Abstract
There are now several independent studies that indicate that the dose-response for the endpoint of radiation-induced neoplastic transformation in vitro is non-linear for low linear energy transfer (LET) radiation. At low doses (<10 cGy) the transformation frequency drops below that seen spontaneously. Importantly, this observation has been made using fluoroscopic energy x-rays, a commonly used modality in diagnostic radiology, the practice of which is responsible for the majority of radiation exposure to the general public. Since the transformation frequency is reduced over a large dose range (0.1 to 10cGy) it is likely that multiple mechanisms are involved and that the relative contribution of these may vary with dose. These include the killing of a subpopulation of cells prone to spontaneous transformation at the lowest doses, and the induction of DNA repair at somewhat higher doses. Protective effects of low doses of low LET radiation on other cancer-relevant endpoints in vitro and in vivo have also been observed by several independent laboratories. These observations strongly suggest that the linear-nonthreshold dose-response model is unlikely to apply to the induction of cancer by low doses of low LET radiation in humans.
Keywords: Low dose, radiation, neoplastic transformation, adaptive response
INTRODUCTION
The risk of cancer induction by low doses (< 10 cGy) of radiation is a much studied and debated topic. Estimation of risk is still largely based on epidemiologic studies assuming a linear-nonthreshold (LNT) dose-response model for ionizing radiation with modifiying factors for dose and dose-rate (DDREF). A recent report from the National Council on Radiation Protection (NCRP Report No. 136, 2001) specifically addressing this topic cautiously concluded that the relevant data do not show any significant departure from a linear-nonthreshold response. The report (p. 211) states that “In conclusion, although the evidence is stronger for high-LET radiation than with low-LET radiation, the weight of evidence, both experimental and theoretical, suggests that the dose-response relationships for many of the biological alterations that are likely precursors to cancer are compatible with linear-nonthreshold functions. The epidemiological evidence, likewise, while necessarily limited to higher doses, suggests that the dose-response relationship for some, but not all, types of cancer may not depart significantly from linear non-threshold functions. The existing data do not exclude other dose-response relationships. Further efforts to clarify the relevant low dose relationships in the low dose domain are strongly warranted”.
The comment that the weight of evidence for the LNT hypothesis is stronger for high than low LET radiations is certainly true and also has support from the known physical action and biological response to high LET radiation. However, the case for low LET radiation and cancer risk as being compatible with a non-threshold response is far from clear. This is implied in the NCRP statement and the text of the document does acknowledge the existence of adaptive responses as likely having some influence on dose and dose-rate effects for low LET radiation.
Since NCRP Report No. 136 (2001) was written, much more data on low dose effects has been accrued. This has largely been a consequence of the US Department of Energy Low Dose Radiation Program. Therefore, some of the statements made in the NCRP (2001) report are in need of revision. For example, in reference to adaptive responses, it is claimed (p. 211) that “they have yet to be elicitable in cells or organisms exposed to less than 10 mGy delivered at a dose-rate of less than 50 mGy min–1”. This statement was actually incorrect as Azzam et al., (1996) had shown that a dose as low as 1 mGy delivered at a dose-rate of 2.4 mGy min–1 could suppress neoplastic transformation frequency in vitro to a level below that seen spontaneously. This observation has subsequently been confirmed by data accrued since the publication of the NCRP report as is discussed below and in a subsequent section.
Neoplastic transformation in vitro is an experimental endpoint long regarded as having relevance to radiation carcinogenesis in vivo (for review see Little 1989). While it is clear that in vitro model cell systems cannot duplicate many of the complexities of the in vivo situation, it is also clear that these in vitro systems have been able to provide data on dose-rate effects, LET effects and chemical modifier effects that are consistent with what is found in vivo. The topic of neoplastic transformation in vitro is discussed in Chapter 7 of NCRP (2001). It is correctly noted that the dose-response curve is complex in shape and subject to variation depending on the particular experimental conditions investigated. It also stated (p. 209) that “Few data are available as of yet on the shape of the dose response for curve at low doses. . . .” This was true for low LET radiation at the time the report was written but again, this is no longer the case. It is mentioned (p. 210) that the lowest dose at which a statistically significant increase in transformation frequency over background has been demonstrated is 10 mGy of 210 kVp x-rays (Borek and Hall 1973). These studies were performed with Syrian hamster embryo cells, a system with advantage of a low spontaneous background of spontaneous transformation, and hence ready ability to detect small changes above background. Interestingly, in the past 20 years or more this system has not been routinely used in studies of radiation-induced neoplastic transformation. This lack of use likely reflects technical difficulties with the system, although the assay has been modified and improved and is used fairly extensively in chemical carcinogenesis (Custer et al., 2000). The NCRP 2001 report (Chapter 7, p. 85) claims that the more widely used C3H10T1/2 transformation assay system is not so readily adapted to low dose studies because of its high background transformation frequency and that the lowest dose used with this system has been 0.1 Gy. As already indicated, this statement is not accurate as in Chapter 7 (p. 96) the important studies of Azzam et al., (1996) who went down to a dose of Co-60 gamma radiation as low as 1 mGy, are discussed. These studies are important in that they were the first to report a low dose suppression of neoplastic transformation frequencies to levels below those seen spontaneously. The NCRP report (p. 96) further implies that these findings with neoplastic transformation in vitro are of particular relevance “since the endpoint observed . . . is closer to carcinogenesis than chromosomal aberrations or mutations”. This is a reasonable statement, yet it stands in contrast to a later statement in the report used in support of the LNT model (Chapter 12, pp. 208–209) that “. . . mutations of types implicated in carcinogenesis . . . have been observed to be inducible at relatively low doses (e.g. <0.01 Gy) with apparently linear-non-threshold dose-response relationships in a various kinds of cells”. This placing of emphasis on mutations is at odds with the earlier statement with respect to the relevance of neoplastic transformation as an endpoint and essentially dismisses the observations of Azzam et al., (1996) on the suppression of neoplastic transformation at low doses. It is likely that the disconnect between dose-response relationships for aberrations and mutations on the one hand, and neoplastic transformation on the other, reflects the additional complexity in damage processing that ultimately results in neoplastic transformation. The results of Azzam et al., (1996) were apparently viewed with caution in NCRP (2001) because of the known existence of a narrow window of sensitivity to transformation in the G2/M phase of the cell cycle and that the low dose of radiation may deplete the population of cells most likely to be transformed by a subsequent dose, or spontaneously. Why this was thought to be reason to view with caution is not clear. Indeed, this comment has since proven to predictive of later findings (Redpath, Short et al., 2003).
The following section shall discuss some recent findings from my laboratory on the response of cells to low doses (<10 cGy) of low LET radiation for the endpoint of neoplastic transformation in vitro, and this will be followed by a more general discussion of low dose effects that support the concept of non-linearity of the dose-response curve for cancer-relevant endpoints and the limitations of epidemiological studies to define the dose-response curve at low doses of low LET radiation.
NEOPLASTIC TRANSFORMATION IN VITRO FOLLOWING LOW DOSES OF LOW LET RADIATION
While this section will deal almost exclusively with low LET radiation, it is important to point out that, consistent with the general conclusion of the NCRP report, in vitro studies of high LET radiation reveal a transformation dose-response curve that is linear down to doses as low as 2 mGy for C3H10T1/2 cells that were in exponential phase at the time of irradiation with 4.3 MeV alpha particles (Bettega et al., 1992). As mentioned earlier, this is not surprising in view of the energy deposition pattern of high LET radiation and current knowledge on subsequent biological responses. However, as already mentioned, the Borek and Hall (1973) study with low doses of low LET 210 kVp x-rays also indicated no threshold. This is in contrast to the studies of Azzam et al., (1996) with low LET Co-60 gamma radiation that showed suppressive effects of low doses of low LET radiation that point to the existence of a threshold. What is the reason for this disagreement between these two low LET studies? In my opinion, it probably lies in the nature of the experimental protocol. The study of Borek and Hall (1973) employed Syrian hamster embryo cells that were irradiated and held at low cell density while that of Azzam et al., (1996) used confluent cultures of C3H10T1/2 cells that were held at confluence for 24 h prior to plating at low cell density for the transformation assay. This post-irradiation holding protocol was used since it is well known that post-irradiation recovery can occur during holding at high cell density, and an adaptive response requires time to develop (Wolff, 1998). It is likely that it is the post-irradiation holding that is the key to observing the suppression of transformation.
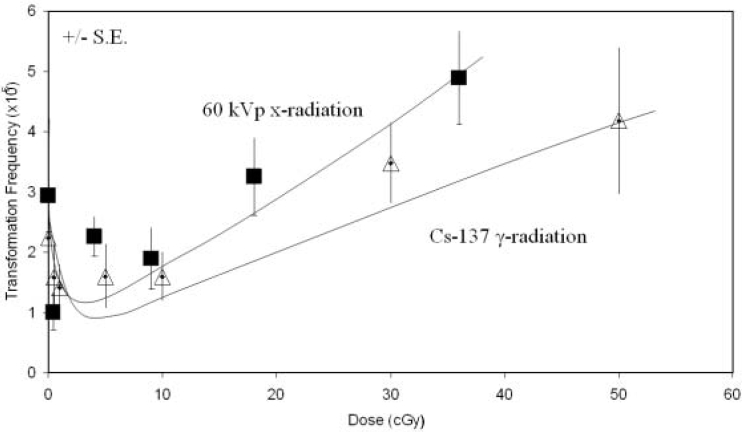
Since the result of Azzam et al., (1996) has potentially important implications in terms of cancer risk following low doses of low LET radiation, particularly if similar effects were to be observed in vivo, we repeated their study using the human cell-based human hybrid cell transformation assay (Redpath et al., 1987, Sun et al., 1988, Mendonca et al., 1992) at a single dose of Cs-137 gamma radiation of 1 cGy and found the same suppressive effect following post-irradiation holding, although of a somewhat lower magnitude (Redpath and Antoniono 1998). We then embarked on developing a full dose response curve, something that was missing at the time of writing of NCRP Report No. 136, and published the results in late 2001. The data showed that after post-irradiation holding there was a significant suppression of transformation at doses of 10 cGy down to 0.1 cGy and that linear extrapolation from higher doses through the origin overestimated the risk of transformation at the low doses (Redpath et al., 2001). The threshold dose at which positive effects of the radiation on induction of transformation began to occur appeared to be between 10 and 20 cGy (Figure 1). This study addressed the aforementioned deficiency on the low dose definition of the shape of the dose-response curve referred to in the NCRP report. We have since shown essentially the same effect for fluoroscopic energy x-rays of the type widely used in diagnostic radiology (Figure 1 and Redpath et al., 2003), and we have evidence that the same is true for mammography energy x-rays (Ko et al., 2004). This is important since it is through the practice of diagnostic radiology that the majority of the public are exposed to ionizing radiation. Mechanistic studies have revealed that at very low doses, hyper-radiosensitivity of a subpopulation of cells prone to spontaneous neoplastic transformation could result in their depletion and thus a reduction in transformation frequency (Redpath, Short et al., 2003). On the other hand, at somewhat higher doses induced DNA repair may account for the suppression (Pant et al., 2003). These mechanisms are consistent with conclusions drawn on the fate of DNA double strand breaks induced by x-irradiation of human cells where at very low doses they are not repaired, thus predicting for cell death, whereas at somewhat higher doses they are repaired (Rothkamm and Lobrich 2003), and with the well established phenomena of low dose hyper-radiosensitivity and induced-radioresistance (see Marples et al., 2004 for a recent review). Thus, for an endpoint that is regarded as having clear cancer relevance (Little 1989, NCRP 2001), namely neoplastic transformation in vitro, it is now apparent that the low LET radiation dose-response is non-linear at low doses and that low dose risk of transformation is overestimated by linear extrapolation from intermediate doses. However, extrapolating this conclusion to cancer risk in vivo must be done with caution. The question then arises is there evidence with other endpoints in other biological systems, particularly in vivo, that point to the same conclusion?
FIGURE 1.
Dose dependence for neoplastic transformation of human hybrid cells by Cs-137 gamma rays and 60 kVp x-rays. Error bars represent +/– SE. Reproduced from Redpath et al (2003) with permission of Taylor & Francis (http://www.tandf.co.uk/journals/titles/09553002.html)
BIOLOGICAL EFFECTS OF LOW DOSES OF LOW LET RADIATION THAT POINT TO A NON-LINEAR DOSE RESPONSE CURVE
A confounding effect that could theoretically counteract the suppressive effect of an adaptive response is the so-called bystander effect. Bystander effects are classically defined as effects of radiation that occur in non-targeted cells (Ballarini et al., 2002, Morgan 2003). They are thought to be independent of dose and to account for the majority of the radiation effect on cells in the dose range 0.01 to 0.5 Gy (Seymour and Mothersill 2000). It is far from clear, however, that such effects necessarily would result in an increased cancer risk at low doses. For example, if the killing of cells prone to neoplastic transformation at low doses was a consequence of a bystander effect, this could result in a suppression of transformation frequency below that seen spontaneously. On the other hand, bystander effects that result in a higher transformation frequency than expected based on the direct absorbed dose have been seen following low doses of high LET radiation (Sawant et al., 2001a). Studies to clarify the role of bystander effects in neoplastic transformation following low doses of low LET radiation are ongoing in several laboratories. A recent paper reported on the influence of cell-to-cell contact on the induction of transformation of C3H10T1/2 cells by α-particles and found a significantly increased transformation frequency for cells irradiated under conditions where >90% of the cells were in contact compared to when <10% were in contact (Mitchell et al., 2004). It was concluded that this increase is likely due to a gap junction intercellular communication (GJIC)-mediated bystander effect. This study did not employ any post-irradiation holding and hence minimized the potential for any modification of the outcome by an adaptive response. On the other hand, in their study demonstrating a suppression of transformation at low doses of low LET radiation, Azzam et al., (1996) also irradiated confluent cultures of gap-junction competent C3H10T1/2 cells. In this case a 24 h post-irradiation holding step was employed prior to seeding the cells for the transformation assay. Clearly, if there were any transformation-inducing bystander effects, they must have been counteracted by an adaptive response. On the other hand, as mentioned above, the bystander effect itself may have been responsible for the suppression of transformation due to killing of a transformation-sensitive subpopulation. The modification of bystander effects by an adaptive response is also an area of current investigation using both transformation (Sawant et al., 2001b) and mutagenesis (Zhou et al., 2003) as endpoints and it is clear that adaptive responses can protect against bystander effects and the final dose-response curve will be a reflection of this. The low dose transformation studies carried out in our laboratory have mainly been carried out with cultures where about 20–30% of the cells were in contact at the time of irradiation, i.e. not optimal for a GJIC-mediated bystander effect, and we found that the GJIC inhibitor, lindane, did not alter the transformation frequency following low doses of low LET radiation (unpublished results). This finding is consistent with the expected minimal GJIC-mediated bystander effect under our standard experimental conditions. Clearly, the absence of any transformation-inducing bystander effects would be the optimal condition under which to observe suppression of transformation as a consequence of an adaptive response. Many of these concepts have been incorporated into models of low dose radiation effects as they pertain to carcinogenesis (Brenner et al., 2001, Schollenberger et al., 2002, Pollycove and Feinendegen 2003, Scott et al., 2003). Again, these are in vitro findings and their in vivo relevance remains to be established.
There is an extensive history of animal studies of radiation carcinogenesis, including studies at relatively low doses of ionizing radiation. Reviews of these data can be found in NCRP (2001) and UNSCEAR (2000). Just as in vitro studies can show cell line dependencies in terms of the shape of dose-response relationships, in vivo studies show significant sex and strain dependencies for the radiation-induction of cancer. Similarly, the human population shows evidence for genetic susceptibility. Unlike in vitro studies, in vivo studies suffer from some of the same statistical problems of measuring low dose effects as do epidemiologic studies. This is particularly true for low LET radiation. This is discussed in Annex G of UNSCEAR (2000). Nonetheless, there is evidence from animal studies of radiation carcinogenesis that suggest that the risk of cancer induction at low doses is lower than would be determined by extrapolation from higher doses and that reducing the dose-rate reduces this risk even further. One of the larger animal studies reported comes from Ullrich and Storer (1979). Their data on myeloid leukemia induction in female RFM mice indicated an increased incidence at doses of 0.5 Gy of Cs-137 gamma radiation and above. While linear, linear-quadratic and threshold quadratic models can be made to fit the data, a threshold of 0.22 Gy fits the data as well as anything else (UNSCEAR 2000). This was in a study that involved nearly 18,000 animals, and yet the low dose information is equivocal because of the low incidence of myeloid leukemias. In the same paper, the incidence of thymic lymphoma showed clear evidence of a threshold-type response, particularly at low dose-rates (Ullrich and Storer 1979). It should be emphasized that even in this large and comprehensive study, the lowest acute exposure dose that was studied was 10 cGy and at this dose no significant increase in tumor incidence was seen for ovarian tumors, pituitary tumors and Harderian gland tumors. With the exception of ovarian tumors, this was also true at a dose of 25 cGy. For chronic exposure, no significant increase in incidence of these tumors was seen at a dose of 50 cGy. The above summary is by no means meant to be extensive, but rather to illustrate the limitations of animal studies in the study of low doses of low LET radiation and also to show that where such data exist, it is at the very least just as compatible with a threshold-type response as with an LNT-type response. Thus, the low dose, low LET, in vitro neoplastic transformation studies are compatible with the low dose in vivo studies, further validating neoplastic transformation in vitro as a cancer-relevant endpoint
In order to examine the effects of lower doses of radiation (<10 cGy) it is necessary to work with animals that are susceptible to cancer formation or to examine effects on tissues that are known to be highly radiosensitive, such as the developing embryo and fetus. There is recent evidence from various in vivo studies of responses that indicate a protective effect of very low doses of radiation under these circumstances. This includes the delay in appearance of tumors in cancer-prone mice (Mitchel et al., 2003) and protection against high dose-induced prenatal death and fetal malformations (Wang et al., 1998). More complex in vivo dose-response relationships are emerging with respect to the dose-rate at which priming doses are delivered in adaptive response studies on fetal malformation in mice (Wang et al., 2004) as well as for chromosome inversion in the mouse spleen following low, very low and ultra low doses of low LET radiation (Hooker et al., 2004). Again, these findings are largely compatible with very low dose in vitro neoplastic transformation studies. All of these point to the likelihood that the dose-response curve is not linear at low doses of low LET radiation, that a threshold is likely, and that this threshold is dose-rate dependent. The caveat is that any threshold will almost certainly be dependent on the tissue at risk, as well as age at exposure. The tissue dependency of a threshold dose, below which an increase in tumor latency is evident, is demonstrated in the study of Mitchel et al., (2003) where clearly different thresholds were apparent for lymphomas versus osteosarcomas.
EPIDEMIOLOGIC STUDIES OF RADIATION-INDUCED CANCER
Given the discussion in the previous sections it is reasonable to ask the question how do the epidemiologic data, as concluded in NCRP Report No. 136 (2001), suggest that the dose-response curve for cancer risk may not significantly depart from linearity? Of course, as has been pointed out earlier (Hoel and Li 1998), simply because a linear-nonthreshold model fits the data does not provide evidence that non-linearities or thresholds in the 0.05 Sv range are not present in the data. Their analysis of the A-bomb survivors cancer incidence data agrees more with a threshold or non-linear dose-response model than a purely linear model. As is the case for laboratory data, there have also been updates on epidemiologic analyses since the publication of NCRP (2001) and they continue to support, within limitations, the LNT stance. An update on radiation-induced breast cancer based on the pooled analysis of eight cohorts supports linearity down to 0.02 Gy (Preston et al., 2002). This is also essentially the conclusion of a recent update of the atomic bomb survivor data, where excess relative risks for all solid cancers combined were significant for dose-ranges above 0.1 Sv, but importantly were not significant for the dose-range 0–0.1 Sv (Preston et al., 2003). The fact that the lower limit of dose at which a significant risk has been measured is a little higher for all solid cancers combined than that found for breast cancer, a cancer that is relatively sensitive to induction by radiation, is almost certainly due to the combination of data for all solid tumors. A recent update on thyroid cancer induction in children acutely irradiated to the scalp for ringworm indicates a dose-response curve that is linear down to a dose of 7 cGy, and that the relative risk at this dose is significantly greater than one (Lubin et al., 2004). This represents a tissue, thyroid, known to be sensitive to radiation-induced cancer, and a population of young age at the time of exposure, both predisposing factors for radiation-induced cancer. A recent review by Brenner et al., (2003) concluded that the most reasonable assumption is that cancer risks from low doses of low LET radiation decrease linearly with dose, although they did add the qualifying statement that the LNT approach could well overestimate cancer risks in some cases and underestimate risk in others. For example, there is strong evidence that a simple LNT model likely overestimates the induction of leukemia in adults (Little and Muirhead, 1998), and for osteosarcomas (White et al., 1993) a threshold dose is likely. The review by Brenner et al., (2003) concluded that for acute exposure the dose-response curve for the induction of solid cancers is linear down to a dose range of approximately 0.01 to 0.05 Sv, and for protracted exposure down to approximately 0.05 to 0.10 Sv. It should be pointed out that the lower limit of the acute exposure dose-range quoted above refers to the case of childhood cancer following intrauterine irradiation where a dose to the fetus of 10 mSv discernibly increases the risk (for recent review, see Wakeford and Little 2003), and not to cancers in exposed adults where this lower limit is considerably higher. This serves to highlight the well known importance specifying the nature of the induced cancer and age at exposure.
It is important to recognize that all of these recent updates continue to support the conclusion of NCRP Report No. 136 (2001) that epidemiological analyses identifying significant risk are largely limited to higher doses of low LET radiation (>5 cGy). With the exception of fetal exposure, they cannot reliably tell what is happening at doses <5 cGy, especially on a tissue by tissue basis, and therefore cannot rule out any departure from linearity at these lower doses, doses that are in the important dose-range relevant to diagnostic radiology.
SUMMARY
Evidence continues to accumulate from in vitro and in vivo laboratory studies of cancer-relevant endpoints across a variety of species and organisms that the linear-nonthreshold dose-response model does not hold for low doses of low LET radiation, and it is difficult to conclude that adult human populations should respond any differently. This lack of adherence to an LNT response is not surprising based on the known complexity of biological response to low LET radiation. The majority of these laboratory data are indicative that cancer risk for low LET radiation may be less than estimated by the LNT model, even after employing a dose and dose-rate effectiveness factor (DDREF), and that a threshold dose is not improbable. This conclusion has support from the statistical model analysis of the A-bomb survivor data (Hoel and Li 1998). However, it is emphasized that the value of any threshold dose will almost certainly be tissue and dose-rate dependent, and for some cancers (breast, thyroid) this could be quite low, while for others (adult leukemia and osteosarcomas) it may be 10 cGy or higher for acute exposure to low LET radiation, and higher still for chronic exposure. The challenge for the future is to continue to attempt to fully incorporate laboratory findings on the mechanisms involved in the response to very low doses of low LET radiation into the estimation of relative risk of cancer induction in both normal and genetically susceptible individuals, as well as define an acceptable level of relative risk on a tissue by tissue basis.
Acknowledgments
The support of the Office Science (BER), U.S. Department of Energy Low Dose Radiation Program Grant Numbers DE-FG07–99ER62876, DE-FG03–02ER63309 and DE-FG02–03ER63648 and the members of my research laboratory are gratefully acknowledged.
REFERENCES
- Azzam EI, De Toledo SM, Raaphorst GP, Mitchel R. Low dose ionizing radiation decreases the frequency of neoplastic transformation to a level below the spontaneous rate in C3H10T1/2 cells. Radiat Res. 1996;146:369–373. [PubMed] [Google Scholar]
- Ballanari F, Biaggi M, Ottolenghi A, Sapora A. Cellular communication and bystander effects: A critical review for modeling low dose radiation action (short survey) Mutat Res. 2002;501:1–12. doi: 10.1016/s0027-5107(02)00010-6. [DOI] [PubMed] [Google Scholar]
- Bettega D, Calozari P, Noris Chiorda G, Tallone-Lombardi L. Transformation of C3H10T1/2 cells with 4.3 MeV α-particles at low doses: Effect of single and fractionated doses. Radiat Res. 1992;131:66–71. [PubMed] [Google Scholar]
- Borek C, Hall EJ. Transformation of mammalian cells in vitro by low doses of x-rays. Nature (London) 1973;243:450–453. doi: 10.1038/243450a0. [DOI] [PubMed] [Google Scholar]
- Brenner DJ, Little JB, Sachs RK. The bystander effect in radiation oncogenesis: A quantitative model. Radiat Res. 2001;155:402–408. doi: 10.1667/0033-7587(2001)155[0402:tbeiro]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Brenner DJ, Doll R, Goodhead DT, Hall EJ, Land CE, Little JB, Lubin JH, Preston DL, Preston RJ, Puskin JS, Ron E, Sachs RK, Samet JM, Setlow RB, Zaider M. Cancer risks attributable to low doses of ionizing radiation: Assessing what we really know. Proc Nat Acad Sci USA. 2003;100:13761–13766. doi: 10.1073/pnas.2235592100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Custer L, Gibson DP, Aardema MJ, LeBoeuf RA. A refined protocol for conducting the low pH 6.7 Syrian hamster embryo (SHE) cell transformation assay. Mutat Res. 2000;455:129–139. doi: 10.1016/s0027-5107(00)00098-1. [DOI] [PubMed] [Google Scholar]
- Hooker A, Bhat M, Day TK, Lane JM, Swinburne SJ, Morley AA, Sykes PJ.2004The linear no-threshold model dose not hold for low dose ionizing radiation Radiat. ResIn press [DOI] [PubMed] [Google Scholar]
- Hoel DG, Li P. Threshold models in radiation carcinogenesis. Health Phys. 1998;75:241–250. doi: 10.1097/00004032-199809000-00002. [DOI] [PubMed] [Google Scholar]
- Ko SJ, Lao X-Y, Molloi S, Elmore E, Redpath JL. Neoplastic transformation in vitro after exposure to low doses of mammographic energy x-rays: Quantitative and mechanistic aspects. Radiat Res. 2004;162:646–654. doi: 10.1667/rr3277. [DOI] [PubMed] [Google Scholar]
- Little JB. The relevance of cell transformation to carcinogenesis in vivo. In: Baverstock KF, Strather JW, editors. Low Dose Radiation: Biological Bases of Risk Assessment. Taylor and Francis; London: 1989. pp. 396–413. [Google Scholar]
- Little MP, Muirhead CR. Curvature in the cancer mortality dose-responses in Japanese atomic bomb survivors: absence of evidence of threshold. Int J Radiat Biol. 1998;74:471–480. doi: 10.1080/095530098141348. [DOI] [PubMed] [Google Scholar]
- Lubin JH, Schafer DW, Ron E, Stovall M, Carroll RJ. A reanalysis of thyroid neoplasms in the Israeli tinea capitis study accounting for dose uncertainties. Radiat Res. 2004;161:359–368. doi: 10.1667/rr3135. [DOI] [PubMed] [Google Scholar]
- Marples B, Wouters BG, Collis SJ, Chalmers AJ, Joiner MC. Low-dose hyper-radiosensitivity: A consequence of ineffective cell cycle arrest of radiation-damaged G2–phase cells. Radiat Res. 2004;161:247–255. doi: 10.1667/rr3130. [DOI] [PubMed] [Google Scholar]
- Mendonca MS, Antoniono RJ, Sun C, Redpath JL. A simplified and rapid staining method for the HeLa x skin fibroblast human hybrid cell neoplastic transformation assay, Radiat Res. 1992;131:345–350. [PubMed] [Google Scholar]
- Mitchel REJ, Jackson JS, Morrison DP, Carlisle SM. Low doses of radiation increase the latency of spontaneous lymphomas and spinal osteosarcomas in cancer-prone, radiation sensitive Trp53 heterozygous mice. Radiat Res. 2003;159:320–327. doi: 10.1667/0033-7587(2003)159[0320:ldorit]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Mitchell SA, Randers-Pehrson G, Brenner DJ, Hall EJ. The bystander response in C3H10T1/2 cells: The influence of cell-to-cell contact. Radiat Res. 2004;161:397–401. doi: 10.1667/rr3137. [DOI] [PubMed] [Google Scholar]
- Morgan WF. Non-targeted and delayed effects of exposure to ionizing radiation: I. Radiation-induced genomic instability and bystander effects in vitro. Radiat Res. 2003;159:567–580. doi: 10.1667/0033-7587(2003)159[0567:nadeoe]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Mothersill C, Seymour C. Radiation-induced bystander effects carcinogenesis and models. Oncogene. 2003;22:7028–7033. doi: 10.1038/sj.onc.1206882. [DOI] [PubMed] [Google Scholar]
- NCRP 2001Evaluation of the Linear-Nonthreshold Dose-Response Model for Ionizing RadiationNational Council on Radiological Protection Report No. 136Bethesda, Maryland [Google Scholar]
- Pant MC, Liao X-Y, Lu Q, Molloi S, Elmore E, Redpath JL. Mechanisms of suppression of neoplastic transformation in vitro by low doses of low LET radiation. Carcinogenesis. 2003;24:1961–1965. doi: 10.1093/carcin/bgg172. [DOI] [PubMed] [Google Scholar]
- Pollycove M, Feinendegen LE. Radiation-induced versus endogeneous DNA damage: possible effects of inducible protective responses in mitigating endogeneous damage. Human Exp Toxicol. 2003;22:290–306. doi: 10.1191/0960327103ht365oa. [DOI] [PubMed] [Google Scholar]
- Preston D, Mattsson A, Holmberg E, Shore R, Hildreth N, Boice J. Radiation effects on breast cancer risk: A pooled analysis of eight cohorts. Radiat Res. 2002;158:220–235. doi: 10.1667/0033-7587(2002)158[0220:reobcr]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Preston D, Shimizu Y, Pierce DA, Suyama A, Mabuchi K. Studies of mortality of atomic bomb survivors. Report 13: Solid cancer and noncancer disease mortality: 1950–1997. Radiat Res. 2003;160:381–407. doi: 10.1667/rr3049. [DOI] [PubMed] [Google Scholar]
- Redpath JL, Sun C, Colman M, Stanbridge EJ. Neoplastic transformation of human hybrid cells by gamma radiation: A quantitative assay. Radiat Res. 1987;110:468–472. [PubMed] [Google Scholar]
- Redpath JL, Short SC, Woodcock M, Johnston PJ. Low dose reduction in transformation frequency compared to unirradiated controls: the role of hyper-radiosensitivity to cell kill. Radiat Res. 2003;159:433–436. doi: 10.1667/0033-7587(2003)159[0433:ldritf]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Redpath JL, Antoniono RJ. Induction of an adaptive response against low dose gamma radiation, Radiat Res. 1998;149:517–520. [PubMed] [Google Scholar]
- Redpath JL, Liang D, Taylor TH, Christie C, Elmore E. The shape of the dose-response curve for radiation-induced neoplastic transformation in vitro: Evidence for an adaptive response against neoplastic transformation at low doses of low-LET radiation. Radiat Res. 2001;156:700–707. doi: 10.1667/0033-7587(2001)156[0700:tsotdr]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Redpath JL, Lu Q, Liao X-Y, Molloi S, Elmore E. Low doses of diagnostic energy x-rays protect against neoplastic transformation in vitro. Int J Radiat Biol. 2003;79:235–240. doi: 10.1080/0955300031000096306. [DOI] [PubMed] [Google Scholar]
- Rothkamm K, Lobrich M. Evidence that human cells lack DNA double-strand break repair in human cells exposed to very low x-ray doses. Proc Nat Acad Sci USA. 2003;100:5057–5062. doi: 10.1073/pnas.0830918100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawant SG, Randhers-Pehrson G, Geard CR, Brenner DJ, Hall EJ. The bystander effect in radiation oncogenesis: I. Transformation of C3H 10T1/2 cells in vitro can be initiated in the unirradiated neighbors of irradiated cells. Radiat Res. 2001a;155:397–401. doi: 10.1667/0033-7587(2001)155[0397:tbeiro]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Sawant SG, Randhers-Pehrson G, Metting NF, Hall EJ. Adaptive response and the bystander effect induced by radiation in C3H 10T1/2 cells in culture. Radiat Res. 2001b;156:177–180. doi: 10.1667/0033-7587(2001)156[0177:aratbe]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Schollenberger H, Mitchel REJ, Azzam EI, Crawford-Brown DJ, Hofman W. Explanation of the protective effects of low doses of γ-radiation with a mechanistic radiobiological model. Int J Radiat Biol. 2002;78:1159–1173. doi: 10.1080/0955300021000034693. [DOI] [PubMed] [Google Scholar]
- Scott BR, Walker DM, Tesfaigi Y, Schollnberger H, Walker V. Mechanistic basis for non-linear dose-response relationships for low-dose radiation-induced stochastic effects. Nonlinearity in Biol Toxicol and Med. 2003;1:93–122. doi: 10.1080/15401420390844492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour CB, Mothersill C. Relative contribution of bystander and targeted cell killing to the low dose region of the radiation dose-response curve. Radiat Res. 2000;153:508–511. doi: 10.1667/0033-7587(2000)153[0508:rcobat]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Sun C, Redpath JL, Colman M, Stanbridge EJ. Further studies of the radiation-induced expression of a tumor-associated antigen in human cell hybrids, Radiat Res. 1988;114:84–93. [PubMed] [Google Scholar]
- Ullrich RL, Storer JB. Influence of γ irradiation on the development of neoplastic disease in mice. III. Dose-rate effects. Radiat Res. 1979;80:325–342. [PubMed] [Google Scholar]
- UNSCEAR . United Nations Scientific Committee on the Effects of Atomic Radiation, Report to the General Assembly. United Nations; New York: 2000. Sources and Effects of Ionizing Radiation. Volume II: Effects. [Google Scholar]
- Wakeford R, Little MP. Risk coefficients for childhood cancer after intrauterine irradiation: a review. Int J Radiat Biol. 2003;79:293–309. doi: 10.1080/0955300031000114729. [DOI] [PubMed] [Google Scholar]
- Wang B, Ohyama H, Nose T, Itsukaichi H, Nakajima T, Yukawa O, Odaka T, Tanaka K, Kojima E, Yamada T, Hayata I. Adaptive response in embryogenesis: I. Dose and timing of radiation for reduction of prenatal death and congenital malformation during the late period of organogenesis. Radiat Res. 1998;150:120–122. [PubMed] [Google Scholar]
- Wang B, Ohyama H, Shang Y, Tanaka K, Aizawa S, Yukawa O, Nakamura N. Adaptive response in embryogenesis: V. Existence of two efficient dose-rate ranges for 0.3 Gy of priming irradiation to adapt mouse fetuses. Radiat Res. 2004;161:264–272. doi: 10.1667/rr3141. [DOI] [PubMed] [Google Scholar]
- White RG, Raabe OG, Culbertson MR, Parks NJ, Samuels SJ, Rosenblatt LS. Bone sarcoma characteristics and distribution on beagles fed strontium-90. Radiat Res. 1993;136:178–189. [PubMed] [Google Scholar]
- Wolff S. The adaptive response in radiobiology: evolving insights and implications. Env Health Perspect. 1998;106(Suppl.1):277–283. doi: 10.1289/ehp.98106s1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Randers-Pehrson G, Geard CR, Brenner DJ, Hall EJ, Hei TK. Interaction between radiation-induced adaptive response and the bystander effect in mutagenesis in mammalian cells. Radiat Res. 2003;160:512–560. doi: 10.1667/rr3083. [DOI] [PMC free article] [PubMed] [Google Scholar]