Abstract
Background
In this prospective observational study, we aim to explore the relationship between age and BIS values at different plasma concentrations of propofol.
Methods
Fifty children aged from 3 to 15 years were included. Anaesthesia was induced using a target controlled infusion of propofol with the Kataria pharmacokinetic model together with a bolus of remifentanil followed by a continuous infusion rate at 0.2 mcg·kg−1·min−1. Target plasma propofol concentration was initially stabilized to 6 mcg·ml−1 and continued for 6 minutes. The target was then decreased and stabilized to 4 mcg·ml−1 and then to 2 mcg·ml−1. BIS values, plasma propofol concentration and EEG were continuously recorded. In order to explore the relationship between variations in propofol concentration and the EEG bispectrum, we used a Multiple Correspondence Analysis (MCA). Results are shown in median (range).
Results
We found no statistical difference between BIS values with propofol 6 mcg·ml−1 23 (12–40) and propofol 4 mcg·ml−1 28 (9–67). At 2 mcg·ml−1, BIS was significantly different 52 (24–71) but a significant correlation between the age of children and BIS values was found (r2=0.66; p<0.01). There was little change in children’s position between 6 mcg·ml−1 and 4 mcg·ml−1 in the structure model of the MCA. From 4 mcg·ml−1 to 2 mcg·ml−1 the position of children moved only on axis 2.
Conclusions
These results showed the difficulty to interpret BIS values because of the absence of significant change for higher plasma propofol concentration variation or because of the link with age for the lower plasma concentration.
Keywords: Adolescent; Aging; physiology; Anesthetics, Intravenous; administration & dosage; blood; pharmacology; Blood Pressure; drug effects; Child; Child, Preschool; Dose-Response Relationship, Drug; Drug Administration Schedule; Electroencephalography; drug effects; Heart Rate; drug effects; Humans; Monitoring, Intraoperative; methods; Propofol; administration & dosage; blood; pharmacology; Prospective Studies
Keywords: anaesthesia, depth, anaesthesia, paediatric, anaesthetics, i.v., propofol, monitoring, bispectral index, bispectrum, plasma concentration, pharmacokinetic model, remifentanil
In the paediatric population, the ability of the Bispectral Index (BIS) to accurately follow variations in anaesthetic agent concentration and evaluate depth of anaesthesia remains controversial. It has been shown in recent studies using various volatile anaesthetic agents that BIS values were linked to the age of children irrespective of the volatile agent used (1–5). In addition, there is good evidence that BIS values are agent dependent for the same level of MAC (minimal alveolar concentration)(4,6,7).
Propofol is widely used for both sedation and general anaesthesia in adults and children. Few studies have attempted to evaluate BIS variation under target controlled infusion (TCI) with propofol in a paediatric population.
In this prospective observational study, we aim to explore the relationship between age and BIS values at different estimated plasma concentrations of propofol and to analyse the EEG bispectrum modifications induced by this hypnotic.
Patients and methods
After approval from the Humans Studies Committee, and with written parental consent, 50 ASA I or II children aged from 3 to 15 years were recruited into our study. Children with central neurological disease and those taking medication acting on the central nervous system were excluded. No children were premedicated. Before arriving in the operating department, an EMLA patch was applied on both hands. Prior to induction of anaesthesia, BIS paediatrics leads (Aspect Medical Systems, Newton, IL, USA) were placed according the manufactures instructions and connected to Aspect XP™ device. EEG leads (3M Red Dot Silver/Silver Chloride model 2269T, 3M Health Care, St Paul, USA) were placed adjacent to the BIS leads.
After intravenous access had been obtained, we pre-oxygenated children and induced anaesthesia with a bolus of remifentanil (1 mcg·kg−1 over 1 minute) followed by a target controlled infusion (TCI) of propofol using an Asena® syringe driver (Alaris® Medical Systems, Alaris Medical UK Ltd, Basingstoke, UK) with the Kataria pharmacokinetic (PK) model (weight-proportional age-adjusted) software (8). Remifentanil was continued at 0.2 mcg·kg−1·min−1 until the end of the study protocol. The initial target plasma propofol concentration was set at 7 mcg·ml−1 to permit intubation without the use of muscle relaxants. After tracheal intubation all children were ventilated in oxygen/air to maintain normocapnoea. Target plasma propofol concentration was then decreased to 6 mcg·ml−1 and after this target was reached, it was maintained for 6 minutes to obtain a stationary EEG bispectrum (defined as the absence of statistical difference in bispectral parameters during the last minute of each steady state). The target plasma concentration was then decreased to 4 mcg·ml−1 and maintained at this level for a further 6 minutes, and then finally the target plasma concentration was decreased to 2 mcg·ml−1 and maintained for the final 6 minutes of the study protocol. During the study period there was no surgical stimulation. No others drugs were administered. BIS values and plasma concentration of propofol were continuously recorded using the Rugloop® device. Raw EEG was recorded using PowerLab™ software (AD Instruments, Castle Hill, NSW, Australia). All recordings started before induction of anaesthesia and were continuous until the end of the study protocol. Raw EEG and EEG bispectrum were mathematically processed as described previously (1). EEG bispectrum was estimated on successive epochs of 20 s using MATLAB© software. Each EEG bispectrum was divided into 36 blocks of frequency of coupling (figure 1). The mean of the bispectrum for each block was then calculated so that each child was represented by 36 descriptors evolving over the time of recording.
Fig 1.
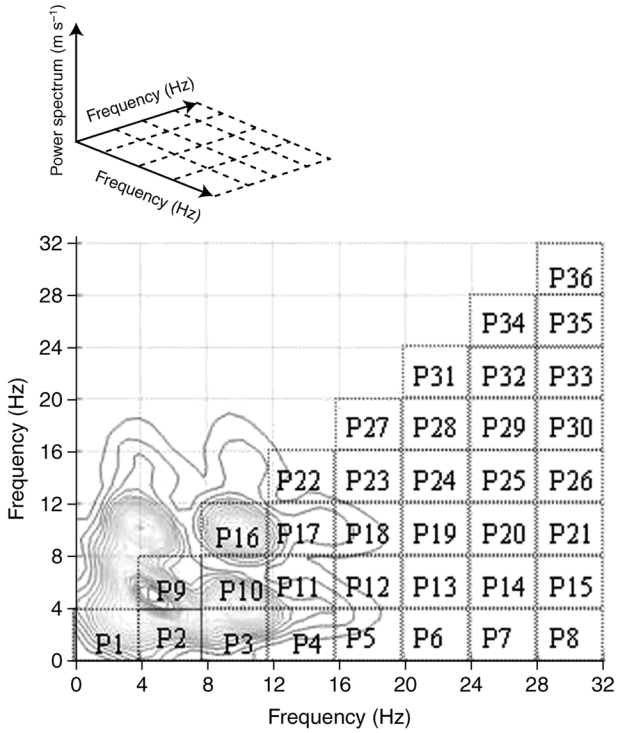
An example of the representation of the 36 frequencies of coupling (Pi) of the bispectrum calculated every 20 s for one child’s EEG (MatBis).
For statistical analysis, correlation between BIS values (taken as the median value during the last 30 s of each plateau phase) and age of children was evaluated by means of a Spearman test. A Wilcoxon test was used to establish significant changes in parameters at various points during the decrease of propofol plasma concentration. In order to explore the relationship between variations in plasma propofol concentrations and the EEG bispectrum, we used a Multiple Correspondence Analysis (MCA). The structured model of the MCA was derived from previous recordings of children anaesthetised with sevoflurane, and was explained in detail in this study (1). The change in position of children within the structured model of the MCA is determined only by changes in the EEG bispectrum during the decrease of plasma propofol concentration. All results are described as median (range). A P-value <0.05 was considered significant. All statistical analyses were performed with the BI©LOGINSERM 1979/1987 software.
Results
Complete recordings were obtained in all 50 children. The median (range) age and weight were 95 months (40–182) and 25 kg (13–60) respectively. Physiological variables at the various target plasma propofol concentrations are shown in Table 1. The BIS values at different plasma propofol concentrations are represented in Figure 2. We found no statistical difference between BIS values under anaesthesia with propofol 6 mcg·ml−1 23 (12–40) and propofol 4 mcg·ml−1 28 (9–67). With propofol 2 mcg·ml−1 BIS was significantly different 52 (24–71). A significant correlation between the age of children and BIS values was found at propofol 2 mcg·ml−1 (r2=0.66; p<0.01) (Figure 3) but not (r2=0.02) and 4 mcg·ml−1 (r2=0.2). In a sub-group of children in whom there were no episodes of burst suppression, correlation between the age of children and BIS values was poorly improved (r2=0.04 at 6 mcg·ml−1 and r2=0.3 at 4 mcg·ml−1).
Table 1.
Physiological variables at different plasma propofol concentrations
| Awake | 6 mcg·ml−1 | 4 mcg·ml−1 | 2 mcg·ml−1 | |
|---|---|---|---|---|
| SBP mmHg | 114 (87–147) | 91 (121-76) | 90 (73–112) | 88 (70–105) |
| DBP mmHg | 60 (42–89) | 47 (39–76) | 46 (30–70) | 43 (25–62) |
| MAP mmHg | 72 (55–94) | 57 (50–86) | 56 (44–79) | 55 (38–77) |
| HR bpm | 82 (45–115) | 81 (40–104) | 75 (49–99) | 72 (59–86) |
SBP: Systolic Blood Pressure; DBP: Diastolic Blood Pressure; MBP: Mean Blood Presure; HR: Heart Rate
Fig 2.
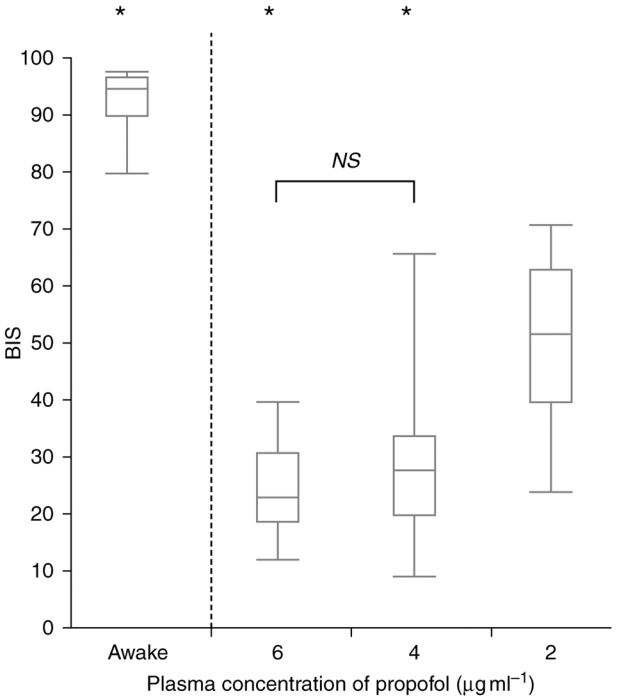
BIS values at different plasma propofol concentrations.
Fig 3.
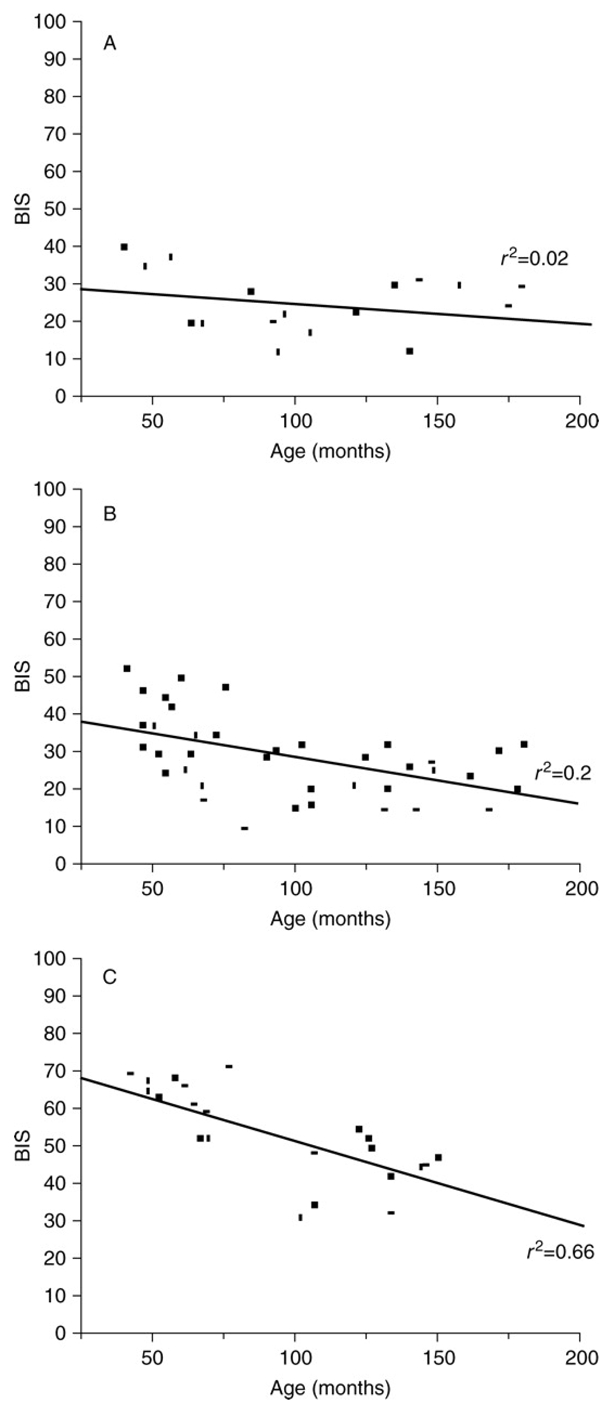
BIS values according to age of children at plasma propofol concentration of 6 mcg·ml−1 on the top, of 4 mcg·ml−1 in the middle and of 2 mcg·ml−1 on the lower graph.
At a target of 6 mcg·ml−1, 55% of the EEG recordings showed episodes of burst suppression. At 4 mcg·ml−1, there were episodes of burst suppression in 19% of EEG recordings. At 2 mcg·ml−1 there were no episodes of burst suppression.
The position of children anaesthetised with propofol at 6, 4 and 2 mcg·ml−1 in the structured model of the MCA is shown in Figure 4. As propofol concentration was decreased there was little change in children’s position between 6 mcg·ml−1 and 4 mcg·ml−1. From 4 mcg·ml−1 to 2 mcg·ml−1 the position of children moved only on axis 2. There was no change in position in the structured model of the MCA during the decrease in propofol concentration in the sub-group of children with no episodes of burst suppression (data not shown).
Fig 4.
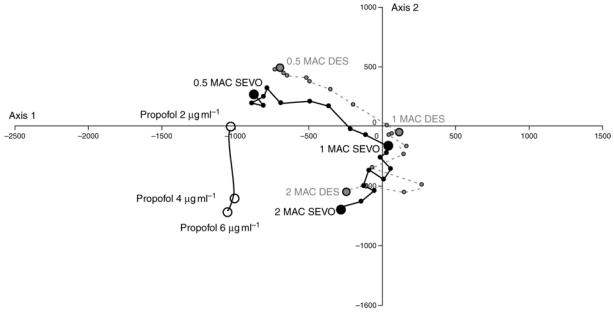
Change in children’s position in the structured model of the multiple correspondence analysis during decrease in plasma propofol concentration. The structure model is the same used for sevoflurane (SEVO) and desflurane (DES) in previous studies (1,4). Larger dots represent steady state. The units of axes F1 and F2 are arbitrary.
Discussion
In our study, BIS values did not change during a decrease in plasma propofol concentration from 6 mcg·ml−1 to 4 mcg·ml−1 using the Kataria PK model (weight-proportional age-adjusted). During a further decrease to 2 mcg·ml−1 BIS values did rise significantly, but at this target concentration of propofol, we found that BIS values were significantly correlated with children’s age.
There are studies that have examined the correlation between BIS values and sedation scores using propofol either by intermittent bolus or variable infusion. However, these sedation scores, for example the Observer’s Assessment of Alertness/Sedation (OAAS) scale or University of Michigan Sedation Scale (UMSS), can be unreliable in a clinical setting, hence the need for an objective, quantitative assessment of the level of sedation (9,10). The use of the BIS monitor to accurately determine the correct level of sedation was initially developed in adults (11). Its validity in a paediatric population is still controversial because of inter-individual variability, and the difficulty in detecting deep sedation (12–15). The large variation of BIS values seen in this study at 2 mcg·ml−1 target-controlled plasma propofol concentration could be mainly due to the influence of age, as we have seen during sevoflurane anaesthesia (1), and also with anaesthesia using desflurane and halothane (4). This alone could explain the inter-individual variability in children. Few studies have examined the relationship between the BIS and anaesthesia with target controlled infusion (TCI) of propofol in a paediatric population. In a recent study, Park and colleagues studied changes of bispectral index when decreasing propofol concentration until awaking in a paediatric population (16). They showed a poor correlation between predicted propofol concentrations and BIS values during emergence. Moreover, they noted large interindividual variations of BIS at any given concentration. These findings have significant clinical implications. For example, the use of a protocol in which the BIS monitor alone is used to guide propofol sedation to a target BIS score of 45 we would suggest is inadequate in a paediatric population as we found this target value to be associated more with general anaesthesia than with sedation (17). Furthermore, to assume that a particular level of BIS in a paediatric population denotes the same pharmacodynamic effect (or depth of anaesthesia) could lead to erroneous results in studies using this monitor to titrate to a standardised anaesthetic effect. In a study by Munoz and colleagues, anaesthesia was titrated to reach a BIS value of 50 in order to calculate and to compare the effect site concentration of propofol in both a paediatric and an adult population (18). No difference was found between effect site concentration (EC50 value) in the two populations, but it would be interesting to calculate this parameter for different sub-populations of age in the paediatric group.
It is possible that the validity of the Kataria PK model could perhaps explain the difference in BIS values with age. However, Kataria and colleagues showed that including age as a covariate produced only a very small improvement of the pharmacokinetic model (in contrast to weight). In our study, even if age of children ranged to 15 year old, the distribution of weight was similar to that of the population used to validate the model. However, we can not exclude that these results could be in part linked to the large variations in plasma concentrations of propofol according to age. Unfortunately, our study was not to validate this model using plasmatic concentration measurement. On the other hand, Park and colleagues (16) have shown same scattering of BIS values in children using another pharmacokinetic model (Marsh).
Another explanation for the variation of BIS with age is the difference in the pharmacodynamics of propofol in younger and older children. The MAC of volatile agents varies with age of children, and so might TCI propofol requirements. To our knowledge, there is no study in a paediatric population that have determined propofol plasma concentration according to age to obtain equivalent level of clinical anaesthesia. However, BIS values in all children were obtained during a stationary EEG bispectrum, suggesting that the pharmacodynamic effect was stable, and secondly we found no influence of age on the BIS at 4 mcg·ml−1 or 6 mcg·ml−1 which would be expected if the only explanation was this difference in pharmacodynamic properties.
In this study we used remifentanil to achieve good intubating conditions, and to represent normal clinical practice with the use of a hypnotic and an analgesic agent to provide general anaesthesia. Remifentanil could be implicated in our findings of a variation of BIS with age. However, in an adult population, Bouillon and colleagues showed that coadministration of remifentanil did not alter the pharmacokinetic of propofol (19). Pharmacodynamically, Minto and colleagues have described age-dependant EEG modification when using remifentanil in adults (20), but the doses of remifentanil used in this study were much higher than those used in our study, and the effect of remifentanil on Bispectral Index remained unclear. The addition of remifentanil to propofol does seem to have a significant clinical effect, with loss of verbal command or eyelash reflex seen at higher BIS value than with propofol alone (21,22). However, Schmidt and colleagues found no difference in BIS values during propofol-remifentanil anaesthesia when remifentanil was stopped (23). Similarly, Wang and colleagues showed no modification of EC50 of propofol when remifentanil is added, even up to a rate of 0.4 mcg·kg−1·min−1 (24). The influence of remifentanil on our results cannot be ignored, but we would argue that it has little effect on the bispectral modification in the dose we used, especially in the absence of noxious stimulation.
Using the Multiple Correspondence Analysis (MCA) it is easy to see the difference between the EEG bispectrum of children anaesthetised with propofol rather than with sevoflurane or desflurane. In the MCA there are certain discriminating frequencies of coupling (Pi) that determine a child’s position in the model. Higher values of P1, P2 and P9 (low frequencies less than 8 Hz) position children towards the right, and higher values of P2, P16 and P17 (intermediate frequencies 8–12 Hz) position children towards the upper half of the model. Under propofol anaesthesia higher frequencies in the EEG bispectrum predominate (greater than 12 Hz) and this explains the left and downward shift in their position compared to the positions seen with sevoflurane and desflurane (with halothane being entirely different altogether). This downward and left-shifted position in the MCA found in children deeply anaesthetised with propofol (4 mcg·ml−1 and 6 mcg·ml−1) could have been in part explained by episodes of burst suppression found in a large proportion of children. However, when we analyzed the position, and change in position, of a sub-group of children in whom no burst suppression was seen, there was no modification to the position of these children in the structured model of the MCA. EEG bispectrum modifications (in certain frequencies of coupling) induced by propofol are therefore significantly different to those induced by the volatile agents. The change in certain frequencies of coupling with decreasing plasma propofol concentration are similar in some respects to the changes we saw from 2 MAC to 1 MAC sevoflurane and desflurane in a previous study (4) along axis 2 in contrast to the changes on axis 1. Given that BIS values in this model are linked to changes along axis 1 (see previous study 1) this could explain why BIS could not detect the decrease of concentration between 6 mcg·ml−1 and 4 mcg·ml−1 We would therefore suggest that the variation in BIS we have seen with the decrease in plasma concentration to 2 mcg·ml−1 cannot be explained by a change in EEG bispectrum detected by this monitor, and is probably due to modification of other aspects of the BIS algorithm. The advantages of bispectral over spectral analysis in determining depth of anaesthesia have been brought into question by Miller and colleagues (25). Our results support this argument in a paediatric population, in particular if frequencies of coupling that are affected when decreasing hypnotic agent are not those used by the BIS monitor.
In summary, when using remifentanil and propofol TCI (Kataria PK model) in a paediatric population, it seems difficult to interpret BIS values because of the absence of significant change for higher plasma concentration variation or because of the link with age for the lower plasma concentration. Moreover, the difference of modifications in frequencies of coupling in the EEG bispectrum induced by propofol anaesthesia certainly emphasizes the importance of specifying the agent being used and the age of children when interpreting values given by monitors using the EEG bispectrum to determine depth of anaesthesia in children. A modification of the BIS algorithm could perhaps improve the performance of this monitor.
Acknowledgments
These works were partly financed by the French Ministry of Research and Technology (Grant: DT 03 B 107- 108-109; CITH Rennes).
References
- 1.Wodey E, Tirel O, Bansard JY, et al. Impact of age on both BIS values and EEG bispectrum during anaesthesia with sevoflurane in children. Br J Anaesth. 2005;94:810–20. doi: 10.1093/bja/aei140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim HS, Oh AY, Kim CS, Kim SD, Seo KS, Kim JH. Correlation of bispectral index with end-tidal sevoflurane concentration and age in infants and children. Br J Anaesth. 2005;95:362–6. doi: 10.1093/bja/aei196. [DOI] [PubMed] [Google Scholar]
- 3.Davidson AJ, Huang GH, Rebmann CS, Ellery C. Performance of entropy and Bispectral Index as measures of anaesthesia effect in children of different ages. Br J Anaesth. 2005;95:674–9. doi: 10.1093/bja/aei247. [DOI] [PubMed] [Google Scholar]
- 4.Tirel O, Wodey E, Harris R, Bansard JY, Ecoffey C, Senhadji L. The impact of age on bispectral index values and EEG bispectrum during anaesthesia with desflurane and halothane in children. Br J Anaesth. 2006;96:480–5. doi: 10.1093/bja/ael034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wallenborn J, Kluba K, Olthoff D. Comparative evaluation of Bispectral Index and Narcotrend Index in children below 5 years of age. Paediatr Anaesth. 2007;17:140–7. doi: 10.1111/j.1460-9592.2006.02036.x. [DOI] [PubMed] [Google Scholar]
- 6.Edwards JJ, Soto RG, Bedford RF. Bispectral Index values are higher during halothane vs. sevoflurane anesthesia in children, but not in infants. Acta Anaesthesiol Scand. 2005;49:1084–7. doi: 10.1111/j.1399-6576.2005.00813.x. [DOI] [PubMed] [Google Scholar]
- 7.Davidson AJ, Czarnecki C. The Bispectral Index in children: comparing isoflurane and halothane. Br J Anaesth. 2004;92:14–7. doi: 10.1093/bja/aeh011. [DOI] [PubMed] [Google Scholar]
- 8.Kataria BK, Ved SA, Nicodemus HF, et al. The pharmacokinetics of propofol in children using three different data analysis approaches. Anesthesiology. 1994;80:104–22. doi: 10.1097/00000542-199401000-00018. [DOI] [PubMed] [Google Scholar]
- 9.Nemethy M, Paroli L, Williams-Russo PG, Blanck TJ. Assessing sedation with regional anesthesia: inter-rater agreement on a modified Wilson sedation scale. Anesth Analg. 2002;94:723–8. doi: 10.1097/00000539-200203000-00045. [DOI] [PubMed] [Google Scholar]
- 10.Motas D, McDermott NB, VanSickle T, Friesen RH. Depth of consciousness and deep sedation attained in children as administered by nonanaesthesiologists in a children’s hospital. Paediatr Anaesth. 2004;14:256–60. doi: 10.1046/j.1460-9592.2003.01184.x. [DOI] [PubMed] [Google Scholar]
- 11.Glass PS, Bloom M, Kearse L, Rosow C, Sebel P, Manberg P. Bispectral analysis measures sedation and memory effects of propofol, midazolam, isoflurane, and alfentanil in healthy volunteers. Anesthesiology. 1997;86:836–47. doi: 10.1097/00000542-199704000-00014. [DOI] [PubMed] [Google Scholar]
- 12.McDermott NB, VanSickle T, Motas D, Friesen RH. Validation of the bispectral index monitor during conscious and deep sedation in children. Anesth Analg. 2003;97:39–43. doi: 10.1213/01.ane.0000067402.02136.a2. [DOI] [PubMed] [Google Scholar]
- 13.Sadhasivam S, Ganesh A, Robison A, Kaye R, Watcha MF. Validation of the bispectral index monitor for measuring the depth of sedation in children. Anesth Analg. 2006;102:383–8. doi: 10.1213/01.ANE.0000184115.57837.30. [DOI] [PubMed] [Google Scholar]
- 14.Shields CH, Styadi-Park G, McCown MY, Creamer KM. Clinical utility of the bispectral index score when compared to the University of Michigan Sedation Scale in assessing the depth of outpatient pediatric sedation. Clin Pediatr (Phila) 2005;44:229–36. doi: 10.1177/000992280504400306. [DOI] [PubMed] [Google Scholar]
- 15.Malviya S, Voepel-Lewis T, Tait AR. A comparison of observational and objective measures to differentiate depth of sedation in children from birth to 18 years of age. Anesth Analg. 2006;102:389– 94. doi: 10.1213/01.ANE.0000184045.01780.73. [DOI] [PubMed] [Google Scholar]
- 16.Park HJ, Kim YL, Kim CS, Kim SD, Kim HS. Changes of bispectral index during recovery from general anesthesia with 2% propofol and remifentanil in children. Paediatr Anaesth. 2007;17:353–7. doi: 10.1111/j.1460-9592.2006.02096.x. [DOI] [PubMed] [Google Scholar]
- 17.Powers KS, Nazarian EB, Tapyrik SA, et al. Bispectral index as a guide for titration of propofol during procedural sedation among children. Pediatrics. 2005;115:1666–74. doi: 10.1542/peds.2004-1979. [DOI] [PubMed] [Google Scholar]
- 18.Munoz HR, Cortinez LI, Ibacache ME, Leon PJ. Effect site concentrations of propofol producing hypnosis in children and adults: comparison using the bispectral index. Acta Anaesthesiol Scand. 2006;50:882–7. doi: 10.1111/j.1399-6576.2006.01062.x. [DOI] [PubMed] [Google Scholar]
- 19.Bouillon T, Bruhn J, Radu-Radulescu L, Bertaccini E, Park S, Shafer S. Non-steady state analysis of the pharmacokinetic interaction between propofol and remifentanil. Anesthesiology. 2002;97:1350– 62. doi: 10.1097/00000542-200212000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Minto CF, Schnider TW, Egan TD, et al. Influence of age and gender on the pharmacokinetics and pharmacodynamics of remifentanil. I. Model development. Anesthesiology. 1997;86:10–23. doi: 10.1097/00000542-199701000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Struys MM, Vereecke H, Moerman A, et al. Ability of the bispectral index, autoregressive modelling with exogenous input-derived auditory evoked potentials, and predicted propofol concentrations to measure patient responsiveness during anesthesia with propofol and remifentanil. Anesthesiology. 2003;99:802–12. doi: 10.1097/00000542-200310000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Lysakowski C, Dumont L, Pellégrini M, Clergue F, Tassonyi E. Effects of fentanyl, alfentanil, remifentanil and sufentanil on loss of consciousness and bispectral index during propofol induction of anaesthesia. Br J Anaesth. 2001;86:523–527. doi: 10.1093/bja/86.4.523. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt GN, Bischoff P, Standl T, et al. Narcotrend, bispectral index, and classical electroencephalogram variables during emergence from propofol/remifentanil anesthesia. Anesth Analg. 2002;95:1324–30. doi: 10.1097/00000539-200211000-00042. [DOI] [PubMed] [Google Scholar]
- 24.Wang LP, McLoughlin P, Paech MJ, Kurowski I, Brandon EL. Low and moderate remifentanil infusion rates do not alter target-controlled infusion propofol concentrations necessary to maintain anesthesia as assessed by bispectral index monitoring. Anesth Analg. 2007;104:325–31. doi: 10.1213/01.ane.0000252966.03103.89. [DOI] [PubMed] [Google Scholar]
- 25.Miller A, Sleigh JW, Barnard J, Steyn-Ross DA. Does bispectral analysis of the electroencephalogram add anything but complexity? Br J Anaesth. 2004;92:8–13. doi: 10.1093/bja/aeh003. [DOI] [PubMed] [Google Scholar]


