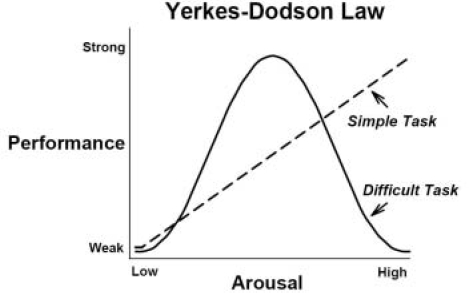
It has been almost a century since the first paper describing a non-linear relationship between arousal and behavioral performance was published (Yerkes and Dodson 1908). This study, an analysis of the influence of task difficulty and stress on discrimination learning in the dancing mouse, stands apart from all others. The paper was published without statistical analyses (statistics had not yet been conceived) and with sample sizes as small as 2 mice per group (an unacceptably low level of power by modern standards). Despite the limitations of their study, the findings of Yerkes and Dodson were subsequently replicated in cats (Dodson, 1915), rats (Broadhurst, 1957; Telegdy and Cohen 1971) and people (Dickman, 2002; Bregman and McAllister 1982; Anderson, 1994), and became part of the lexicon of the field of psychology as the “Yerkes-Dodson Law” (Young, 1936; Eysenk, 1955). In brief, Yerkes and Dodson found that when mice were given a simple discrimination task their performance improved linearly with increases in arousal. With more difficult tasks, the performance of the mice improved with moderate with increases in arousal, but at the highest levels of arousal their performance was impaired, forming an overall non-linear (inverted-U) shaped relationship between arousal and performance. This task-dependent influence on the shape of arousal-performance curves is illustrated in Figure 1.
FIGURE 1.
Illustration of the linear and non-linear components of the Yerkes-Dodson Law based on task difficulty. Performance in both types of tasks is seen to increase in response to a change from low to moderate levels of arousal. Under conditions of high arousal (or specifically “vigor” (Dickman, 2002)), subjects show a high degree of performance in simple tasks, but are impaired in performance in difficult tasks. In general, “simple tasks” would conform to a training condition which involves focused attention to a highly distinct stimulus. Performance on this type of task routinely produces strong and relatively accurate memories under highly arousing conditions, as typified by phenomena such as “weapon focus” and “flashbulb memories”, as well as enhanced memory for central events occurring in an arousing scene (Christianson, 1992; Conway et al., 1994; Safer et al., 1998; Pickel, 1998). By contrast, more demanding tasks that involve divided attention, working memory, attention to cues outside of the source of arousal, or those that involve subtle differences between stimuli in discrimination tasks would conform to the “difficult task” distinction. High levels of arousal typically impair performance in these types of tasks (Leon and Revelle 1985; Burke et al., 1992; Christianson, 1992). Ultimately, our understanding of the complex relationships among task difficulty, arousal and memory will be substantiated by studying the activation or inhibition of different brain memory systems by different levels of task difficulty and arousal (Barch et al., 1997; Arnsten, 1998; Diamond et al., 2001; Braver et al., 2001; Kensinger et al., 2003; Kensinger and Corkin 2004; Birnbaum et al., 2004).
The Yerkes-Dodson Law has served as a continuous influence on cognitive research, with decades of debate involving both support and criticism of its heuristic value (Broadhurst, 1957; Broadhurst, 1959; Brown, 1965; Deffenbacher, 1982; Christianson, 1992; Baumler, 1994; Teigen, 1994; Watters et al., 1997; Dutton and Carroll 2001; Hanoch and Vitouch 2004). Thus, the original work of Yerkes and Dodson and the “Law” it spawned provided the foundation for decades of research into why behavioral performance can be either enhanced or impaired when subjects are under strong pressure (high arousal).
Current research into non-linearities involving arousal and brain function have been conducted at advanced methodological levels that were unimaginable at the time of the birth of the Yerkes-Dodson Law. Neurobiological research has investigated non-linearities in a broad range of investigations of behavior and brain functioning, including functional MRI analysis of working memory (Callicott et al., 1999), hormone-memory interactions (Gold and Van Buskirk 1975; Introini-Collison et al., 1994; Stefani et al., 1999), synaptic plasticity (Diamond et al., 1992; Kerr et al., 1994), receptor ligand-memory interactions (Conrad et al., 1999; Okuda et al., 2004) and neuroprotection in response to metabolic insults (Bar-Joseph et al., 1994; Kaltschmidt et al., 1999; Abraham et al., 2000). It is in this context that I invited researchers with expertise in the study of non-linear relationships involving brain and arousal to submit manuscripts to the journal. The final product is an outstanding effort, with 7 papers contributed by distinguished researchers from 5 different countries. Their work is presented in two special issues devoted to the theme of non-linear relationships involving brain functioning, arousal and behavioral performance.
The current issue contains three papers from researchers in the laboratories of Dr. Corrado Bucherelli of the University of Florence, in Florence, Italy, Dr. Sonia Lupien of the Douglas Hospital Research Center and McGill University, in Montreal, Canada, and Dr. Cheryl Conrad of Arizona State University, in Tempe, Arizona, USA. The first paper by Drs. Baldi and Bucherelli provides a broad perspective on the neural and endocrine basis of non-linearities between behavioral performance and arousal. These investigators present a synthesis, derived from decades of research on rodents and humans, which enhances our understanding of the complex modulation of memory by arousal. Specifically, they have discussed the non-linear relationship between memory and the actions of hormones of the sympathetic nervous system, e.g., epinephrine, and hypothalamic-pituitary-adrenal (HPA) axis, e.g., adrenocorticotropic hormone (ACTH) and glucocorticoids (GCs; corticosterone in the rat and cortisol in people).
The second and third papers in this issue by Drs. Lupien and Conrad focus primarily on the acute effects of GCs on cognition in rodents and people. Both papers discuss evidence that an intermediate level of glucocorticoids enhance, and high levels of GCs impair, memory-related functioning of the hippocampus, a structure that has long been known to be highly sensitive to stress and to be critically involved in learning and memory (Eichenbaum, 2001; Poldrack and Packard 2003). Each of these papers provides balance in pointing out that elevated levels of stress or glucocorticoids may also enhance attention and memory, depending on whether the information is a part of, or outside of, the stress context, as well as whether the stress occurs at the time of acquisition versus the retrieval phase of memory (see also Roozendaal, 2000 for related discussion). This added level of complexity, i.e., that the shape of the function between GC levels and memory is context-dependent, is consistent with the primary feature of the Yerkes and Dodson Law which states that high levels of stress or arousal may enhance performance under some conditions and impair performance under other conditions (Figure 1). Thus, the expression of the relationship between GCs (as well as other physiological measures) and cognition can be either linear or non-linear, depending on variables such as the level of task difficulty, involvement of hippocampus versus non-hippocampal structures, as well as contextual and temporal variables (Cordero and Sandi 1998; Akirav et al., 2004; Okuda et al., 2004).
The second issue in this series will contain papers from the laboratories of Dr. Julian Thayer, of the National Institute on Aging, in Baltimore, Maryland, USA, Dr. Gal Richter-Levin, of the University of Haifa, in Haifa, Israel, Dr. Paul Luiten, of the University of Groningen, in Groningen, The Netherlands and Dr. David Diamond, of the University of South Florida and Veterans Hospital, in Tampa, Florida, USA. The paper by Dr. Thayer is perhaps the most eclectic work in this volume, providing a novel perspective on non-linear relationships in different neural systems. Specifically, this work integrates findings on non-linearities in inhibitory processes in different brain structures, e.g., prefrontal cortex and amygdala, as well as GABA receptor dynamics and synaptic plasticity, all within the broad framework of the neural control of the heart as a model system of inhibitory control.
The paper by Drs. Akirav and Richter-Levin focuses on interactions between neural structures. It is difficult enough to understand how brain structures, in isolation, are involved in learning and memory. These investigators have taken on the additional challenge to understand how the amygdala interacts with the hippocampus to influence memory processing. The ideas developed in their paper are consistent with the secondary theme in both of the special issues, that the expression of non-linearities in amygdala-hippocampus interactions is, among other factors, context-dependent.
The final two papers describe GC actions on neuron survival and memory. Their area of commonality is that they both demonstrate that very low or very high levels of GCs can produce adverse effects on hippocampal functioning. Specifically, Drs. Abraham, Meerlo and Luiten reviewed a substantial literature demonstrating that GCs play a potent role in both the survival and death of hippocampal neurons. They have shown that GCs can activate mechanisms involved in neurodegeneration, as well as neuroprotection, depending on the concentration of the steroid. The recurring theme of context-dependency of GC actions on the hippocampus is addressed here, as well. These authors point out, for example, that the adverse effects of high levels of GCs on neuronal death can be nullified by environmental manipulations, such as calorie restriction.
The volume concludes with a paper by Drs. Park, Campbell, Woodson, Smith, Fleshner and Diamond. These authors studied spatial memory in rats under conditions in which GC levels were manipulated behaviorally, by exposing the rats to a predator, or pharmacologically, by administering metyrapone, a GC synthesis inhibitor. They found that an intermediate level of GCs correlated with optimal memory, and very low or high GC levels correlated with impaired memory. Once again, the issue of context-dependency was raised because elevated GC levels, alone, were insufficient to impair spatial memory. Spatial memory was impaired only when elevated GC levels occurred in conjunction with a behavioral stress state (see also Woodson et al., 2003; Okuda et al., 2004 for related findings).
In summary, the explicit theme of these two issues is the non-linear relationship between arousal and brain functioning. However, it should be noted that increasing levels of arousal do not necessarily produce non-linear effects on the brain or behavior, despite the fact that this is how the Yerkes-Dodson Law is routinely portrayed in Psychology textbooks (e.g., Rice, 1999). The influence of the difficulty of the task on the shape of the arousal-performance curve was emphasized by Yerkes and Dodson (1908), as well as in later discussion by Dodson (1917) and in an early Psychology textbook (Young, 1936). However, recognition of the dual non-linear/linear feature of the Yerkes-Dodson Law by contemporary workers is only rarely acknowledged. Even scholars in the field of emotion, brain and memory have relegated the linear component to the status of a mere footnote (Christianson, 1992) or they have disregarded it entirely (Loftus, 1980; Metcalfe and Jacobs 1998; Mendl, 1999; Aston-Jones et al., 2000). The papers in these two issues help to meld neurobiology and psychology, within the context of the complete, i.e., original, version of the Yerkes-Dodson Law, by demonstrating that the ultimate shape of arousal-performance dose-response curves (as linear or curvilinear) depends on intervening variables, such as the nature of the task and contextual influences.
In closing I would like to note that all of the papers in these two special issues of the journal were peer-reviewed, and I thank the reviewers for their time, effort and constructive criticisms of preliminary versions of the manuscripts. I would also like to thank Dr. Ed Calabrese, the editor-in-chief and driving force behind the study of non-linear phenomena throughout the animal and plant kingdoms, for inviting me to serve as the guest editor of this volume. I would also like to thank Barbara Callahan for her assistance with the formatting of the manuscripts.
REFERENCES
- Abraham I, Harkany T, Horvath KM, Veenema AH, Penke B, Nyakas C, Luiten PG. Chronic corticosterone administration dose-dependently modulates Abeta(1–42)- and NMDA-induced neurodegeneration in rat magnocellular nucleus basalis. J Neuroendocrinol. 2000;12:486–494. doi: 10.1046/j.1365-2826.2000.00475.x. [DOI] [PubMed] [Google Scholar]
- Akirav I, Kozenicky M, Tal D, Sandi C, Venero C, Richter-Levin G. A facilitative role for corticosterone in the acquisition of a spatial task under moderate stress. Learn.Mem. 2004;11:188–195. doi: 10.1101/lm.61704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KJ. Impulsivity, Caffeine, and Task-Difficulty—A Within-Subjects Test of the Yerkes-Dodson Law. Personality and Individual Differences. 1994;16:813–829. [Google Scholar]
- Arnsten AF.The biology of being frazzled Science6–12–19982801711–1712. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Cohen J. Locus coeruleus and regulation of behavioral flexibility and attention. Prog.Brain Res. 2000;126:165–182. doi: 10.1016/S0079-6123(00)26013-5. [DOI] [PubMed] [Google Scholar]
- Bar-Joseph A, Berkovitch Y, Adamchik J, Biegon A. Neuroprotective activity of HU-211, a novel NMDA antagonist, in global ischemia in gerbils. Mol.Chem.Neuropathol. 1994;23:125–135. doi: 10.1007/BF02815406. [DOI] [PubMed] [Google Scholar]
- Barch DM, Braver TS, Nystrom LE, Forman SD, Noll DC, Cohen JD. Dissociating working memory from task difficulty in human prefrontal cortex. Neuropsychologia. 1997;35:1373–1380. doi: 10.1016/s0028-3932(97)00072-9. [DOI] [PubMed] [Google Scholar]
- Baumler G. On the Validity of the Yerkes-Dodson Law. Studia Psychologica. 1994;36:205–210. [Google Scholar]
- Birnbaum SG, Yuan PX, Wang M, Vijayraghavan S, Bloom AK, Davis DJ, Gobeske KT, Sweatt JD, Manji HK, Arnsten AF.Protein kinase C overactivity impairs prefrontal cortical regulation of working memory Science10–29–2004306882–884. [DOI] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Kelley WM, Buckner RL, Cohen NJ, Miezin FM, Snyder AZ, Ollinger JM, Akbudak E, Conturo TE, Petersen SE. Direct comparison of prefrontal cortex regions engaged by working and long-term memory tasks. Neuroimage. 2001;14:48–59. doi: 10.1006/nimg.2001.0791. [DOI] [PubMed] [Google Scholar]
- Bregman NJ, McAllister HA. Motivation and Skin Temperature Biofeedback—Yerkes-Dodson Revisited. Psychophysiology. 1982;19:282–285. doi: 10.1111/j.1469-8986.1982.tb02564.x. [DOI] [PubMed] [Google Scholar]
- Broadhurst PL. Emotionality and the Yerkes-Dodson Law. Journal of Experimental Psychology. 1957;54:345–352. doi: 10.1037/h0049114. [DOI] [PubMed] [Google Scholar]
- Broadhurst PL. A Confirmation of the Yerkes-Dodson Law and Its Relationship to Emotionality in the Rat. Acta Psychologica. 1959;15:603–604. [Google Scholar]
- Brown WP. The Yerkes-Dodson Law Repealed. Psychological Reports. 1965;17:663–666. doi: 10.2466/pr0.1965.17.2.663. [DOI] [PubMed] [Google Scholar]
- Burke A, Heuer F, Reisberg D. Remembering emotional events. Memory.& Cognition. 1992;20:277–290. doi: 10.3758/bf03199665. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Mattay VS, Bertolino A, Finn K, Coppola R, Frank JA, Goldberg TE, Weinberger DR. Physiological characteristics of capacity constraints in working memory as revealed by functional MRI. Cerebral Cortex. 1999;9:20–26. doi: 10.1093/cercor/9.1.20. [DOI] [PubMed] [Google Scholar]
- Christianson SA. Emotional stress and eyewitness memory: a critical review. Psychol.Bull. 1992;112:284–309. doi: 10.1037/0033-2909.112.2.284. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Lupien SJ, McEwen BS. Support for a bimodal role for Type II adrenal steroid receptors in spatial memory. Neurobiology of Learning and Memory. 1999;72:39–46. doi: 10.1006/nlme.1998.3898. [DOI] [PubMed] [Google Scholar]
- Conway MA, Anderson SJ, Larsen SF, Donnelly CM, McDaniel MA, McClelland AG, Rawles RE, Logie RH. The formation of flashbulb memories. Mem.Cognit. 1994;22:326–343. doi: 10.3758/bf03200860. [DOI] [PubMed] [Google Scholar]
- Cordero MI, Sandi C.A role for brain glucocorticoid receptors in contextual fear conditioning: dependence upon training intensity Brain Res3–9–199878611–17. [DOI] [PubMed] [Google Scholar]
- Deffenbacher KA. Eyewitness Recall, the Yerkes-Dodson Law, and Optimal-Level Theory. Bulletin of the British Psychological Society. 1982;35:A105. [PubMed] [Google Scholar]
- Diamond DM, Bennett MC, Fleshner M, Rose GM. Inverted-U relationship between the level of peripheral corticosterone and the magnitude of hippocampal primed burst potentiation. Hippocampus. 1992;2:421–430. doi: 10.1002/hipo.450020409. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Park CR, Puls MJ, Rose GM. 2001. Differential effects of stress on hippocampal and amygdaloid LTP: Insight into the neurobiology of traumatic memories. In: Holscher C, editor. Neuronal Mechanisms of Memory Formation. Cambridge University Press; New York: pp. 379–403. [Google Scholar]
- Dickman SJ. Dimensions of arousal: Wakefulness and vigor. Human Factors. 2002;44:429–442. doi: 10.1518/0018720024497673. [DOI] [PubMed] [Google Scholar]
- Dodson JD. Relative values of reward and punishment in habit formation. Psychobiology. 1917;1:231–276. [Google Scholar]
- Dutton A, Carroll M. Eyewitness testimony: Effects of source of arousal on memory, source-monitoring, and metamemory judgments. Australian Journal of Psychology. 2001;53:83–91. [Google Scholar]
- Eichenbaum H.The hippocampus and declarative memory: cognitive mechanisms and neural codes Behav.Brain Res12–14–2001127199–207. [DOI] [PubMed] [Google Scholar]
- Gold PE, Van Buskirk RB. Facilitation of time-dependent memory processes with posttrial epinephrine injections. Behav.Biol. 1975;13:145–153. doi: 10.1016/s0091-6773(75)91784-8. [DOI] [PubMed] [Google Scholar]
- Hanoch Y, Vitouch O. When less is more—Information, emotional arousal and the ecological reframing of the Yerkes-Dodson law. Theory & Psychology. 2004;14:427–452. [Google Scholar]
- Introini-Collison IB, Castellano C, McGaugh JL. Interaction of GABAergic and beta-noradrenergic drugs in the regulation of memory storage. Behav.Neural Biol. 1994;61:150–155. doi: 10.1016/s0163-1047(05)80068-8. [DOI] [PubMed] [Google Scholar]
- Kaltschmidt B, Uherek M, Wellmann H, Volk B, Kaltschmidt C.Inhibition of NF-kappaB potentiates amyloid beta-mediated neuronal apoptosis Proc.Natl.Acad.Sci.U.S A8–3–1999969409–9414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensinger EA, Clarke RJ, Corkin S.What neural correlates underlie successful encoding and retrieval? A functional magnetic resonance imaging study using a divided attention paradigm J Neurosci3–15–2003232407–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensinger EA, Corkin S.Two routes to emotional memory: Distinct neural processes for valence and arousal Proceedings of the National Academy of Sciences of the United States of America3–2–20041013310–3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr DS, Huggett AM, Abraham WC. Modulation of Hippocampal Long-Term Potentiation and Long-Term Depression by Corticosteroid Receptor Activation. Psychobiology. 1994;22:123–133. [Google Scholar]
- Leon MR, Revelle W. Effects of anxiety on analogical reasoning: a test of three theoretical models. J Pers.Soc.Psychol. 1985;49:1302–1315. doi: 10.1037//0022-3514.49.5.1302. [DOI] [PubMed] [Google Scholar]
- Loftus EF. Memory, surprising insights into how we remember and why we forget. 1980. Reading, Massachusetts: Addison-Wesley; [Google Scholar]
- Mendl M. Performing under pressure: stress and cognitive function. Applied Animal Behaviour Science. 1999;65:221–244. [Google Scholar]
- Metcalfe J, Jacobs WJ. Emotional memory: The effects of stress on “cool” and “hot” memory systems. Psychology of Learning and Motivation. 1998;38:187–222. [Google Scholar]
- Okuda S, Roozendaal B, McGaugh JL. Glucocorticoid effects on object recognition memory require training-associated emotional arousal. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:853–858. doi: 10.1073/pnas.0307803100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickel KL. Unusualness and threat as possible causes of “weapon focus”. Memory. 1998;6:277–295. doi: 10.1080/741942361. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Packard MG. Competition among multiple memory systems: converging evidence from animal and human brain studies. Neuropsychologia. 2003;41:245–251. doi: 10.1016/s0028-3932(02)00157-4. [DOI] [PubMed] [Google Scholar]
- Rice PL. Stress and Health. Pacific Grove, California: Brooks/Cole; 1999. [Google Scholar]
- Roozendaal B. 1999 Curt P. Richter award. Glucocorticoids and the regulation of memory consolidation. Psychoneuroendocrinology. 2000;25:213–238. doi: 10.1016/s0306-4530(99)00058-x. [DOI] [PubMed] [Google Scholar]
- Safer MA, Christianson SA, Autry MW, Osterlund K. Tunnel memory for traumatic events. Applied Cognitive Psychology. 1998;12:99–117. [Google Scholar]
- Stefani MR, Nicholson GM, Gold PE. ATP-sensitive potassium channel blockade enhances spontaneous alternation performance in the rat: a potential mechanism for glucose-mediated memory enhancement. Neuroscience. 1999;93:557–563. doi: 10.1016/s0306-4522(99)00128-1. [DOI] [PubMed] [Google Scholar]
- Teigen KH. Yerkes-Dodson—A Law for All Seasons. Theory & Psychology. 1994;4:525–547. [Google Scholar]
- Telegdy GA, Cohen JS.Cue Utilization and Drive Level in Albino Rat Journal of Comparative and Physiological Psychology 197175248& [Google Scholar]
- Watters PA, Martin F, Schreter Z. Caffeine and cognitive performance: The nonlinear Yerkes-Dodson Law. Human Psychopharmacology-Clinical and Experimental. 1997;12:249–257. [Google Scholar]
- Woodson JC, Macintosh D, Fleshner M, Diamond DM. Emotion-induced amnesia in rats: working memory-specific impairment, corticosterone-memory correlation, and fear versus arousal effects on memory. Learn.Mem. 2003;10:326–336. doi: 10.1101/lm.62903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerkes RM, Dodson JD. The relation of strength of stimulus to rapidity of habit-formation. J.Comp.Neurol.Psychol. 1908;18:459–482. [Google Scholar]
- Young PT. Motivation of Behavior. 1936. London: Wiley; [Google Scholar]