Abstract
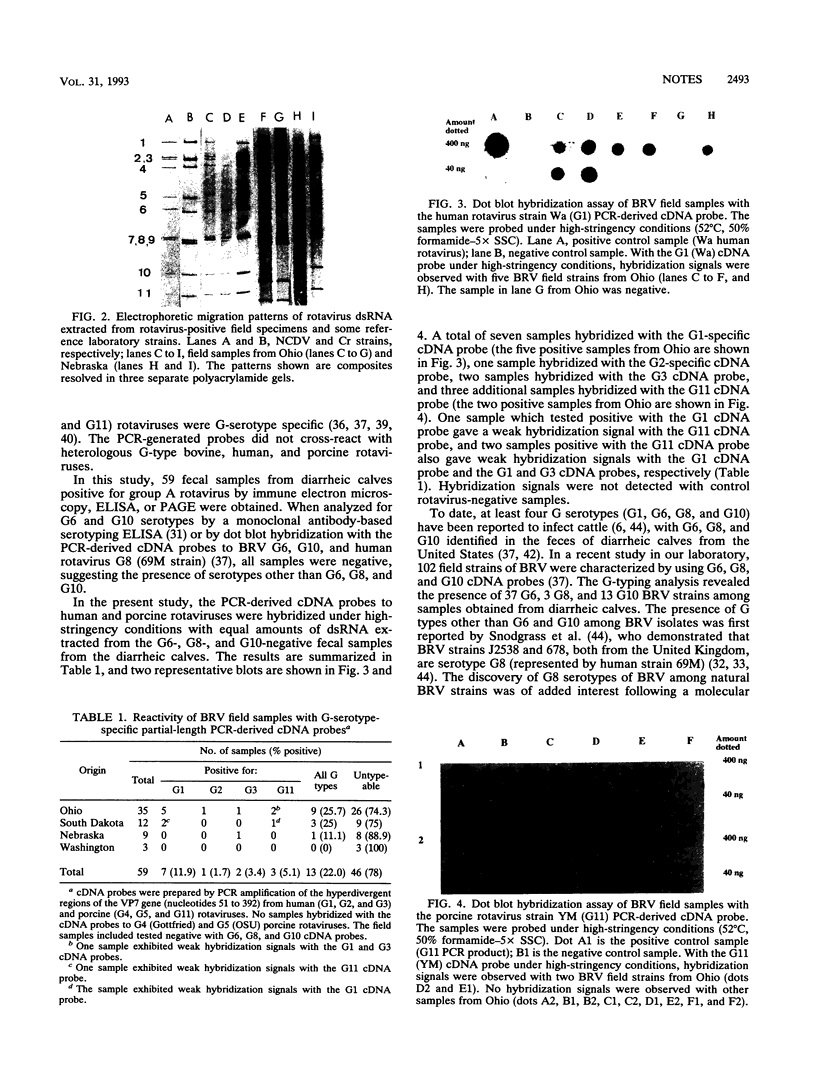
On the basis of antigenic variability in the VP7 outer capsid glycoprotein, at least 14 G serotypes exist for group A rotaviruses. Serotypic diversity exists among bovine rotaviruses (BRV), with serotypes G1, G6, G8, and G10 reported for cattle. Although G1 and G8 rotaviruses were originally described for humans, the recent isolation of G6 and G10 rotaviruses from humans further emphasizes the serotypic similarity between human and bovine rotaviruses and the possible zoonotic potential of rotaviruses. Results of our previous studies have indicated that more than 24% of BRV-positive field samples from diarrheic calves were nonreactive with cDNA probes or monoclonal antibodies to serotypes G6, G8, and G10. In this study, cDNA probes were prepared by polymerase chain reaction amplification of the hyperdivergent regions of the VP7 genes (nucleotides 51 to 392) from human (G1, G2, and G3) and porcine (G4, G5, and G11) rotaviruses. These probes were used in a dot blot hybridization assay to further characterize the G types of 59 BRV strains (fecal samples from diarrheic calves in Ohio, Nebraska, Washington, and South Dakota) that were nonreactive with cDNA probes to G6, G8, and G10. Rotaviruses belonging to serotypes G1 (n = 7), G2 (n = 1), G3 (n = 2), and G11 (n = 3) were identified among the BRV field samples. The BRV associated with these G types accounted for 22% of the samples tested; the other 78% of these samples remained untypeable with these probes. To our knowledge, this is the first report in the United States of the identification among BRV isolates of rotavirus serotypes G1, G2, G3, and G11.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert M. J., Unicomb L. E., Bishop R. F. Cultivation and characterization of human rotaviruses with "super short" RNA patterns. J Clin Microbiol. 1987 Jan;25(1):183–185. doi: 10.1128/jcm.25.1.183-185.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias C. F., Ruiz A. M., López S. Further antigenic characterization of porcine rotavirus YM. J Clin Microbiol. 1989 Dec;27(12):2871–2873. doi: 10.1128/jcm.27.12.2871-2873.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beards G., Xu L., Ballard A., Desselberger U., McCrae M. A. A serotype 10 human rotavirus. J Clin Microbiol. 1992 Jun;30(6):1432–1435. doi: 10.1128/jcm.30.6.1432-1435.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinzoni R. B., Mattion N. M., Matson D. O., Blackhall J., La Torre J. L., Scodeller E. A., Urasawa S., Taniguchi K., Estes M. K. Porcine rotaviruses antigenically related to human rotavirus serotypes 1 and 2. J Clin Microbiol. 1990 Mar;28(3):633–636. doi: 10.1128/jcm.28.3.633-636.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinzoni R. C., Blackhall J. O., Mattion N. M., Estes M. K., Snodgrass D. R., LaTorre J. L., Scodeller E. A. Serological characterization of bovine rotaviruses isolated from dairy and beef herds in Argentina. J Clin Microbiol. 1989 Nov;27(11):2619–2623. doi: 10.1128/jcm.27.11.2619-2623.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackhall J., Bellinzoni R., Mattion N., Estes M. K., La Torre J. L., Magnusson G. A bovine rotavirus serotype 1: serologic characterization of the virus and nucleotide sequence determination of the structural glycoprotein VP7 gene. Virology. 1992 Aug;189(2):833–837. doi: 10.1016/0042-6822(92)90617-x. [DOI] [PubMed] [Google Scholar]
- Bohl E. H., Saif L. J., Theil K. W., Agnes A. G., Cross R. F. Porcine pararotavirus: detection, differentiation from rotavirus, and pathogenesis in gnotobiotic pigs. J Clin Microbiol. 1982 Feb;15(2):312–319. doi: 10.1128/jcm.15.2.312-319.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning G. F., Chalmers R. M., Fitzgerald T. A., Snodgrass D. R. Serological and genomic characterization of L338, a novel equine group A rotavirus G serotype. J Gen Virol. 1991 May;72(Pt 5):1059–1064. doi: 10.1099/0022-1317-72-5-1059. [DOI] [PubMed] [Google Scholar]
- Browning G. F., Fitzgerald T. A., Chalmers R. M., Snodgrass D. R. A novel group A rotavirus G serotype: serological and genomic characterization of equine isolate FI23. J Clin Microbiol. 1991 Sep;29(9):2043–2046. doi: 10.1128/jcm.29.9.2043-2046.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning G. F., Snodgrass D. R., Nakagomi O., Kaga E., Sarasini A., Gerna G. Human and bovine serotype G8 rotaviruses may be derived by reassortment. Arch Virol. 1992;125(1-4):121–128. doi: 10.1007/BF01309632. [DOI] [PubMed] [Google Scholar]
- Brüssow H., Offit P. A., Sidoti J. Neutralizing antibodies to heterologous animal rotavirus serotypes 5, 6, 7, and 10 in sera from Ecuadorian children. J Clin Microbiol. 1991 May;29(5):869–873. doi: 10.1128/jcm.29.5.869-873.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brüssow H., Snodgrass D., Fitzgerald T., Eichhorn W., Gerhards R., Bruttin A. Antigenic and biochemical characterization of bovine rotavirus V1005, a new member of rotavirus serotype 10. J Gen Virol. 1990 Nov;71(Pt 11):2625–2630. doi: 10.1099/0022-1317-71-11-2625. [DOI] [PubMed] [Google Scholar]
- Clark H. F., Hoshino Y., Bell L. M., Groff J., Hess G., Bachman P., Offit P. A. Rotavirus isolate WI61 representing a presumptive new human serotype. J Clin Microbiol. 1987 Sep;25(9):1757–1762. doi: 10.1128/jcm.25.9.1757-1762.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes M. K., Cohen J. Rotavirus gene structure and function. Microbiol Rev. 1989 Dec;53(4):410–449. doi: 10.1128/mr.53.4.410-449.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores J., Greenberg H. B., Myslinski J., Kalica A. R., Wyatt R. G., Kapikian A. Z., Chanock R. M. Use of transcription probes for genotyping rotavirus reassortants. Virology. 1982 Sep;121(2):288–295. doi: 10.1016/0042-6822(82)90168-4. [DOI] [PubMed] [Google Scholar]
- Flores J., Sears J., Schael I. P., White L., Garcia D., Lanata C., Kapikian A. Z. Identification of human rotavirus serotype by hybridization to polymerase chain reaction-generated probes derived from a hyperdivergent region of the gene encoding outer capsid protein VP7. J Virol. 1990 Aug;64(8):4021–4024. doi: 10.1128/jvi.64.8.4021-4024.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerna G., Sarasini A., Parea M., Arista S., Miranda P., Brüssow H., Hoshino Y., Flores J. Isolation and characterization of two distinct human rotavirus strains with G6 specificity. J Clin Microbiol. 1992 Jan;30(1):9–16. doi: 10.1128/jcm.30.1.9-16.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerna G., Sarasini A., Zentilin L., Di Matteo A., Miranda P., Parea M., Battaglia M., Milanesi G. Isolation in Europe of 69 M-like (serotype 8) human rotavirus strains with either subgroup I or II specificity and a long RNA electropherotype. Arch Virol. 1990;112(1-2):27–40. doi: 10.1007/BF01348983. [DOI] [PubMed] [Google Scholar]
- Green K. Y., Midthun K., Gorziglia M., Hoshino Y., Kapikian A. Z., Chanock R. M., Flores J. Comparison of the amino acid sequences of the major neutralization protein of four human rotavirus serotypes. Virology. 1987 Nov;161(1):153–159. doi: 10.1016/0042-6822(87)90181-4. [DOI] [PubMed] [Google Scholar]
- Hardy M. E., Gorziglia M., Woode G. N. Amino acid sequence analysis of bovine rotavirus B223 reveals a unique outer capsid protein VP4 and confirms a third bovine VP4 type. Virology. 1992 Nov;191(1):291–300. doi: 10.1016/0042-6822(92)90191-q. [DOI] [PubMed] [Google Scholar]
- Hardy M. E., Woode G. N., Xu Z. C., Gorziglia M. Comparative amino acid sequence analysis of VP4 for VP7 serotype 6 bovine rotavirus strains NCDV, B641, and UK. J Virol. 1991 Oct;65(10):5535–5538. doi: 10.1128/jvi.65.10.5535-5538.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring A. J., Inglis N. F., Ojeh C. K., Snodgrass D. R., Menzies J. D. Rapid diagnosis of rotavirus infection by direct detection of viral nucleic acid in silver-stained polyacrylamide gels. J Clin Microbiol. 1982 Sep;16(3):473–477. doi: 10.1128/jcm.16.3.473-477.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y., Sereno M. M., Midthun K., Flores J., Kapikian A. Z., Chanock R. M. Independent segregation of two antigenic specificities (VP3 and VP7) involved in neutralization of rotavirus infectivity. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8701–8704. doi: 10.1073/pnas.82.24.8701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y., Wyatt R. G., Greenberg H. B., Flores J., Kapikian A. Z. Serotypic similarity and diversity of rotaviruses of mammalian and avian origin as studied by plaque-reduction neutralization. J Infect Dis. 1984 May;149(5):694–702. doi: 10.1093/infdis/149.5.694. [DOI] [PubMed] [Google Scholar]
- Kalica A. R., Greenberg H. B., Wyatt R. G., Flores J., Sereno M. M., Kapikian A. Z., Chanock R. M. Genes of human (strain Wa) and bovine (strain UK) rotaviruses that code for neutralization and subgroup antigens. Virology. 1981 Jul 30;112(2):385–390. doi: 10.1016/0042-6822(81)90285-3. [DOI] [PubMed] [Google Scholar]
- Kassuba A., Saif L. J., Greenberg H. B. Subgroup classification of porcine group-A rotaviruses, using monoclonal antibodies in an enzyme-linked immunosorbent assay. Am J Vet Res. 1990 Jun;51(6):938–944. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matsuda Y., Nakagomi O., Offit P. A. Presence of three P types (VP4 serotypes) and two G types (VP7 serotypes) among bovine rotavirus strains. Arch Virol. 1990;115(3-4):199–207. doi: 10.1007/BF01310530. [DOI] [PubMed] [Google Scholar]
- Matsuno S., Hasegawa A., Mukoyama A., Inouye S. A candidate for a new serotype of human rotavirus. J Virol. 1985 May;54(2):623–624. doi: 10.1128/jvi.54.2.623-624.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Blavat G. Identification of the two rotavirus genes determining neutralization specificities. J Virol. 1986 Jan;57(1):376–378. doi: 10.1128/jvi.57.1.376-378.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohshima A., Takagi T., Nakagomi T., Matsuno S., Nakagomi O. Molecular characterization by RNA-RNA hybridization of a serotype 8 human rotavirus with "super-short" RNA electropherotype. J Med Virol. 1990 Feb;30(2):107–112. doi: 10.1002/jmv.1890300206. [DOI] [PubMed] [Google Scholar]
- Parwani A. V., Rosen B. I., Flores J., McCrae M. A., Gorziglia M., Saif L. J. Detection and differentiation of bovine group A rotavirus serotypes using polymerase chain reaction-generated probes to the VP7 gene. J Vet Diagn Invest. 1992 Apr;4(2):148–158. doi: 10.1177/104063879200400206. [DOI] [PubMed] [Google Scholar]
- Reynolds D. J., Morgan J. H., Chanter N., Jones P. W., Bridger J. C., Debney T. G., Bunch K. J. Microbiology of calf diarrhoea in southern Britain. Vet Rec. 1986 Jul 12;119(2):34–39. doi: 10.1136/vr.119.2.34. [DOI] [PubMed] [Google Scholar]
- Rosen B. I., Saif L. J., Jackwood D. J., Gorziglia M. Hybridization probes for the detection and differentiation of two serotypes of porcine rotavirus. Vet Microbiol. 1990 Sep;24(3-4):327–339. doi: 10.1016/0378-1135(90)90181-t. [DOI] [PubMed] [Google Scholar]
- Rosen B. I., Saif L. J., Jackwood D. J., Gorziglia M. Serotypic differentiation of group A rotaviruses with porcine rotavirus gene 9 probes. J Clin Microbiol. 1990 Nov;28(11):2526–2533. doi: 10.1128/jcm.28.11.2526-2533.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass D. R., Fitzgerald T., Campbell I., Scott F. M., Browning G. F., Miller D. L., Herring A. J., Greenberg H. B. Rotavirus serotypes 6 and 10 predominate in cattle. J Clin Microbiol. 1990 Mar;28(3):504–507. doi: 10.1128/jcm.28.3.504-507.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass D. R., Hoshino Y., Fitzgerald T. A., Smith M., Browning G. F., Gorziglia M. Identification of four VP4 serological types (P serotypes) of bovine rotavirus using viral reassortants. J Gen Virol. 1992 Sep;73(Pt 9):2319–2325. doi: 10.1099/0022-1317-73-9-2319. [DOI] [PubMed] [Google Scholar]
- Snodgrass D. R., Ojeh C. K., Campbell I., Herring A. J. Bovine rotavirus serotypes and their significance for immunization. J Clin Microbiol. 1984 Sep;20(3):342–346. doi: 10.1128/jcm.20.3.342-346.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass D. R., Terzolo H. R., Sherwood D., Campbell I., Menzies J. D., Synge B. A. Aetiology of diarrhoea in young calves. Vet Rec. 1986 Jul 12;119(2):31–34. doi: 10.1136/vr.119.2.31. [DOI] [PubMed] [Google Scholar]
- Taniguchi K., Urasawa T., Kobayashi N., Gorziglia M., Urasawa S. Nucleotide sequence of VP4 and VP7 genes of human rotaviruses with subgroup I specificity and long RNA pattern: implication for new G serotype specificity. J Virol. 1990 Nov;64(11):5640–5644. doi: 10.1128/jvi.64.11.5640-5644.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi K., Urasawa T., Pongsuwanna Y., Choonthanom M., Jayavasu C., Urasawa S. Molecular and antigenic analyses of serotypes 8 and 10 of bovine rotaviruses in Thailand. J Gen Virol. 1991 Dec;72(Pt 12):2929–2937. doi: 10.1099/0022-1317-72-12-2929. [DOI] [PubMed] [Google Scholar]
- Terrett L. A., Saif L. J., Theil K. W., Kohler E. M. Physicochemical characterization of porcine pararotavirus and detection of virus and viral antibodies using cell culture immunofluorescence. J Clin Microbiol. 1987 Feb;25(2):268–272. doi: 10.1128/jcm.25.2.268-272.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urasawa S., Urasawa T., Wakasugi F., Kobayashi N., Taniguchi K., Lintag I. C., Saniel M. C., Goto H. Presumptive seventh serotype of human rotavirus. Arch Virol. 1990;113(3-4):279–282. doi: 10.1007/BF01316680. [DOI] [PubMed] [Google Scholar]
- Ward R. L., Knowlton D. R., Schiff G. M., Hoshino Y., Greenberg H. B. Relative concentrations of serum neutralizing antibody to VP3 and VP7 proteins in adults infected with a human rotavirus. J Virol. 1988 May;62(5):1543–1549. doi: 10.1128/jvi.62.5.1543-1549.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woode G. N., Kelso N. E., Simpson T. F., Gaul S. K., Evans L. E., Babiuk L. Antigenic relationships among some bovine rotaviruses: serum neutralization and cross-protection in gnotobiotic calves. J Clin Microbiol. 1983 Aug;18(2):358–364. doi: 10.1128/jcm.18.2.358-364.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]