4A). However, hematopoietic recovery occurred by 1 week after injection. Similarly, red pulp in the spleen significantly decreased and white pulp in the spleen became atrophic at 24 h and 48 h after injection (Figure 4B). In the 24 h sample, there was diffuse necrosis of lymphoid cells intermixed with proliferating lymphocytes. The white pulp became atrophic (approximately 25% of normal cellularity) and red pulp was depleted down to about 50% of normal cellularity at 24 h. Megakaryocytes remained. However, recovery of hematopoiesis in the spleen was also observed in the specimen 1 week after injection. There were no pathological abnormalities in the liver. In the five animals on which necropsies were performed, there were no abnormalities noted in the liver or kidney.
Figure 4. Pathological changes in the spleen and bone marrow after injection of 211At-MAb.
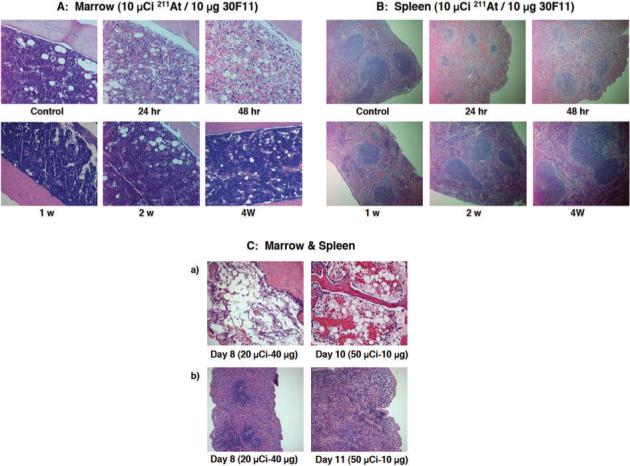
Pathological examination of bone marrow from femurs (Panel A) and the spleens (Panel B) was performed on mice sacrificed 24, 48 h, 1, 2, 4 weeks after injection of 10 μCi/10 μg 211At-MAb. Pathological examination of the bone marrow from femur or sternum was conducted on necropsy samples on day 8 and day 10 after 20 μCi/40 μg or 50 μCi/10 μg 211At-MAb were administered, respectively (Panel C; row a) and the spleens on day 8 and day 11 after 20 μCi/40 μg or 50 μCi/10 μg 211At-MAb were administered, respectively (Panel C; row b).
Necropsies were performed on mice that received 20 μCi 211At on 40 μg of MAb or 50 μCi 211At on 10 μg of MAb to investigate the cause of spontaneous death. Based on the autopsy and pathological examination, it was likely that all the mice in these two groups injected with 211At labeled MAb died of complications of severe pancytopenia (such as anemia or sepsis). In contrast to the group receiving 10 μCi 211At, the bone marrow cellularity remained very sparse at day 8 or 10 in groups receiving 20−50 μCi 211At, documenting protracted myelosuppression (Figure 4C, row a). At day 8−11, the red and white pulps of the spleen were depleted down to 25% of normal cellularity and megakaryocytes almost disappeared in the groups receiving 20 μCi 211At. In the groups receiving 50 μCi 211At, the red and white pulps were depleted down to 5−10% of normal cellularity and megakaryocytes were rarely observed (Figure 4C, row b). There was no evidence of extramedullary hematopoiesis in the liver.
Discussion
The current study demonstrated that 211At was more effective than 213Bi at producing myelosuppression for the same quantity of injected radioactivity. All mice injected with 20 or 50 μCi 211At but none with the same quantities of 213Bi had lethal myeloablation. Severe reversible acute hepatic toxicity occurred with the highest doses of 213Bi, but not with lower doses of 213Bi or with any dose of 211At. No renal toxicity occurred with either radionuclide. The data suggest that smaller μCi quantities of 211At-labeled anti-CD45 antibody are sufficient to achieve myelosuppression and myeloablation with less non-hematological toxicity compared with 213Bi-labeled antibody.
Our previous study showed that the donor chimerism levels in dogs conditioned with 213Bi-labeled anti-TCRαβ MAb (CA15.9D95) were lower than those observed in dogs conditioned with 213Bi-labeled pan-hematopoietic anti-CD45 MAb (CA12.10C12) (10, 11, 20). The results suggested that 213Bi-labeled anti-CD45 MAb was more effective in killing host residual cells, such as natural killer (NK) cells, that are responsible for graft rejection. CD45 was an excellent target because it is ubiquitously expressed on both nonmalignant and malignant hematopoietic cells (21-23). However, the short half-life of 213Bi presents a significant problem since it mandates the use of large 225Ac/213Bi generators and multiple preparations/injections per patient to obtain adequate doses of clinical materials for patient treatment. A further consideration is the fact that, at present, there is a very high cost to obtain a generator of sufficient size to conduct clinical studies. Therefore, a clinical study was not feasible at the current time using 213Bi. Additionally, the longer half-life of 211At has logistical and, potentially, therapeutic benefits.
211At is available at our institution by irradiation of a bismuth target with an α-particle beam from a cyclotron. An important consideration for initiating the investigation with 211At was the fact that recent upgrades on the cyclotron and target station used to produce 211At make it possible to prepare the quantities required for clinical studies, and this can be done at a much lower cost relative to producing 213Bi. Perhaps more importantly, the consideration for studies where 213Bi is replaced by 211At is the fact that the half-life of 211At (t1/2 = 7.21 h) is ∼9.5× longer than that of 213Bi (t1/2 = 45.6 min). This difference in half-life has some important benefits. One benefit is that there are 9.5 times the number of radioactive atoms as that of 213Bi in each mCi of 211At injected. Thus, for the same number of mCi of 211At as 213Bi, much higher doses can be obtained, or considerably lower quantities of 211At might be used to deliver a therapeutic dose. Another benefit of the longer half-life is the fact that a smaller percentage of the injected radioactivity will decay during the period of targeting hematopoietic cells, potentially resulting in more specific delivery of the radiation.
In the current study, labeling MAb 30F11 with the two radionuclides required use of different chemical modifications that could potentially affect the tissue distribution. Further, biodistribution of the radiolabeled MAb conjugates (30F11-CHX-A″ and 30F11-ADTM) were only relevant over the period where most of the radioactivity decays, potentially making the relative biodistributions very different given the different half-lives of the two isotopes. To label 213Bi, MAb 30F11 was conjugated with a benzylisothiocyanate-cyclohexyl derivative of diethylenetetraaminepentaacetic acid (IB-CHX-A″) which had been used in our previous canine conditioning studies. MAb conjugates of IB-CHX-A″ were rapidly labeled (5 min) in high yield (>80%) and provided good in vivo stability during the period of 213Bi decay. Stability of the label had been a problem for antibodies labeled with 211At (24). Although 211At-labeled benzoate esters could be used for stable labeling some MAbs, an investigation of 30F11 labeled with N-hydroxysuccinimidyl 3-[211At]astatobenzoate (25) indicated that it was not stable in vivo (unpublished). Therefore, an alternate 211At-labeling conjugate, a recently developed reagent (15, 26), N-(15-(aminoacyldecaborate)-4,7.10-trioxatridecanyl)-3-maleimide (ADTM), was used. The use of MAb-ADTM conjugates provided high radiochemical yields (70−80%) through direct labeling of 211At, and provided high in vivo stability to deastatination.
The biodistribution studies showed that the much higher radiation doses delivered by 211At depleted the hematopoietic cells and accounted for the differences seen in the spleen weights. While a large portion of the difference in radiation doses was provided by the fact that there are 9.49 times more 211At atoms per μCi administered, it appears that another factor of 2× in the dose may be due to the longer half-life of 211At, permitting more localization of MAb to the spleen prior to decay. Thus, from the tissue radiation dose estimates, 500 μCi of 213Bi would deliver less than half the dose to the target (spleen) of 50 μCi 211At if the radionuclides were on 10 μg of MAb. As might be expected from the biodistribution and tissue dose estimates, the blood count data indicated that myelosuppression was more effective with the 211At-MAb for the same μCi amount of 213Bi-MAb.
In this study, severe hepatic toxicity appeared in the mice receiving the highest dose (50 μCi) of 213Bi-MAb, presumably due to the abundance of hematopoietic cells and Kupffer cells in the liver expressing the CD45 antigen. There may also be a non-specific dose contribution as immunoglobulins from the blood stream are known to be trapped in the liver, resulting in the radiolabeled MAb being trapped even though CD45 is not expressed on hepatocytes (7). In the previous canine study, the dog receiving the highest dose of 8.8 mCi/kg 213Bi labeled anti-CD45 MAb also showed marked elevation of hepatic enzymes and evidence of liver failure with the development of ascites due to toxicity from the radioimmunotherapy (10). Based on the present and previous data, hepatic toxicity of 213Bi-labeled anti-CD45 MAb can be considered dose-limiting. The observed hepatic toxicity in the 213Bi studies was likely caused by deposition of 213Bi-labeled MAb in the liver. The nature of conjugate molecule (i.e. CHX-A″ or ADTM) to label the MAb with radionuclide might also contribute to hepatic deposition, but there are no data suggesting that either conjugate specifically localizes to liver.
The overall objective of our continuing research effort is to determine if MAbs labeled with an α-particle emitting radionuclide can deliver a marrow-ablative dose without the other organ toxicities associated with high-dose conditioning regimens. Our earlier studies demonstrated that 213Bi-labeled anti-CD45 MAb provided adequate myelosuppression to obtain stable chimeras in the dog model. From previous dog data it was estimated that 1.5−2 mCi 213Bi/kg on 0.5 mg anti-CD45 MAb/kg would likely be required in patients to obtain stable engraftment. This study demonstrated that 211At was more effective at myelosuppression for the same quantity of radioactivity injected than 213Bi without significant non-hematopoietic toxicity. Based on the fact that there are fewer barriers to clinical studies with 211At, and the encouraging results obtained in this investigation, further studies in the dog model with 211At-labeled anti-CD45 MAb are underway.
Acknowledgments
The authors wish to thank Dai Nguyen for her help in conducting the biodistribution study, and Sue E Knoblaugh and George E Sale for pathological evaluation. The authors also wish to thank Helen Crawford, Bonnie Larson and Sue Carbonneau for manuscript preparation.
Grant Support: This study was supported by grants from the National Institutes of Health including CA118940, CA015704, CA109663, and CA095448, and by the Frederick Kullman and Penny E. Petersen Memorial Foundations. H.N. was funded by the Graduate School of Medicine, Osaka City University, Osaka, Japan. J.M.P. is supported by Career Development Awards from the Lymphoma Research Foundation and the Damon Runyon Cancer Foundation.
References
- 1.Matthews DC, Appelbaum FR, Eary JF, et al. Development of a marrow transplant regimen for acute leukemia using targeted hematopoietic irradiation delivered by 131I-labeled anti-CD45 antibody, combined with cyclophosphamide and total body irradiation. Blood. 1995;85:1122–31. [PubMed] [Google Scholar]
- 2.Matthews DC, Martin PJ, Nourigat C, Appelbaum FR, Fisher DR, Bernstein ID. Marrow ablative and immunosuppressive effects of 131 I-anti-CD45 antibody in congenic and H2-mismatched murine transplant models. Blood. 1999;93:737–45. [PubMed] [Google Scholar]
- 3.Matthews DC, Appelbaum FR, Eary JF, et al. Phase I study of 131I-Anti-CD45 antibody plus cyclophosphamide and total body irradiation for advanced acute leukemia and myelodysplastic syndrome. Blood. 1999;94:1237–47. [PubMed] [Google Scholar]
- 4.Pagel JM, Appelbaum FR, Eary JF, et al. 131I-anti-CD45 antibody plus busulfan and cyclophosphamide before allogeneic hematopoietic cell transplantation for treatment of acute myeloid leukemia in first remission. Blood. 2006;107:2184–91. doi: 10.1182/blood-2005-06-2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fisher DR. Alpha-particle emitters in medicine. In: Adelstein SJ, Kassis AI, Burt RW, editors. Dosimetry of Administered Radionucles. The American College of Nuclear Physicians; Washington, DC: 1989. pp. 194–214. [Google Scholar]
- 6.Wilbur DS. Potential use of alpha emitting radionuclides in the treatment of cancer. Antibody, Immunoconjugates, and Radiopharmaceuticals. 1991;4:85–97. [Google Scholar]
- 7.Couturier O, Supiot S, Degraef-Mougin M, et al. Cancer radioimmunotherapy with alpha-emitting nuclides. European Journal of Nuclear Medicine and Molecular Imaging. 2005;32:601–14. doi: 10.1007/s00259-005-1803-2. (Review) [DOI] [PubMed] [Google Scholar]
- 8.Mulford DA, Scheinberg DA, Jurcic JG. The promise of targeted {alpha}-particle therapy. J Nucl Med. 2005;46(Suppl 1):199S–204S. (Review) [PubMed] [Google Scholar]
- 9.Zweit J. Radionuclides and carrier molecules for therapy. Physics in Medicine and Biology. 1996;41:1905–14. doi: 10.1088/0031-9155/41/10/004. (Review) [DOI] [PubMed] [Google Scholar]
- 10.Sandmaier BM, Bethge WA, Wilbur DS, et al. Bismuth 213-labeled anti-CD45 radioimmunoconjugate to condition dogs for nonmyeloablative allogeneic marrow grafts. Blood. 2002;100:318–26. doi: 10.1182/blood-2001-12-0322. [DOI] [PubMed] [Google Scholar]
- 11.Bethge WA, Wilbur DS, Storb R, et al. Radioimmunotherapy with Bismuth-213 as conditioning for nonmyeloablative allogeneic hematopoietic cell transplantation in dogs: a dose deescalation study. Transplantation. 2004;78:352–9. doi: 10.1097/01.tp.0000128853.62545.b2. [DOI] [PubMed] [Google Scholar]
- 12.Bethge WA, Sandmaier BM. Targeted cancer therapy and immunosuppression using radiolabeled monoclonal antibodies. Semin Oncol. 2004;31:68–82. doi: 10.1053/j.seminoncol.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Zalutsky MR, Reardon DA, Pozzi OR, Vaidyanathan G, Bigner DD. Targeted alpha-particle radiotherapy with 211At-labeled monoclonal antibodies. Nucl Med Biol. 2007;34:779–85. doi: 10.1016/j.nucmedbio.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruffner KL, Martin PJ, Hussell S, et al. Immunosuppressive effects of 131I-anti-CD45 antibody in unsensitized and donor antigen-presensitized H2-matched, minor antigen-mismatched murine transplant models. Cancer Res. 2001;61:5126–31. [PubMed] [Google Scholar]
- 15.Wilbur DS, Chyan MK, Hamlin DK, Vessella RL, Wedge TJ, Hawthorne MF. Reagents for astatination of biomolecules. 2. Conjugation of anionic boron cage pendant groups to a protein provides a method for direct labeling that is stable to in vivo deastatination. Bioconjugate Chem. 2007;18:1226–40. doi: 10.1021/bc060345s. [DOI] [PubMed] [Google Scholar]
- 16.Wilbur DS, Vessella RL, Stray JE, Goffe DK, Blouke KA, Atcher RW. Preparation and evaluation of para-[211At]astatobenzoyl labeled anti-renal cell carcinoma antibody A6H F(ab')2. In vivo distribution comparison with para-[125I]iodobenzoyl labeled A6H F(ab')2. Nucl Med Biol. 1993;20:917–27. doi: 10.1016/0969-8051(93)90092-9. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan HM, Brewer NR, Blair WH. Physiology. In: Foster HL, Small JD, Fox JG, editors. The Mouse in Biomedical Research. Academic Press; New York, NY: 1983. pp. 248–92. [Google Scholar]
- 18.Loevinger R, Budinger TF, Watson EE. MIRD Primer for Absorbed Dose Calculations. Revised Edition Society of Nuclear Medicine; Reston, VA: 1991. [Google Scholar]
- 19.Eckerman KF, Endo A. MIRD: Radionuclide Data and Decay Schemes. Society of Nuclear Medicine; Reston, VA: 2008. [Google Scholar]
- 20.Bethge WA, Wilbur DS, Storb R, et al. Selective T-cell ablation with bismuth-213-labeled anti-TCRαβ as nonmyeloablative conditioning for allogeneic canine marrow transplantation. Blood. 2003;101:5068–75. doi: 10.1182/blood-2002-12-3867. [DOI] [PubMed] [Google Scholar]
- 21.van der Jagt RHC, Badger CC, Appelbaum FR, et al. Localization of radiolabeled antimyeloid antibodies in a human acute leukemia xenograft tumor model. Cancer Res. 1992;52:89–94. [PubMed] [Google Scholar]
- 22.Andres TL, Kadin ME. Immunologic markers in the differential diagnosis of small round cell tumors from lymphocytic lymphoma and leukemia. Am J Clin Pathol. 1983;79:546–52. doi: 10.1093/ajcp/79.5.546. [DOI] [PubMed] [Google Scholar]
- 23.Caldwell CW, Patterson WP, Hakami N. Alterations of HLe-1 (T200) fluorescence intensity on acute lymphoblastic leukemia cells may relate to therapeutic outcome. Leuk Res. 1987;11:103–6. doi: 10.1016/0145-2126(87)90110-x. [DOI] [PubMed] [Google Scholar]
- 24.Wilbur DS. [211AT]astatine-labeled compound stability: issues with released [211At]astatide and development of labeling reagents to increase stability. Current Radiopharmaceuticals. 2008;1:144–76. [Google Scholar]
- 25.Zalutsky MR, Garg PK, Friedman HS, Bigner DD. Labeling monoclonal antibodies and F(ab')2 fragments with the alpha-particle-emitting nuclide astatine-211: preservation of immunoreactivity and in vivo localizing capacity. Proc Natl Acad Sci USA. 1989;86:7149–53. doi: 10.1073/pnas.86.18.7149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steffen AC, Almqvist Y, Chyan MK, et al. Biodistribution of 211At labeled HER-2 binding affibody molecules in mice. Oncology Reports. 2007;17:1141–7. [PubMed] [Google Scholar]


