Abstract
E-cadherin based adherens junctions are finely regulated by multiple cellular signaling events. Here we show that the Ras-related Rap1 GTPase is enriched in regions of nascent cell-cell contacts and strengthens E-cadherin junctions: constitutively active Rap1 expressing MDCK cells exhibit increased junctional contact and resisted calcium depletion-induced cell-cell junction disruption. E-cadherin disengagement activated Rap1 and this correlated with E-cadherin association with the Rap GEFs, C3G and PDZ-GEF I. PDZ-GEF I associated with E-cadherin and β-catenin whereas C3G interaction with E-cadherin did not involve β-catenin. Knockdown of PDZ-GEF I in MDCK cells decreased Rap1 activity following E-cadherin junction disruption. We hereby show that Rap1 plays a role in the maintenance and repair of E-cadherin junctions and is activated via an “outside-in” signaling pathway initiated by E-cadherin and mediated at least in part by PDZ-GEF I.
Keywords: Rap1, PDZ-GEF, Guanine nucleotide exchange factor, adherens junction, E-cadherin, C3G
Epithelial cells establish structurally and functionally distinct cell-cell adhesions with their neighbors. Three types of cell junctions, tight junctions (TJs), adherens junctions (AJs) and desmosomes contribute to cell-cell adhesion and specialized proteins mediate each of these interactions. Adherens junctions are the first cell-cell contacts to form and their establishment is necessary for the subsequent formation of tight junctions and desmosomes [Adams et al., 1996; Gumbiner et al., 1988; Pokutta and Weis, 2002]. Transmembrane E-cadherin is the major component of epithelial cell adherens junctions. The extracellular domains of E-cadherin form calcium dependent homophilic interactions with cadherin molecules on an adjacent cell [Pokutta et al., 1994] while its cytoplasmic tail acts as a docking site for β-catenin [Chen et al., 1999] [Pokutta and Weis, 2002], α-catenin and the actin cytoskeleton [Adams et al., 1996; Vasioukhin et al., 2000].
In addition to serving a structural role, adherens junctions also regulate the growth, proliferation and migration of epithelial cells [Perez-Moreno et al., 2003]. They are essential during both normal growth and developmental processes as well as in pathological situations such as carcinoma growth and metastasis. The formation and maintenance of epithelial cell junctions are dynamic processes and undergo active regulation by both the extracellular milieu and intracellular signals. The Rho family GTPases, Rac and Cdc42 play a critical role in the regulation of E-cadherin based AJs [Fukata and Kaibuchi, 2001; Kaibuchi et al., 1999]. E-cadherin in turn activates Rac and Cdc42 during the initiation of cell-cell contacts to drive the process of junction formation and maintenance of the mature contact [Arthur et al., 2002; Braga, 2000; Braga et al., 1999; Braga et al., 1997; Nakagawa et al., 2001; Noren et al., 2001]. Here, we have analyzed junctional regulation by another small GTPase, Rap1.
Rap1 is a member of the Ras-family of GTP binding proteins [Pizon et al., 1988] that cycles between active GTP- and inactive GDP-bound states. The hydrolysis of bound GTP is facilitated by GTPase activating proteins or GAPs. The release of the bound GDP to permit loading with GTP is mediated by guanine nucleotide exchange factors or GEFs. Multiple Rap1 GEFs exist that enable it to be controlled by a variety of extracellular stimuli [Quilliam et al., 2002; Stork, 2003]. Rap1 was originally discovered as a Ras homolog and as a suppressor of Ras induced signaling [Pizon et al., 1988] [Kitayama et al., 1989]. Subsequent studies demonstrated it to have varied signaling functions in the cell [Stork, 2003] [Bos et al., 1997] [Caron, 2003]. A major breakthrough in understanding Rap1 function came with the discovery that it regulates integrin-mediated adhesion of cells to the extra-cellular matrix, reviewed in [Bos, 2005; Caron, 2003; Hattori and Minato, 2003].
A potential role for Rap1 in cell junction regulation was also realized when Boettner et al reported that it localized with the tight junction protein AF-6 in MCF-7 breast epithelial cells [Boettner et al., 2000]. Additional studies in Drosophila demonstrated that loss of Rap1 results in the failure of DE-cadherin, β-, and α-catenin to redistribute to nascent junctions following cytokinesis. Furthermore, in wild type cells Rap1 was found to concentrate in the junction between two sister cells, showing that Rap might be involved in the re-distribution of cadherins to the newly formed adherens junctions of daughter cells [Knox and Brown, 2002].
In this study we show that constitutively activated Rap1 promotes E-cadherin based junctions in MDCK epithelial cells and resists disruption of existing junctions. Disruption of AJs promotes Rap1 activation and this process is mediated via β-catenin and PDZ-GEF I/RapGEF2.
MATERIALS AND METHODS
Cell culture and Transfection
MDCK cells provided by Dr. Simon Atkinson (Indiana University School of Medicine) and HEK293T cells were cultured in 5% CO2, 37°C in DMEM (BioWhittaker, Walkersville, NJ) supplemented with 10% FBS (Hyclone, Logan, UT or Atlanta Biologicals, Lawrenceville, GA). FRT cells were provided by Dr. Rodriguez-Boulan (Cornell University, New York) and grown in Ham's F12/Coon's medium (Sigma, St Louis, MO) supplemented with 5% FBS (Atlanta Biologicals). HUVECs provided by Dr. David Ingram (Indiana University School of Medicine) were grown in complete EGM-2 media (BioWhittaker). HEK293T cells were transfected with Fugene 6 (Roche, Indianapolis, IN) or calcium phosphate. MDCK cells were transfected with plasmids using Cytofectene (Biorad, Hercules, CA) or Lipofectamine 2000 (Invitrogen, Carlsbad, CA) when transfecting with siRNA. siRNA were added at the time of seeding the cells into culture dishes. Stable MDCK cell transfectants expressing GFP-Rap1 were established by selection of cells on 150 μg/ml G418 (Invitrogen) while cells stably expressing HA-tagged Rap1 were selected and maintained in 100 μg/ml Hygromycin B (Roche). Individual transfectants were ring cloned and the expression of the proteins confirmed by immunoblotting.
Antibodies and Reagents
Antibodies were purchased as HA monoclonal antibody – Covance (Denver, PA), FLAG M2 antibody and monoclonal anti-uvomorulin clone DECMA-1 - Sigma, β catenin and E-cadherin cytoplasmic tail antibody - BD Biosciences (San Jose, CA), Rap1 antibody – ViroMed (Minneapolis, MN), pcDNA3-E-Cadherin and E-cadherin extracellular domain antibody (rr-1) were kind gifts from Drs. Jim Marrs and Shobha Gopalakrishnan (Indiana University School of Medicine). Rap1A, C3G and RalGDS-RBD encoding plasmids have been described previously Rebhun et al 2000. PDZ-GEF I antibody was a generous gift from Drs. Naoki Mochizuki and Atsuko Sakurai (National Cardiovascular Center Research Institute, Osaka, Japan). Recombinant Human Hepatocyte Growth Factor (294-HG) was purchased from R&D systems (Minneapolis, MN), Rat tail collagen I was from BD Biosciences and PP2 from CalBiochem (La Jolla, CA) and bafilomycin A1 from Sigma.. siRNA for canine PDZ-GEF I were designed and synthesized by Applied Biosystems, Austin, TX. Three siRNA sequences were synthesized for the 5′ region of the PDZ-GEF I sequence (NCBI ID gi: 73978027 or XM 848711) of which 2 were effective. PDZ-GEF I siRNA #2 sense 5′ – GCAGCAGUCUUUCUGAUAUtt -3′, antisense 5′– AUAUCAGAAAGACUGCUGCtt -3′; siRNA #3 sense 5′ – GGAAGAAGGAGAAAUUGUUtt -3′, antisense 5′– AACAAUUUCUCCUUCUUCCtc -3′. Control non-targeting siRNA #2 was purchased from Dharmacon/ThermoFisher, Chicago, IL).
Calcium Switch
MDCK cells were serum starved overnight in DMEM and 4 mM EGTA was added to the media for 30 min to chelate calcium in the medium, leading to the disruption of E-cadherin homophilic interactions. 4 mM CaCl2 was re-added at specified time points following the 30 min of EGTA to induce re-formation of E-cadherin junctions. Alternatively, the cells were serum starved overnight in DMEM containing 100 μM calcium and switched to DMEM containing 1.8 mM calcium at the specified points of time (low calcium switch).
Rap1 activity assay
Cells were washed twice in ice cold phosphate buffered saline (PBS), and lysed by incubating the cells at 4°C for 5 min in 1 ml of lysis buffer containing 50 mM Tris HCl pH 7.4, 10% glycerol, 200 mM NaCl, 2.5 mM MgCl2, 1% IGEPAL (Sigma) and protease inhibitors – 1 mM p-methylsulfonylfluoride (PMSF) and 0.05 trypsin inhibitor units/ml of Aprotinin. The cells were harvested and centrifuged at 14,000 rpm for 5 min. 850 μl of the cleared lysate were then rocked with 30 μg of GST-RalGDS-RBD-bound beads for 45 min at 4°C to pull down GTP bound Rap1. The beads were then washed 3 times with the above described buffer. Rap1 was detected by running the samples on SDS page gels and western blotting with Rap1 specific antibody [Castro et al., 2005].
Immunofluorescence
MDCK cells were seeded on 12 mm round cover slips (Carolina Biologicals, Burlington, NC). The cells were fixed using 4% formaldehyde for 10 min at room temperature or overnight at 4°C and permeabilized using 0.2% Triton X-100, 5 min. The cells were blocked using 0.2% BSA, 0.5 mM MgCl2, 0.45 mM CaCl2, 10% goat serum, 50 mM NH4Cl, 25 mM glycine, and 25 mM lysine for 1 hr, washed twice in PBS containing 0.2% BSA and 0.5 mM MgCl2, 0.45 mM CaCl2 and incubated with specified antibodies for 1 hr, followed by two washes and 1 hr incubation with Alexafluor 594-conjugated goat anti-mouse IgG (Invitrogen). After two final washes, cover slips were mounted onto slides using mounting medium containing DAPI (Vector labs, Burlingame, CA). Confocal images were taken using a Zeiss LSM-510 confocal microscope equipped with Laser lines 364, 488, and 543 nm and PMT detectors.
Live cell imaging
MDCK cells expressing GFP tagged proteins were cultured on glass bottom plates (MatTEK, Ashland, MA) and imaged live using a LSM 510 laser scanning confocal microscope. During imaging cells were maintained in growth media containing 50 mM HEPES to maintain physiological pH and kept at 37°C using a heated stage.
Adherens junction formation in cells after trypsinization and re-plating
MDCK cells stably expressing GFP-Rap1A were plated at 70-80% confluency. The next day they were trypsinized for 2 min at 37°C and triturated aggressively to disrupt any cell-cell contacts in growth media containing 20% serum to neutralize the trypsin. The cells were then centrifuged at 800 rpm for 5 min and re-suspended in 10 ml of growth media containing 20% serum and 50 mM HEPES. 1-2 ml of the cell suspension was plated on 35 mm glass bottom plates pre-coated with collagen (1-2 mg/ml) and allowed to re-form junctions.
Co-immuno precipitation
HEK293T cells were transfected with the appropriate expression vectors. After 48 hr the cells were washed twice with ice-cold PBS and lysed using CSK buffer containing 50 mM NaCl, 300 mM sucrose, 10 mM PIPES pH 6.8, 3 mM MgCl2, 0.5% Triton X-100 and protease inhibitors – 1 mM PMSF and 0.05 trypsin inhibitor units per ml of Aprotinin. Cells were harvested by scraping and the lysate cleared by centrifuging at 14,000 rpm for 10 min at 4°C. Supernatant was pre-cleared using 10 μl of protein A/G agarose beads (Santa Cruz Biotech, Santa Cruz CA) and tumbled with 1 μl of the specified antibody for 1 hr at 4°C. 30 μl of protein A/G agarose beads were then added and tumbled for an additional 1.5 hr. Beads were washed 6 times with cold CSK buffer and proteins were eluted by boiling with SDS loading buffer. Co-Immunoprecipitations in MDCK cells were performed by plating 450,000 cells on 100 mm dishes and after 48 hr lysed and assayed as described above for HEK293T cells. PDZ-GEF I antibody was used at 1:100 dilution for co-immunoprecipitation from MDCK cells.
Statistical analysis
Pooled data from at least 3 independent experiments were normalized to total protein and significance (P<0.05) determined by Student's t-test.
RESULTS
Rap1 is localized to cell-cell junctions in epithelial cells
Rap1 has been shown to localize to the peri-nuclear region of mesenchymal cells [Beranger et al., 1991; Sato et al., 1994]. However, when we examined the localization of epitope-tagged Rap1 in the Madin Darby canine kidney (MDCK) epithelial cell line, it was found to be highly concentrated at cell-cell junctions (unpublished results). To address its role in this novel sub-cellular locale we stably expressed HA-tagged wild type Rap1A as well as constitutively active (63E) and dominant negative (17N) mutants in MDCK cells. Rap1A was localized to cell-cell contacts independent of its activation status although Rap1A-17N showed more intracellular distribution than the other Rap1 proteins (Fig 1, A-C). Interestingly, when the Rap1A-63E mutant was stably expressed in MDCK cells they underwent a dramatic change in morphology; the colonies became highly compacted, suggesting an increase in cell-cell contact (Fig 1C). In contrast, the Rap1A-17N expressing cells had a slightly flattened phenotype. The compacted phenotype of Rap1A-63E cells was only observed following the trypsinization and replating of drug-resistant colonies, suggesting that an initial disruption of junctions was required to trigger it.
Fig. 1. Rap1 localizes to regions of cell–cell contact in epithelial cells.
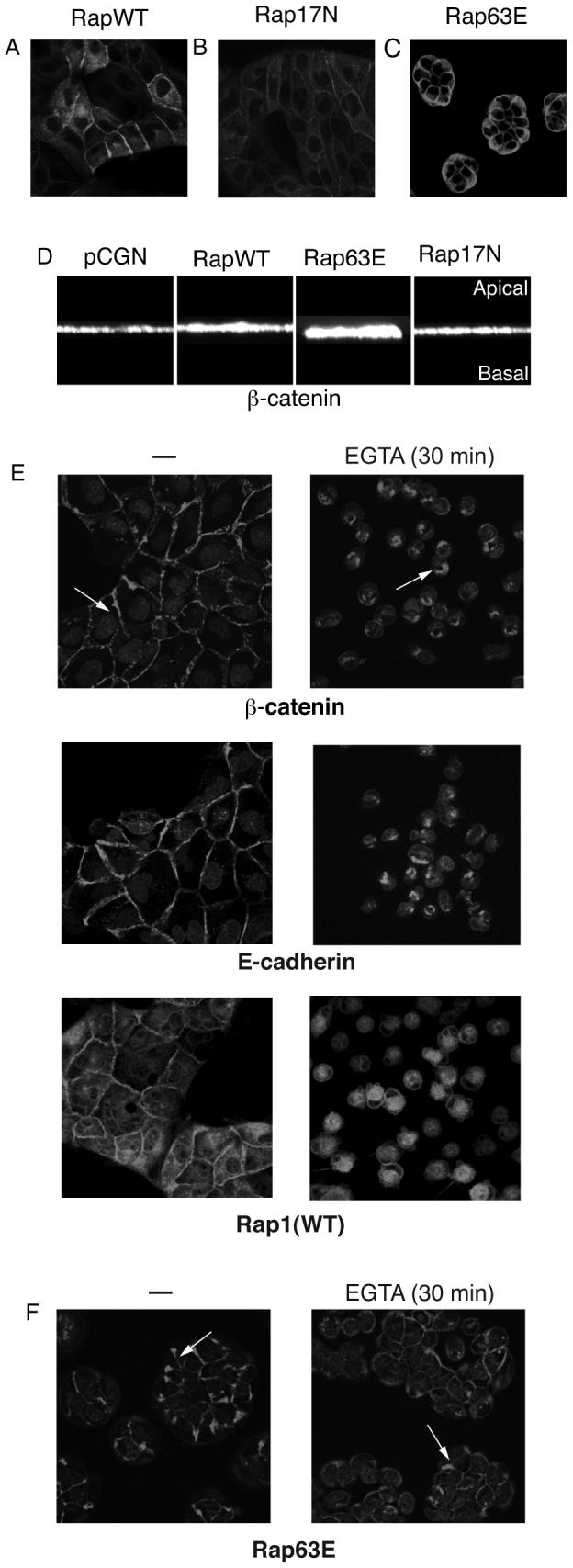
MDCK cells stably expressing HA-tagged wild type Rap1A, Rap1A-WT (A), dominant negative Rap1A-17N (B) or constitutively active Rap1A-63E (C) were stained using HA antibody and imaged using confocal microscopy. D: The height of adherens junctions was determined by detection of β-catenin. The images were analyzed using the VOX program (VayTek Inc., Fairfield, IA) to obtain a 90° rotation and vertical perception of β-catenin localization in three-dimensional space. E: Rap1A-WT expressing MDCK cells were subject to calcium switch and β-catenin, E-cadherin and HA-tagged Rap1A detected by immunofluorescence microscopy. Following extracellular calcium chelation, adherens junctions were disrupted and proteins endocytosed. Whereas the Rap1A-WT cells underwent complete dissolution of adherens junctions and endocytosis of β-catenin (arrow) Rap1A-63E cells retained most cell–cell contacts and β-catenin on their membrane (F). Note also the increased β-catenin staining at cell–cell contacts in Rap1A-63E expressing colonies before treatment with EGTA. Data representative of three independent experiments.
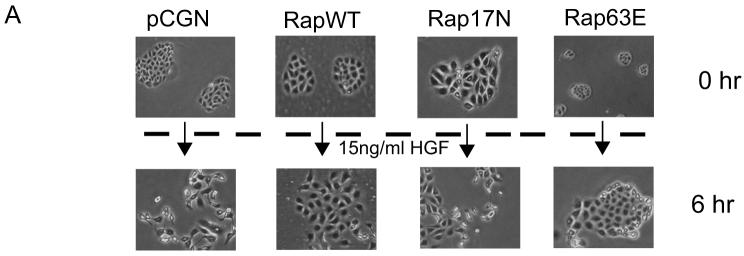
The constitutively active Rap1A-63E mutant appeared to increase the length of junctional contacts (cells were taller based on confocal microscopy; Fig 1D). Therefore, to further evaluate if Rap1 activation indeed strengthened cell-cell adhesions we used the “calcium switch” method as described in “Materials and Methods”. Loss of extracellular calcium causes a change in the conformation of E-cadherin's extracellular domains leading to the loss of cadherin-cadherin binding and eventually the disruption of adherens junctions ([Pokutta et al., 1994]. MDCK cells expressing Rap1A-WT underwent cell-cell junction disruption upon calcium chelation with EGTA, resulting in the internalization of β-catenin, E-cadherin and Rap1A (Fig 1E). In contrast, Rap1A-63E expressing cells maintained about 60% of their junctions and remained together in colonies (Fig 1F). Scatter factor/hepatocyte growth factor (HGF) also disrupts E-cadherin based adherens junctions in epithelial cells and induces scattering of the cells on tissue culture dishes [Konishi et al., 1991]. In accordance with the assumption that Rap63E strengthened cell-cell junctions, MDCK cells that stably expressed Rap1A-63E became flatter following exposure to HGF, resembling untreated control cells, but showed no disruption of cadherin based junctions even after 12 hours of HGF treatment. Meanwhile, Rap1A-WT expressing cells underwent cell-cell junction disruption and scattering similar to cells transfected with just empty vector or Rap1A-17N (Fig 2). These data indicated that Rap1 might play an essential role in either the formation or maintenance of cell-cell junctions in epithelial cells.
Fig. 2. Constitutively active Rap1 strengthens E-cadherin junctions.
A: MDCK cells stably transfected with empty pCGN vector, or vector encoding wild type Rap1A (RapWT), constitutively active Rap1A mutant (Rap63E) or dominant negative Rap1 mutant (Rap17N) were treated with HGF for 6 h. Cells transfected with pCGN, pCGN Rap1A-WT or Rap1A-17N underwent dissolution of cell–cell junctions followed by cell scattering whereas Rap1A-63E expressing cells did not undergo junction disruption. All images were obtained at the same magnification. Representative of at least three independent experiments.
Rap1 is localized to regions of cell-cell contact during formation of nascent cell junctions
Epithelial cells initiate cell-cell junctions through localized regions of cell contact that are then zippered to form a continuous band of adherens junction [Adams et al., 1998; Jamora and Fuchs, 2002]. To evaluate the role of Rap1 in the formation of initial cell-cell contacts and nascent cell junctions we generated MDCK cells expressing EGFP-tagged Rap1A and monitored Rap1A localization following trypsinization and replating of cells at high density. As shown in Fig 3, Rap1A was localized mostly to the interior of the cells prior to their contacting each other, with additional Rap1A being visible at the plasma membrane. However, as soon as the cells encountered each other, the intracellular Rap1A pool began to polarize (see upward pointing arrows) and re-locate to the region of new cell-cell contact. This suggested that Rap1A might be involved in the early steps of cell junction formation.
Fig. 3. Rap1 localizes to nascent cell–cell contacts during cell junction formation.
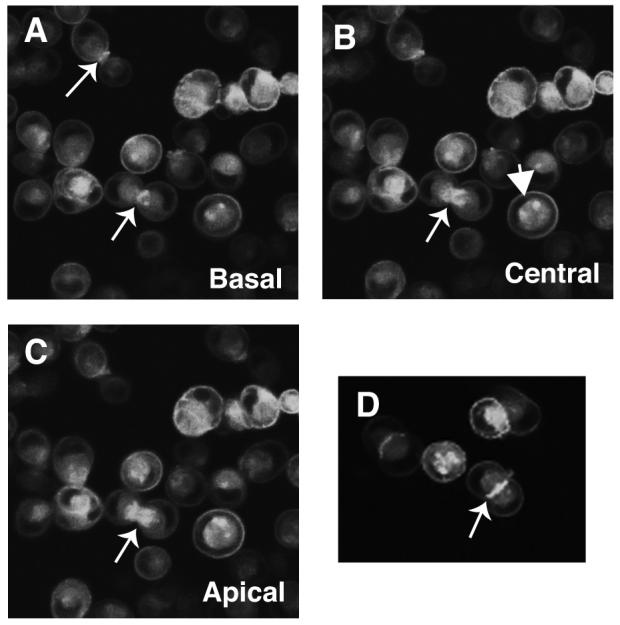
MDCK cells stably expressing GFP-Rap1A-WT were trypsinized, vigorously pipetted to disrupt cell–cell junctions, and re-plated. Localization of GFP-Rap1A in these cells was then followed using live cell confocal microscopy. A–C: Images collected on the same microscopic field of the plate from near the basal (A) to near the apical (C) surface of the cells. GFP-Rap1A was mainly intracellular with a ring of Rap1 on the plasma membrane (down facing arrows) in single cells whereas GFP-Rap1A is highly enriched when cells make contact and begin to establish cell junctions (upward facing arrow). D: Field showing two cells establishing a nascent cell–cell contact and GFP-Rap1A enriched at the cell junction. Data representative of three or more independent experiments.
Rap1 is activated during disruption of E-cadherin based junctions in MDCK cells
Localization of Rap1A at regions of cell-cell contact and its enrichment in nascent junctions intrigued us to further understand how Rap1 signaling is affected by E-cadherin engagement. We first monitored Rap1 activity during the disruption and re-formation of E-cadherin junctions in MDCK cells. Two different “calcium switch” protocols were used to disrupt and re-form the adherens junctions and Rap1-GTP measured at various time points by RalGDS-RBD pull down. When EGTA was used to chelate the extracellular calcium, Rap1 was activated within 2 min of calcium depletion and this activation was sustained for at least 30 min (Fig 4A). Re-addition of calcium by providing fresh medium containing 1.8 mM CaCl2 resulted in the rapid inactivation of Rap1. The second approach was to maintain cells overnight in medium containing 100 μM calcium and switch to regular media containing 1.8 mM CaCl2. Reducing the calcium concentration resulted in sustained elevation of Rap-GTP levels overnight (Fig 4B, lane 2) and this decreased towards baseline within 15 min of re-addition of extracellular calcium (Fig 4B, lanes 3-5).
Fig. 4. Rap1 is activated during disruption of E-cadherin based adherens junctions.
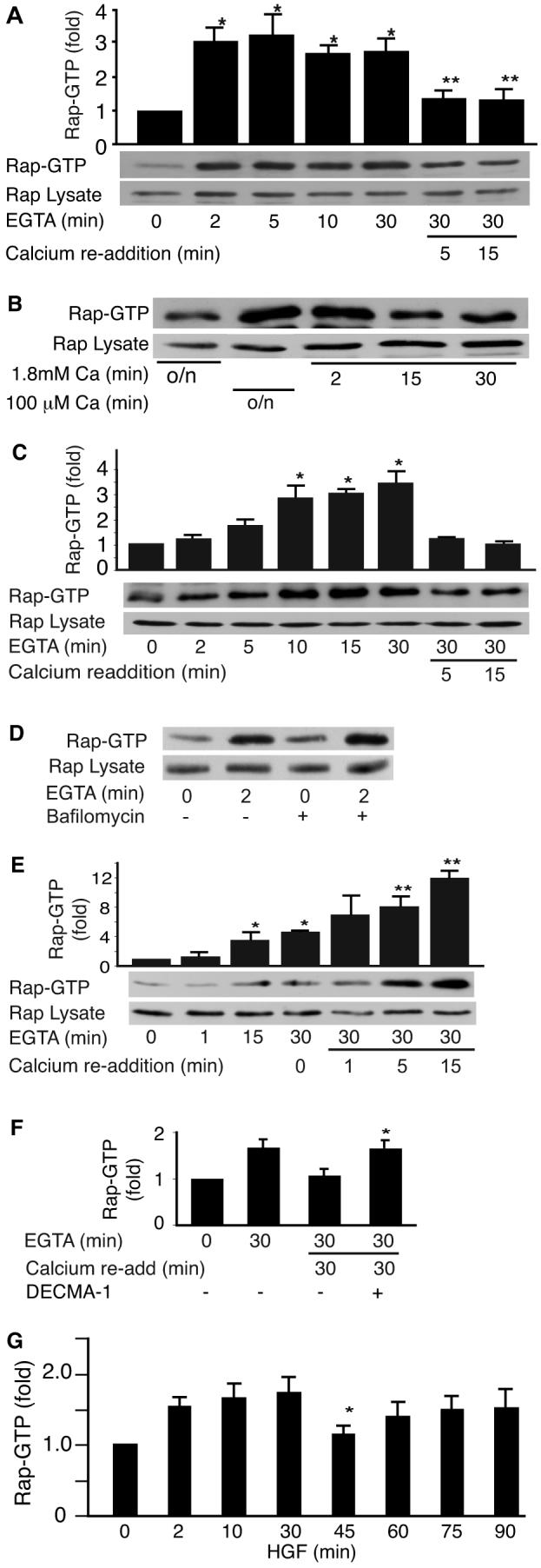
A: Calcium switch induced activation of Rap1. MDCK cell junctions were disrupted by addition of 4 mM EGTA to the medium to chelate calcium and reformed by adding back 4 mM CaCl2 (calcium switch). Data representative of at least 10 independent experiments. A representative blot and pooled data from 5 to 9 experiments is shown, mean +/− SEM, P<0.01 versus t=0 (*) or t=30 (**) min. B: Rap1 activation during low calcium switch. Rap1 was activated following overnight incubation of MDCK cells in low calcium (100 μM) medium (lane 2). This activation was reversed when cells were returned to regular calcium medium for 2, 15, or 30 min (lanes 3–5). C: Rap1 was activated by Ca2+ removal in Fisher rat thyroid (FRT) epithelial cells. P<0.05 versus t=0, n=3. D: 30 min pretreatment with 100 nM bafilomycin did not prevent calcium chelation from rapidly activating Rap1. Representative of three separate experiments. E: Rap1 activation following calcium switch in HUVECs. A minimal activation of Rap1 during the disruption of adherens junctions was seen in endothelial cells (lanes 3 and 4 P<0.05) with a stronger activation of Rap1 during re-addition of 1.8 mM CaCl2 (lanes 6–8, P<0.05 vs. t=30 min). F: Incubation of cells with E-cadherin antibody, DECMA-1 to prevent homotypic binding prevented Rap1 deactivation. P<0.05 versus goat IgG control, n=3. G: HGF induced activation of Rap1. MDCK cells were treated with HGF (15 ng/ml) for the specified times and Rap1GTP levels measured. Graph shows biphasic increase in RapGTP following treatment with HGF (mean +/− SEM) from four independent experiments. P<0.025 versus t=30 min.
The pattern of activation of Rap1 was similar in the MCF-7 and MCF10A epithelial cell lines (unpublished results). In FRT cells there was a 10-15 min delay in Rap activation (Fig 3C). This is likely due to the failure of Rap1 to localize to junctions in this cell line [Balzac et al., 2005]. Consistent with this notion, bafilomycin did not prevent the rapid activation of Rap1 in MDCK cells upon Ca2+ chelation (Fig 4D). Interestingly, human umbilical vein endothelial cells (HUVECS) showed the typical increase in Rap1-GTP accumulation during EGTA treatment but a further increase in Rap1-GTP was abserved upon the re-addition of calcium (Fig 4E). This was in contrast to the decrease seen in all four epithelial cell lines but consistent with our previous studies [Wittchen et al., 2005]. Incubation of MDCK cells with an E-cadherin-neutralizing antibody, DECMA-1, inhibited Rap1-GTP levels from returning to baseline upon readdition of CaCl2. This supported the notion that E-cadherin disengagement is responsible for the activation of Rap1 (Fig 4F).
In order to rule out any discrepancies that could be caused by changes in extracellular calcium on the intracellular milieu, we also examined the effect of HGF induced cadherin dis-engagement on Rap1 activation. As with calcium depletion, disruption of cell junctions by HGF correlated with an increase in Rap1-GTP levels. Upon exposure of MDCK cells to HGF (15 ng/ml) Rap1 was activated within 2 min and Rap1-GTP levels were sustained for at least 4 hours.
Interestingly, Rap1 activation by HGF was biphasic with a brief, highly reproducible decrease occurring approximately 45-60 min post stimulation (Fig 4G). Phasic activation of Rap1 suggested that Rap1 might be first responding to the disruption of junctions and later perhaps separately contributing to the integrin-dependent migratory process that is induced by HGF [Lamorte et al., 2002; Sakkab et al., 2000].
PDZ-GEF I and C3G are candidate GEFs for activation of Rap1 during disassembly of cell-cell junctions
PDZ-GEF I is a Rap GEF that additionally contains PDZ and RA (Ras association) domains plus a C-terminal PDZ domain-binding motif [de Rooij et al., 1999; Liao et al., 1999; Rebhun et al., 2000]. PDZ-GEF I has been previously shown to form a complex with β-catenin [Kawajiri et al., 2000] and the scaffold protein, MAGI-1. This association was significantly enhanced by chronic calcium chelation to disrupt cell-cell junctions [Mino et al., 2000; Ohtsuka et al., 1999]. To address whether PDZ-GEF I might regulate Rap1 at cell junctions, we first examined whether the GEF associates with E-cadherin. As shown in Fig 5A, following co-transfection of 293T cells with plasmids encoding human E-cadherin and Flag-tagged PDZ-GEF I, E-cadherin was co-precipitated by the M2 anti-Flag antibody. We also examined the association of another GEF, C3G, that was recently reported to interact with the C-terminal tail of E-cadherin [Hogan et al., 2004]. Following co-expression of the human E-cadherin and Flag-tagged C3G in 293T cells we also observed co-precipitation of these two proteins (Fig 5A). Coexpression of E-cadherin with each GEF resulted in increased protein levels, suggesting that they may stabilize each other.
Fig. 5. E-cadherin co-precipitates with PDZ-GEF I and C3G.
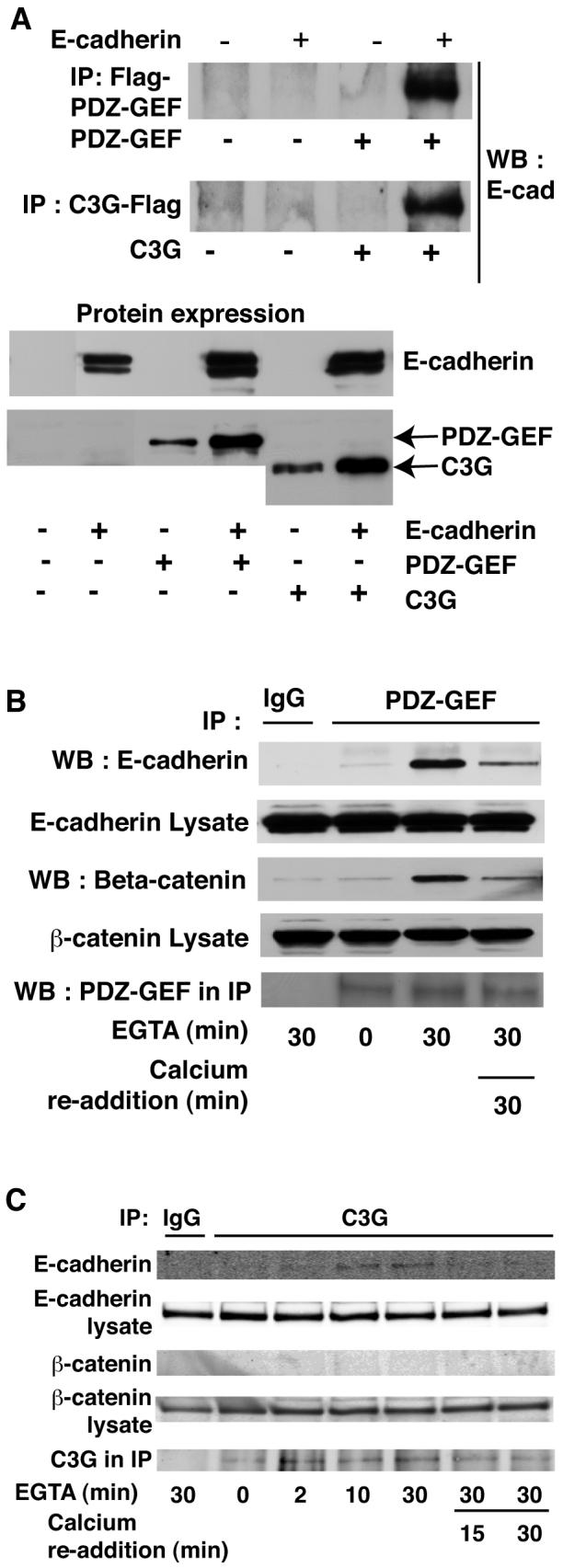
A: E-cadherin was exogenously expressed in 293T cells along with either Flag tagged PDZ-GEF I or C3G. Flag antibody was used to precipitate GEFs prior to blotting for E-cadherin. E-cadherin co-precipitated with both PDZ-GEF I (top panel) and C3G (middle panel). Expression of proteins is shown below. B: Endogenous E-cadherin and β-catenin associate with PDZ-GEF I in MDCK cells. MDCK cells were subject to calcium switch and PDZ-GEF antibody used to immunoprecipitate the endogenous GEF. Association of endogenous E-cadherin with PDZ-GEF was greatly enhanced upon disruption of junctions with EGTA for 30 min but returned to near basal levels following Ca2+ re-addition. β-Catenin also coprecipitated with the E-cadherin and PDZ-GEF I complex (lower panels). C: E-cadherin but not β-catenin co-immunoprecipitated with C3G in MDCK cells. MDCK cells were calcium switched as above and C3G antibody used for immunoprecipitation. Data representative of at least three independent experiments.
If these interactions are relevant to Rap activation in epithelial cells, they should occur between endogenous proteins and be modulated by the status of cell junctions. To address these possibilities, we performed a calcium switch experiment in MDCK cells, precipitated the endogenous GEFs using specific antibodies, and blotted for E-cadherin association. PDZ-GEF I and E-cadherin showed minimal interaction under basal conditions (1.8 mM calcium), but when junctions were disrupted E-cadherin showed dramatically increased association with PDZ-GEF I that returned towards baseline upon re-addition of calcium (Fig 5B, upper panels). Similarly, C3G showed minimal association with E-cadherin under basal conditions but efficiently co-precipitated the cell junction protein upon calcium withdrawal (Fig 5C).
PDZ-GEF I also co-precipitated β-catenin from MDCK cells upon calcium switching (Fig 5B, lower panels) whereas C3G/E-cadherin complexes did not include β-catenin (Fig 5C). This is consistent with the notion that the interaction of PDZ-GEF I with E-cadherin might occur via a β-catenin/MAGI-1 complex [Kawajiri et al., 2000]. Attempts to detect MAGI-1 in the PDZ-GEF I/β-catenin/E-cadherin complexes were unproductive due to low expression of MAGI-1 in MDCK cells and unavailability of high affinity MAGI-1 antibodies to detect the scaffold protein in these cells.
Knock down of PDZ-GEF I in MDCK cells results in the loss of Rap1 activity
Having demonstrated an interaction between PDZ-GEF I, β-catenin and E-cadherin in MDCK cells, we wished to determine if PDZ-GEF I is responsible for mediating Rap1 activation during E-cadherin junction disruption. Therefore, we used siRNA to knock down PDZ-GEF I protein in MDCK cells. Two siRNAs designed against canine PDZGEF I knocked down its protein expression in MDCK cells by 50-60% 48 hr post transfection compared to a non-targeting siRNA (Fig 6A). 48 hr after transfecting with control or a PDZ-GEF I siRNA, MDCK cells were calcium-depleted and Rap1 activity determined using the RalGDS-RBD pull down assay as before. The PDZ-GEF I siRNA-transfected cells showed attenuated Rap1GTP levels (approximately 50%) compared to the control siRNA transfected cells (Fig 6B, C) indicating that PDZ-GEF I is involved in the E-cadherin-induced Rap1 activation following the disruption of cell-cell adhesions.
Fig. 6. Knockdown of MDCK cell PDZ-GEF I reduces the ability of cell junction disruption to activate Rap1.
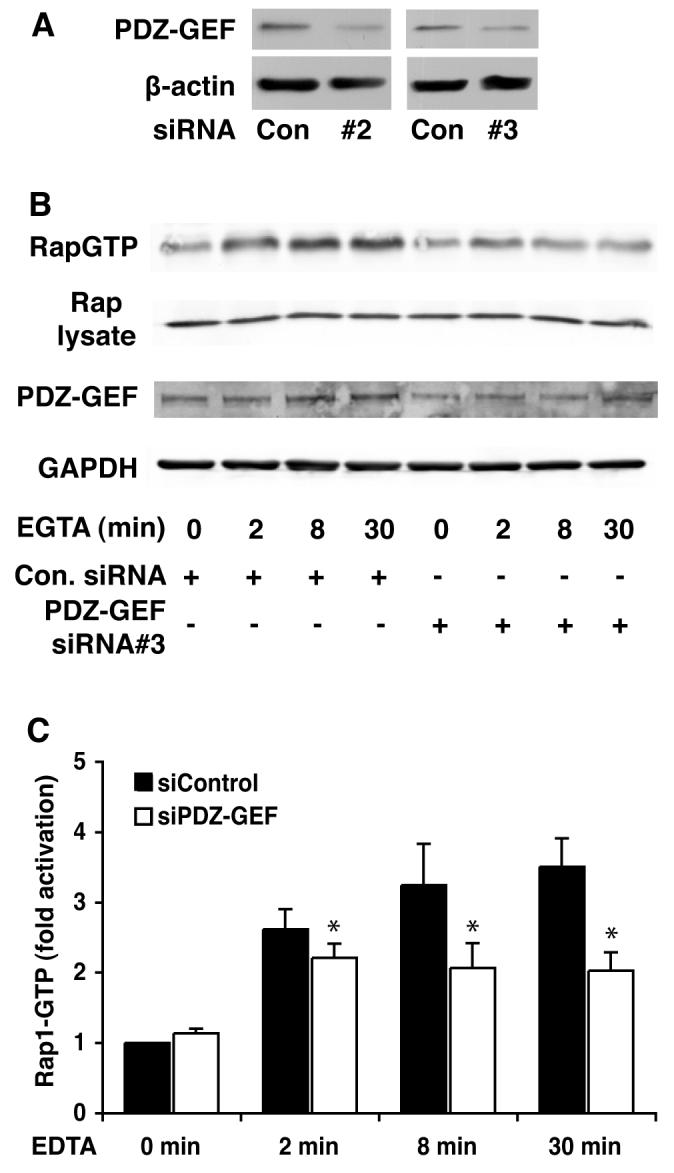
A: Transfection of two different PDZGEF siRNAs resulted in decreased expression of the GEF 48 hr later. B: MDCK cells were transfected with control or two different PDZ-GEF I siRNAs (75 mM siRNA#3 shown). Forty-eight hours later calcium switch was performed as indicated and RapGTP levels measured using the RalGDS-RBD pull down. Glyceraldehyde 3 phosphate dehydrogenease and total Rap1 expression levels indicate equal loading. C: Graph shows quantitation of the data represented in (B) (mean +/− SEM from three separate experiments using siRNA #3).
DISCUSSION
Adherens junctions are dynamic. Their formation and maintenance are finely regulated by growth factors and their receptors, kinase cascades, phosphatases, heterotrimeric G proteins, small GTPases, and scaffold proteins [Braga, 2002; Burridge et al., 2006; Jamora and Fuchs, 2002; Le et al., 2002; Lock and Stow, 2005; Roura et al., 1999]. Here, we have demonstrated a role of the small GTPase, Rap1, and an upstream GEF in regulating E-cadherin based adherens junctions in an epithelial model system.
Rap localizes to cell-cell contacts and strengthens them
GFP-Rap1A localized to regions of cell-cell contact in MDCK cells and was enriched at cell junctions from the early stages of cell contact, suggesting a key role for Rap1 in junction formation. Stable expression of constitutively active Rap1A-63E in MDCK cells promoted a dramatic change in their morphology; colonies were highly compacted and there was increased β-catenin localization within junctions despite no significant change in total protein content. Interestingly, enhanced junction formation was only seen upon stable expression of activated Rap1 and is consistent with studies using mouse osteosarcoma cells that lack the Rap GEF DOCK4 [Yajnik et al., 2003]. Since RapGAP also inhibited junction formation in MCF7 cells following a calcium switch but did not disrupt preexisting junctions [Hogan et al., 2004] our result are consistent with Rap1 playing a role in the formation of new cell-cell contacts rather than in the maintenance of mature ones. The reason for this requirement is unclear but suggests that the ability of Rap1 to promote enhanced junctional assembly is prompted by their disruption, i.e. Rap1 activation is triggered as a repair mechanism.
MDCK cells expressing Rap1A-63E showed resistance to HGF-induced cell scattering and calcium depletion induced cell junction disruption. This is consistent with the ability of the Rap GEF, Epac1 to resist scattering upon transfection into MDCK cells [Price et al., 2004] and supports the notion that Rap1 activity might also resist the junction separation. Recent studies have shown that activated Rap1 enhances the binding of AF-6 to p120 catenin that in turn binds to E-cadherin and prevents its endocytosis from the plasma membrane [Hoshino et al., 2005; Sato et al., 2006]. Rap has also been shown to activate Rho family GEFs and GAPs to modulate the actin cytoskeleton [Arthur et al., 2004; Fukuyama et al., 2005; Yamada et al., 2005] which is essential for the formation and maintenance of E-cadherin junctions. Thus Rap1 may utilize multiple mechanisms to promote junction stability.
Rap1 is activated following disruption of E-cadherin adherens junctions
Rap1 was activated when junctions were disrupted, either by chelation of extracellular calcium or by HGF stimulation. This suggested that Rap1's action was associated with repair and might explain why the compacted phenotype of Rap1A-63E expressing cells was only observed subsequent to their first trypsinization. EGTA-induced activation of Rap1 was E-cadherin dependent since incubation of cells with DECMA-1, an inhibitory antibody that binds the cadherin extracellular domains to prevent homotypic binding, prevented CaCl2 from reversing the effect of EGTA. This is consistent with recent findings in FRT cells [Balzac et al., 2005]. Therefore Rap1 is both regulating cell junctions (inside-out signaling) and being activated by E-cadherin via a classic outside-in signaling pathway.
Although Rap1 had previously been reported to be activated by HGF [Sakkab et al., 2000] no time course had been performed in epithelial cells. It is interesting to note that the activation of Rap1 by HGF was biphasic and detectable for over an hour compared to the transient response observed in 293 HEK cells [Sakkab et al., 2000]. Following disruption of cell junctions, HGF promotes integrin-dependent migration. Since Rap regulates cell spreading and migration via Rho family GTPases [Arthur et al., 2004; Bos, 2005], the second phase of Rap1 activation may contribute to HGF-induced scattering.
Although disruption of cell junctions in endothelial cells promoted an increase in RapGTP similarly to that seen in MDCK cells, a further increase in Rap1 activation rather than return toward baseline was seen upon re-addition of extracellular calcium. It would therefore appear that although Rap1 is activated upon breaking junctions in both cell types, Rap is differentially regulated by E- versus VE-cadherin engagement. Whereas treatment of epithelial cells with RapGAP failed to disrupt existing junctions [Hogan et al., 2004], it promoted loss of junctional integrity in HUVECs [Cullere et al., 2005; Sakurai et al., 2006; Wittchen et al., 2005] suggesting a more major role for Rap1 in maintenance of cell-cell contacts in endothelial cells.
C3G has been recently reported to directly bind to E-cadherin [Hogan et al., 2004] and to couple the engagement of nectins, which may precede cadherin binding, to Rap activation [Fukuyama et al., 2005]. On the other hand, PDZ-GEF I has been implicated in the activation of Rap1 in endothelial cells [Sakurai et al., 2006]. However, the differential activation of Rap GEFs can not explain the cell type differences in Rap activation sincewe found both GEFs to associate with E-cadherin upon junctional disruption in MDCK cells. The time course of Rap1 activation was delayed in FRT compared to other epithelial cells. In contrast to other cell lines tested, most Rap1 is located on intracellular membranes in FRT cells, requiring endocytosis of junctional components before Rap1 can become activated following extracellular calcium chelation [Balzac et al., 2005]. While it has been reported that endocytosis must occur prior to Rap1 activation by neurotrophins [Hisata et al., 2007; York et al., 1998] it has also been contended that Rap1 is activated at the plasma membrane in other cells [Bivona et al., 2004]. The failure of bafilomycin to block Rap1 activation in MDCK (this report) versus FRT cells [Balzac et al., 2005] supports our notion that in MDCK cells Rap1 localizes to cell-cell junctions where it is rapidly activated by an E-cadherin-associated RapGEF.
We observed an interaction between C3G and E-cadherin upon breaking MDCK cell junctions that did not coincide with the precipitation of β-catenin. This is consistent with the notion that C3G directly binds E-cadherin [Hogan et al., 2004] or is coupled via Src and Crk. In agreement with Balzac et al [Balzac et al., 2005] we found that the Src inhibitor PP2 could attenuate Rap activation (unpublished results). However, since phosphorylation by Src enhances C3G exchange activity [Ichiba et al., 1999], this does not preclude a direct E-cadherin-C3G complex. Association between PDZ-GEF I and E-cadherin was also greatly enhanced when cell-cell junctions were dissolved and resulted in co-precipitation with β-catenin. This association was only seen using anti-PDZ-GEF I as the immunoprecipitating antibody, presumably due to steric hindrance by the anti-cadherin antibody that recognizes the intracellular domain, and likely explains why PDZ-GEF I was not previously reported to associate with E-cadherin in MCF-7 cells [Hogan et al., 2004].
Previous studies have shown PDZ-GEF I binding to the multi-PDZ domain-containing scaffold protein, MAGI-1, MAGI binding to β-catenin, and the localization of each of these protein to regions of cell-cell contact in epithelial cells and hypocampal neurons [Hisata et al., 2007]. Recent findings also suggest that Rap1 activation by PDZ-GEF in epithelial cells is critical during Drosophila development [Boettner and Van Aelst, 2007]. However, a role for PDZ-GEF I in cell junction formation had not been established [Dobrosotskaya and James, 2000; Ide et al., 1999; Kawajiri et al., 2000; Mino et al., 2000]. Here we show that knock down of PDZ-GEF I in MDCK cells results in decreased Rap1 activation during junction disassembly. The partial Rap1 activation persisting after PDZ-GEF I siRNA transfection may be attributable to the incomplete knockdown of GEF expression, resulting from only ∼50% transfection efficiency, or the continued presence of C3G. We have been unsuccessful in identifying effective canine C3G siRNAs to address this latter possibility. Though we could not detect MAGI-1 in our PDZ-GEF I/E-cadherin complexes we speculate that it plays an essential role in localizing PDZ-GEF I to the junctional complex: Recent studies by Sakurai et al show MAGI-1 binding to β-catenin and PDZ-GEF I, and localizing it to cell-cell adhesion sites in human endothelial cells. Depletion of MAGI-1 by siRNA resulted in decreased Rap1 activation and impaired cell-cell adhesion. However, Sakurai et al could not knockdown PDZ-GEF I in HUVECs without cell detachment [Sakurai et al., 2006]. We now show that Rap1 is localized to regions of epithelial cell-cell adhesion, strengthens these junctions and is activated upon adherens junction disruption. This effect is at least in part mediated by the recruitment and activation of PDZ-GEF I.
ACKNOWLDEGEMENTS
We thank Atsuko Sakurai and Noaki Mochizuki for the generous provision of PDZ-GEF I antibody, Simon Atkinson for MDCK cells and valuable discussion, Jim Marrs and Shobha Gopalakrishnan for rr-1 antibody, Enrique Rodriguez-Boulan for FRT cells and David Ingram for HUVECs. This work was supported by grants from the Indiana University School of Medicine Biomedical Research Fund and the NIH (CA 108647) to LAQ.
Funded by:
Indiana University School of Medicine Biomedical Research Fund
NIH; CA108647.
REFERENCES
- Adams CL, Chen YT, Smith SJ, Nelson WJ. Mechanisms of epithelial cell-cell adhesion and cell compaction revealed by high-resolution tracking of E-cadherin-green fluorescent protein. J Cell Biol. 1998;142:1105–19. doi: 10.1083/jcb.142.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams CL, Nelson WJ, Smith SJ. Quantitative analysis of cadherin-catenin-actin reorganization during development of cell-cell adhesion. J Cell Biol. 1996;135:1899–911. doi: 10.1083/jcb.135.6.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur WT, Noren NK, Burridge K. Regulation of Rho family GTPases by cell-cell and cell-matrix adhesion. Biol Res. 2002;35:239–46. doi: 10.4067/s0716-97602002000200016. [DOI] [PubMed] [Google Scholar]
- Arthur WT, Quilliam LA, Cooper JA. Rap1 promotes cell spreading by localizing Rac guanine nucleotide exchange factors. J Cell Biol. 2004;167:111–22. doi: 10.1083/jcb.200404068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balzac F, Avolio M, Degani S, Kaverina I, Torti M, Silengo L, Small JV, Retta SF. E-cadherin endocytosis regulates the activity of Rap1: a traffic light GTPase at the crossroads between cadherin and integrin function. J Cell Sci. 2005;118:4765–83. doi: 10.1242/jcs.02584. [DOI] [PubMed] [Google Scholar]
- Beranger F, Goud B, Tavitian A, de Gunzburg J. Association of the Ras-antagonistic Rap1/Krev-1 proteins with the Golgi complex. Proc Natl Acad Sci U S A. 1991;88:1606–10. doi: 10.1073/pnas.88.5.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bivona TG, Wiener HH, Ahearn IM, Silletti J, Chiu VK, Philips MR. Rap1 up-regulation and activation on plasma membrane regulates T cell adhesion. J Cell Biol. 2004;164:461–70. doi: 10.1083/jcb.200311093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettner B, Govek EE, Cross J, Van Aelst L. The junctional multidomain protein AF-6 is a binding partner of the Rap1A GTPase and associates with the actin cytoskeletal regulator profilin. Proc Natl Acad Sci U S A. 2000;97:9064–9. doi: 10.1073/pnas.97.16.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettner B, Van Aelst L. The Rap GTPase activator Drosophila PDZ-GEF regulates cell shape in epithelial migration and morphogenesis. Mol Cell Biol. 2007;27:7966–80. doi: 10.1128/MCB.01275-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos JL. Linking Rap to cell adhesion. Curr Opin Cell Biol. 2005;17:123–8. doi: 10.1016/j.ceb.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Bos JL, Franke B, M'Rabet L, Reedquist K, Zwartkruis F. In search of a function for the Ras-like GTPase Rap1. FEBS Lett. 1997;410:59–62. doi: 10.1016/s0014-5793(97)00324-4. [DOI] [PubMed] [Google Scholar]
- Braga V. Epithelial cell shape: cadherins and small GTPases. Exp Cell Res. 2000;261:83–90. doi: 10.1006/excr.2000.5050. [DOI] [PubMed] [Google Scholar]
- Braga VM. Cell-cell adhesion and signalling. Curr Opin Cell Biol. 2002;14:546–56. doi: 10.1016/s0955-0674(02)00373-3. [DOI] [PubMed] [Google Scholar]
- Braga VM, Del Maschio A, Machesky L, Dejana E. Regulation of cadherin function by Rho and Rac: modulation by junction maturation and cellular context. Mol Biol Cell. 1999;10:9–22. doi: 10.1091/mbc.10.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga VM, Machesky LM, Hall A, Hotchin NA. The small GTPases Rho and Rac are required for the establishment of cadherin-dependent cell-cell contacts. J Cell Biol. 1997;137:1421–31. doi: 10.1083/jcb.137.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K, Sastry SK, Sallee JL. Regulation of cell adhesion by protein-tyrosine phosphatases. I. Cell-matrix adhesion. J Biol Chem. 2006;281:15593–6. doi: 10.1074/jbc.R500030200. [DOI] [PubMed] [Google Scholar]
- Caron E. Cellular functions of the Rap1 GTP-binding protein: a pattern emerges. J Cell Sci. 2003;116:435–40. doi: 10.1242/jcs.00238. [DOI] [PubMed] [Google Scholar]
- Castro AF, Rebhun JF, Quilliam LA. Measuring Ras-family GTP levels in vivo--running hot and cold. Methods. 2005;37:190–6. doi: 10.1016/j.ymeth.2005.05.015. [DOI] [PubMed] [Google Scholar]
- Chen YT, Stewart DB, Nelson WJ. Coupling assembly of the E-cadherin/beta-catenin complex to efficient endoplasmic reticulum exit and basal-lateral membrane targeting of E-cadherin in polarized MDCK cells. J Cell Biol. 1999;144:687–99. doi: 10.1083/jcb.144.4.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullere X, Shaw SK, Andersson L, Hirahashi J, Luscinskas FW, Mayadas TN. Regulation of vascular endothelial barrier function by Epac, a cAMP-activated exchange factor for Rap GTPase. Blood. 2005;105:1950–5. doi: 10.1182/blood-2004-05-1987. [DOI] [PubMed] [Google Scholar]
- de Rooij J, Boenink NM, van Triest M, Cool RH, Wittinghofer A, Bos JL. PDZ-GEF1, a guanine nucleotide exchange factor specific for Rap1 and Rap2. J Biol Chem. 1999;274:38125–30. doi: 10.1074/jbc.274.53.38125. [DOI] [PubMed] [Google Scholar]
- Dobrosotskaya IY, James GL. MAGI-1 interacts with beta-catenin and is associated with cell-cell adhesion structures. Biochem Biophys Res Commun. 2000;270:903–9. doi: 10.1006/bbrc.2000.2471. [DOI] [PubMed] [Google Scholar]
- Fukata M, Kaibuchi K. Rho-family GTPases in cadherin-mediated cell-cell adhesion. Nat Rev Mol Cell Biol. 2001;2:887–97. doi: 10.1038/35103068. [DOI] [PubMed] [Google Scholar]
- Fukuyama T, Ogita H, Kawakatsu T, Fukuhara T, Yamada T, Sato T, Shimizu K, Nakamura T, Matsuda M, Takai Y. Involvement of the c-Src-Crk-C3G-Rap1 signaling in the nectin-induced activation of Cdc42 and formation of adherens junctions. J Biol Chem. 2005;280:815–25. doi: 10.1074/jbc.M411099200. [DOI] [PubMed] [Google Scholar]
- Gumbiner B, Stevenson B, Grimaldi A. The role of the cell adhesion molecule uvomorulin in the formation and maintenance of the epithelial junctional complex. J Cell Biol. 1988;107:1575–87. doi: 10.1083/jcb.107.4.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori M, Minato N. Rap1 GTPase: functions, regulation, and malignancy. J Biochem (Tokyo) 2003;134:479–84. doi: 10.1093/jb/mvg180. [DOI] [PubMed] [Google Scholar]
- Hisata S, Sakisaka T, Baba T, Yamada T, Aoki K, Matsuda M, Takai Y. Rap1-PDZ-GEF1 interacts with a neurotrophin receptor at late endosomes, leading to sustained activation of Rap1 and ERK and neurite outgrowth. J Cell Biol. 2007;178:843–60. doi: 10.1083/jcb.200610073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan C, Serpente N, Cogram P, Hosking CR, Bialucha CU, Feller SM, Braga VM, Birchmeier W, Fujita Y. Rap1 regulates the formation of E-cadherin-based cell-cell contacts. Mol Cell Biol. 2004;24:6690–700. doi: 10.1128/MCB.24.15.6690-6700.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino T, Sakisaka T, Baba T, Yamada T, Kimura T, Takai Y. Regulation of E-cadherin endocytosis by nectin through afadin, Rap1, and p120ctn. J Biol Chem. 2005;280:24095–103. doi: 10.1074/jbc.M414447200. [DOI] [PubMed] [Google Scholar]
- Ichiba T, Hashimoto Y, Nakaya M, Kuraishi Y, Tanaka S, Kurata T, Mochizuki N, Matsuda M. Activation of C3G guanine nucleotide exchange factor for Rap1 by phosphorylation of tyrosine 504. J Biol Chem. 1999;274:14376–81. doi: 10.1074/jbc.274.20.14376. [DOI] [PubMed] [Google Scholar]
- Ide N, Hata Y, Nishioka H, Hirao K, Yao I, Deguchi M, Mizoguchi A, Nishimori H, Tokino T, Nakamura Y, Takai Y. Localization of membrane-associated guanylate kinase (MAGI)-1/BAI-associated protein (BAP) 1 at tight junctions of epithelial cells. Oncogene. 1999;18:7810–5. doi: 10.1038/sj.onc.1203153. [DOI] [PubMed] [Google Scholar]
- Jamora C, Fuchs E. Intercellular adhesion, signalling and the cytoskeleton. Nat Cell Biol. 2002;4:E101–8. doi: 10.1038/ncb0402-e101. [DOI] [PubMed] [Google Scholar]
- Kaibuchi K, Kuroda S, Fukata M, Nakagawa M. Regulation of cadherin-mediated cell-cell adhesion by the Rho family GTPases. Curr Opin Cell Biol. 1999;11:591–6. doi: 10.1016/s0955-0674(99)00014-9. [DOI] [PubMed] [Google Scholar]
- Kawajiri A, Itoh N, Fukata M, Nakagawa M, Yamaga M, Iwamatsu A, Kaibuchi K. Identification of a novel beta-catenin-interacting protein. Biochem Biophys Res Commun. 2000;273:712–7. doi: 10.1006/bbrc.2000.3002. [DOI] [PubMed] [Google Scholar]
- Kitayama H, Sugimoto Y, Matsuzaki T, Ikawa Y, Noda M. A ras-related gene with transformation suppressor activity. Cell. 1989;56:77–84. doi: 10.1016/0092-8674(89)90985-9. [DOI] [PubMed] [Google Scholar]
- Knox AL, Brown NH. Rap1 GTPase regulation of adherens junction positioning and cell adhesion. Science. 2002;295:1285–8. doi: 10.1126/science.1067549. [DOI] [PubMed] [Google Scholar]
- Konishi T, Takehara T, Tsuji T, Ohsato K, Matsumoto K, Nakamura T. Scatter factor from human embryonic lung fibroblasts is probably identical to hepatocyte growth factor. Biochem Biophys Res Commun. 1991;180:765–73. doi: 10.1016/s0006-291x(05)81131-3. [DOI] [PubMed] [Google Scholar]
- Lamorte L, Royal I, Naujokas M, Park M. Crk adapter proteins promote an epithelial-mesenchymal-like transition and are required for HGF-mediated cell spreading and breakdown of epithelial adherens junctions. Mol Biol Cell. 2002;13:1449–61. doi: 10.1091/mbc.01-10-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le TL, Joseph SR, Yap AS, Stow JL. Protein kinase C regulates endocytosis and recycling of E-cadherin. Am J Physiol Cell Physiol. 2002;283:C489–99. doi: 10.1152/ajpcell.00566.2001. [DOI] [PubMed] [Google Scholar]
- Liao Y, Kariya K, Hu CD, Shibatohge M, Goshima M, Okada T, Watari Y, Gao X, Jin TG, Yamawaki-Kataoka Y, Kataoka T. RA-GEF, a novel Rap1A guanine nucleotide exchange factor containing a Ras/Rap1A-associating domain, is conserved between nematode and humans. J Biol Chem. 1999;274:37815–20. doi: 10.1074/jbc.274.53.37815. [DOI] [PubMed] [Google Scholar]
- Lock JG, Stow JL. Rab11 in recycling endosomes regulates the sorting and basolateral transport of E-cadherin. Mol Biol Cell. 2005;16:1744–55. doi: 10.1091/mbc.E04-10-0867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mino A, Ohtsuka T, Inoue E, Takai Y. Membrane-associated guanylate kinase with inverted orientation (MAGI)-1/brain angiogenesis inhibitor 1-associated protein (BAP1) as a scaffolding molecule for Rap small G protein GDP/GTP exchange protein at tight junctions. Genes Cells. 2000;5:1009–16. doi: 10.1046/j.1365-2443.2000.00385.x. [DOI] [PubMed] [Google Scholar]
- Nakagawa M, Fukata M, Yamaga M, Itoh N, Kaibuchi K. Recruitment and activation of Rac1 by the formation of E-cadherin-mediated cell-cell adhesion sites. J Cell Sci. 2001;114:1829–38. doi: 10.1242/jcs.114.10.1829. [DOI] [PubMed] [Google Scholar]
- Noren NK, Niessen CM, Gumbiner BM, Burridge K. Cadherin engagement regulates Rho family GTPases. J Biol Chem. 2001;276:33305–8. doi: 10.1074/jbc.C100306200. [DOI] [PubMed] [Google Scholar]
- Ohtsuka T, Hata Y, Ide N, Yasuda T, Inoue E, Inoue T, Mizoguchi A, Takai Y. nRap GEP: a novel neural GDP/GTP exchange protein for rap1 small G protein that interacts with synaptic scaffolding molecule (S-SCAM) Biochem Biophys Res Commun. 1999;265:38–44. doi: 10.1006/bbrc.1999.1619. [DOI] [PubMed] [Google Scholar]
- Perez-Moreno M, Jamora C, Fuchs E. Sticky business: orchestrating cellular signals at adherens junctions. Cell. 2003;112:535–48. doi: 10.1016/s0092-8674(03)00108-9. [DOI] [PubMed] [Google Scholar]
- Pizon V, Chardin P, Lerosey I, Olofsson B, Tavitian A. Human cDNAs rap1 and rap2 homologous to the Drosophila gene Dras3 encode proteins closely related to ras in the ‘effector’ region. Oncogene. 1988;3:201–4. [PubMed] [Google Scholar]
- Pokutta S, Herrenknecht K, Kemler R, Engel J. Conformational changes of the recombinant extracellular domain of E-cadherin upon calcium binding. Eur J Biochem. 1994;223:1019–26. doi: 10.1111/j.1432-1033.1994.tb19080.x. [DOI] [PubMed] [Google Scholar]
- Pokutta S, Weis WI. The cytoplasmic face of cell contact sites. Curr Opin Struct Biol. 2002;12:255–62. doi: 10.1016/s0959-440x(02)00318-4. [DOI] [PubMed] [Google Scholar]
- Price LS, Hajdo-Milasinovic A, Zhao J, Zwartkruis FJ, Collard JG, Bos JL. Rap1 regulates E-cadherin-mediated cell-cell adhesion. J Biol Chem. 2004;279:35127–32. doi: 10.1074/jbc.M404917200. [DOI] [PubMed] [Google Scholar]
- Quilliam LA, Rebhun JF, Castro AF. A growing family of guanine nucleotide exchange factors is responsible for activation of Ras-family GTPases. Prog Nucleic Acid Res Mol Biol. 2002;71:391–444. doi: 10.1016/s0079-6603(02)71047-7. [DOI] [PubMed] [Google Scholar]
- Rebhun JF, Castro AF, Quilliam LA. Identification of guanine nucleotide exchange factors (GEFs) for the Rap1 GTPase. Regulation of MR-GEF by M-Ras-GTP interaction. J Biol Chem. 2000;275:34901–8. doi: 10.1074/jbc.M005327200. [DOI] [PubMed] [Google Scholar]
- Roura S, Miravet S, Piedra J, Garcia de Herreros A, Dunach M. Regulation of E-cadherin/Catenin association by tyrosine phosphorylation. J Biol Chem. 1999;274:36734–40. doi: 10.1074/jbc.274.51.36734. [DOI] [PubMed] [Google Scholar]
- Sakkab D, Lewitzky M, Posern G, Schaeper U, Sachs M, Birchmeier W, Feller SM. Signaling of hepatocyte growth factor/scatter factor (HGF) to the small GTPase Rap1 via the large docking protein Gab1 and the adapter protein CRKL. J Biol Chem. 2000;275:10772–8. doi: 10.1074/jbc.275.15.10772. [DOI] [PubMed] [Google Scholar]
- Sakurai A, Fukuhara S, Yamagishi A, Sako K, Kamioka Y, Masuda M, Nakaoka Y, Mochizuki N. MAGI-1 is required for Rap1 activation upon cell-cell contact and for enhancement of vascular endothelial cadherin-mediated cell adhesion. Mol Biol Cell. 2006;17:966–76. doi: 10.1091/mbc.E05-07-0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato KY, Polakis PG, Haubruck H, Fasching CL, McCormick F, Stanbridge EJ. Analysis of the tumor suppressor activity of the K-rev-1 gene in human tumor cell lines. Cancer Res. 1994;54:552–9. [PubMed] [Google Scholar]
- Sato T, Fujita N, Yamada A, Ooshio T, Okamoto R, Irie K, Takai Y. Regulation of the assembly and adhesion activity of E-cadherin by nectin and afadin for the formation of adherens junctions in Madin-Darby canine kidney cells. J Biol Chem. 2006;281:5288–99. doi: 10.1074/jbc.M510070200. [DOI] [PubMed] [Google Scholar]
- Stork PJ. Does Rap1 deserve a bad Rap? Trends Biochem Sci. 2003;28:267–75. doi: 10.1016/S0968-0004(03)00087-2. [DOI] [PubMed] [Google Scholar]
- Vasioukhin V, Bauer C, Yin M, Fuchs E. Directed actin polymerization is the driving force for epithelial cell-cell adhesion. Cell. 2000;100:209–19. doi: 10.1016/s0092-8674(00)81559-7. [DOI] [PubMed] [Google Scholar]
- Wittchen ES, Worthylake RA, Kelly P, Casey PJ, Quilliam LA, Burridge K. Rap1 GTPase inhibits leukocyte transmigration by promoting endothelial barrier function. J Biol Chem. 2005;280:11675–82. doi: 10.1074/jbc.M412595200. [DOI] [PubMed] [Google Scholar]
- Yajnik V, Paulding C, Sordella R, McClatchey AI, Saito M, Wahrer DC, Reynolds P, Bell DW, Lake R, van den Heuvel S, Settleman J, Haber DA. DOCK4, a GTPase activator, is disrupted during tumorigenesis. Cell. 2003;112:673–84. doi: 10.1016/s0092-8674(03)00155-7. [DOI] [PubMed] [Google Scholar]
- Yamada T, Sakisaka T, Hisata S, Baba T, Takai Y. RA-RhoGAP, Rap-activated Rho GTPase-activating protein implicated in neurite outgrowth through Rho. J Biol Chem. 2005;280:33026–34. doi: 10.1074/jbc.M504587200. [DOI] [PubMed] [Google Scholar]
- York RD, Yao H, Dillon T, Ellig CL, Eckert SP, McCleskey EW, Stork PJ. Rap1 mediates sustained MAP kinase activation induced by nerve growth factor. Nature. 1998;392:622–6. doi: 10.1038/33451. [DOI] [PubMed] [Google Scholar]