Abstract
Objectives
Ovarian epithelial carcinoma can be subdivided into separate histological subtypes including clear cell, endometrioid, mucinous, and serous. These carcinoma subtypes may represent distinctive pathways of tumorigenesis and disease development. This distinction could potentially be reflected in the levels of tumor produced factors that enter into the circulation and serve as biomarkers of malignant growth. Here, we analyze levels of circulating biomarkers from a diverse set of patients diagnosed with ovarian carcinoma to identify biomarker trends and relationships associated with distinct carcinoma histotypes and divergent tumorigenic pathways.
Methods
We utilize multiplexed bead-based immunoassays to measure serum levels of a diverse array of fifty-eight biomarkers from the sera of patients diagnosed with various histological subtypes of ovarian carcinoma and benign lesions. The biomarkers studied include cancer antigens, oncogenes, cytokines, chemokines, receptors, growth and angiogenic factors, proteases, hormones, and apoptosis and adhesion related molecules. Levels of each biomarker are compared statistically across carcinoma subtypes as well as with benign cases.
Results
A total of 21 serum biomarkers differ significantly between patients diagnosed with ovarian carcinomas and benign cases. Nine of these biomarkers are specific for carcinomas identified as clear cell, endometrioid, or mucinous in histology, while two biomarkers are specific for the serous histology. In a direct comparison of the histology groups, ten biomarkers are found to be subtype specific. Identified biomarkers include traditional and emerging tumor markers, cytokines and receptors, hormones, and adhesion- and metastasis-related proteins.
Conclusions
We demonstrate here that the divergent histology-based tumorigenic pathways proposed for ovarian epithelial carcinomas are associated with distinct profiles of circulating biomarkers. Continued investigation into the relationships between these factors should reveal new insights into the complex mechanisms underlying ovarian epithelial tumorigenesis.
Keywords: ovarian carcinoma, tumor histology, serum biomarkers, ovarian tumorigenesis
Introduction
For women in the United States, ovarian cancer ranks eighth among cancers, excluding skin cancer, in terms of incidence, but moves up to fifth in a ranking of age-adjusted mortality [1]. Ovarian carcinomas, tumors of the surface epithelium, are by far the most common form of ovarian cancer [2]. The notion that ovarian carcinomas arise from the surface epithelium or postovulatory inclusion cysts following chronic exposure to hormones is met with widespread agreement [3], however a growing number of clinicians and researchers are beginning to appreciate a far greater heterogeneity concerning the development of ovarian epithelial carcinoma (OEC). Ovarian carcinomas can be classified into the histological subtypes of serous, clear cell, endometrioid, and mucinous which correspond to the different types of epithelia present in the female reproductive tract [4, 5]. Serous tumors, which carry the poorest prognosis, are the most common form of ovarian carcinoma and make up roughly half of all diagnoses [6]. Serous tumors are histologically similar to cancers of the fallopian tube, and range from cystic papillary tumors to solid masses [6]. Endometrioid tumors, accounting for 15–20% of ovarian carcinomas, are characterized by endometrial-like glandular structures [7]. Mucinous tumors often contain cysts and glands lined by mucin-rich cells and constitute 10% of ovarian carcinomas [8]. Clear cell tumors represent 4–12% of ovarian carcinomas and are comprised of clear and hobnailed cells with an immature glomerular pattern [9].
Within the broad spectrum of disease states represented by OEC, there is accumulating clinical, translational, and genetic evidence for the existence of two distinct classes of carcinogenesis [2]. These classes have been termed type I, tumors comprising low-grade serous, mucinous, endometrioid, malignant Brenner, and clear cell carcinoma, and type II, tumors including high-grade serous carcinoma, malignant mixed mesodermal tumors (carcinosarcomas), and undifferentiated carcinoma [2, 10]. Type I tumors typically present as early stage neoplasms that pursue an indolent course which may last more than 20 years [11–13]. Recent findings have traced the development of type I tumors through a stepwise series of well-described precursor lesions [10]. Benign cystadenomas and adenofibromas are believed to give rise to so-called borderline tumors which in turn develop into the type I tumors described above. In contrast to type I tumors, type II tumors are not associated with any recognizable precursors and apparently develop de novo from the surface epithelium or inclusion cysts of the ovary [14]. Type II carcinomas present as late stage, high grade neoplasms that are clinically aggressive, evolve rapidly and metastasize early, and are associated with a poor prognosis [2, 13]. Type II tumors are relatively chemosensitive in comparison to type I tumors [2].
Mutation screening and gene expression profiling have identified a number of molecular alterations and differences in gene expression that distinguish type I ovarian tumors from type II. These distinctions suggest a difference in prognosis and treatment response between the two groups [15, 16]. Most prominent among observed genetic alterations are mutations in the BRAF and KRAS oncogenes, which occur in 28–35% of type I tumors but are largely nonexistent in type II tumors [17]. Mutations in the tumor suppressor gene PTEN and the CTNNB1 gene, which encodes β-catenin, are also more prevalent in type I tumors, particularly endometrioid carcinomas [18–20]. Mutations in TP53 are common in type II carcinomas but relatively rare in type I tumors [21–25]. Gene expression profiling and immunohistochemical analyses have identified numerous factors that are overexpressed in type II tumors when compared to type I including AKT2, human leukocyte antigen-G (HLA-G), apolipoprotein E, p53, MIB1, and bcl-2 [26–29].
Here we present an analysis of a diverse array of biomarkers found in the serum of women diagnosed with ovarian cancer. Biomarker levels are compared among patients grouped according to carcinoma subtype as well as with those presenting with benign disease to identify markers that may contribute to or result from a particular carcinogenic pathway. In this manner, we seek to contribute to the evolving body of evidence related to ovarian epithelial tumorigenesis.
Materials and Methods
Human Serum Samples
Serum samples from 157 patients diagnosed with ovarian cancer as well as 130 women with benign ovarian lesions were provided by the Gynecological Oncology Group (GOG) (Cleveland, OH) without individual identification of patients. Procedures for serum collection, processing, and storage have been previously described [30]. Written informed consent was obtained for each subject. The diagnostic breakdown of the study population is presented in Table 1 and represents a diverse spectrum of disease subtypes. Benign cases include a broad spectrum of non-malignant lesions representing a variety of histological origins. Patients diagnosed with endometriosis were not included in this study. Patients diagnosed with clear cell, endometrioid, and mucinous carcinomas are grouped together under the heading of “CEM Carcinoma.”
Table 1.
Clinical Characteristics of Study Population
| Diagnosis | N | Age Range |
|---|---|---|
| Benign | 133 | 24–87 |
| CEM Carcinoma | 100 | 27–87 |
| Clear Cell Carcinoma | 24 | |
| Endometrioid Carcinoma | 46 | |
| Mucinous Carcinoma | 30 | |
| Stage I & II | 83 | |
| Stage III & IV | 17 | |
| Grade 1 | 17 | |
| Grade 2 | 11 | |
| Grade 3 | 16 | |
| Unknown Grade | 56 | |
| Serous Carcinoma | 57 | 48–87 |
| Stage I & II | 27 | |
| Stage III & IV | 30 | |
| Grade 1 | 2 | |
| Grade 2 | 21 | |
| Grade 3 | 29 | |
| Unknown Grade | 5 |
Multiplexed Bead-Based Immunoassay
The xMAP™ bead-based technology (Luminex Corp., Austin, TX) permits simultaneous analysis of numerous analytes in a single sample. Fifty-eight bead-based xMAP™ immunoassays for a variety of known or potential biomarkers for ovarian and other epithelial cancers were utilized in the present study (Table 2). Assays were performed according to the manufacturers’ protocol or as described previously [30]. Samples were analyzed using the Bio-Plex suspension array system (Bio-Rad Laboratories, Hercules, CA). For each analyte, 100 beads were analyzed and the median fluorescence intensity was determined. Analysis of experimental data was performed using five-parameter logistic curve fitting to standard analyte values.
Table 2.
Complete List of Biomarkers
| Cancer Antigens/Oncogenes | α-fetoprotein, CA 19-9, CA 125, CA 15-3, CA 72-4, CEA, ErbB2 |
| Cytokines/Chemokines/Receptors | Eotaxin, fractalkine, GM-CSF, IFNγ, IL-10, IL-12p70, IL-13, IL-1β, IL-1Rα, IL-2, IL-2R, IL-4, IL-5, IL-6, IL-6R, IL-7, IL-8, IP-10, MIF, MIP-1β, sCD40L, TNFα, TNF-R1, TNF-R2 |
| Growth/Angiogenic Factors | EFGR, IGFBP-1, TGFα |
| Proteases | Kallikrein 10, MMP-2, MMP-3, MMP-9 |
| Hormones | ACTH, FSH, GH, LH, prolactin, TSH |
| Adipokines | Adiponectin |
| Apoptosis-related molecules | Cyfra 21-1, DR5, sFas, sFasL |
| Adhesion molecules | sICAM-1, sVCAM-1, tTG, tPAI-1 |
| Other | HE4, osteopontin, SMRP, transthyretin, MPO |
Assays for CA 19-9, CA 125, CA 15-3, CA 72-4, CEA, ErbB2, Kallikrein 10, EGFR, Cyfra 21-1, SMRP, tTG, HE4, osteopontin, transthyretin, and IGFBP-1 were developed in the UPCI Luminex® Core Facility [31]. The inter-assay variability of each assay was 5% to 11% and the intra-assay variability was 2% to 9%. Assays for eotaxin, Mip-1β, IP-10, IL-2R, IL-1Rα, IL-6R, DR5, TNF-R1, and TNF-R2 were obtained from Invitrogen (Camarillo, CA). Assays for MMP-2, MMP-3, and MMP-9 were obtained from R&D Systems (Minneapolis, MN). All other listed assays were obtained from Millipore (St. Charles, MO).
Statistical Analysis
The Mann-Whitney nonparametric t test was used to evaluate the significance of differences in serum biomarker levels expressed as observed concentrations between patients diagnosed with benign ovarian lesions and various ovarian carcinoma subtypes. The level of significance was p<0.05.
Results
Analysis of Serum Biomarker Levels Across Ovarian Epithelial Carcinoma Subtypes
Sera from patients presenting with clear cell, endometrioid, and mucinous carcinomas, hereafter termed (CEM), were considered jointly as this group was presumed to represent type I ovarian carcinomas. Patients diagnosed with serous carcinoma presented with tumors that were almost uniformly high grade. Thus, this group was presumed to represent type II carcinomas and was considered separately. Serum biomarker levels from each of these groups were compared to each other as well as to those from patients diagnosed with benign ovarian lesions. These results are presented in Table 3.
Table 3.
Serum Biomarker Levels Across Ovarian Epithelial Carcinoma Subtypes
| Biomarker Levels (mean pg/ml ± 95% CI) | Mann-Whitney Significance | |||||
|---|---|---|---|---|---|---|
| Benign | CEM Carcinoma | Serous Carcinoma | Benign vs. CEM | Benign vs. Serous | CEM vs. Serous | |
| CA 1251 | 14.15±4.61 | 49.65±15.04 | 123.9±51.17 | *** | ** | *** |
| CA 72-41 | 2.04±.285 | 13.5±7.9 | 4.39±2.06 | *** | ** | |
| CD40L | 19457±4732 | 23522±4994 | 16414±5929 | * | ||
| Cyfra 21-1 | 891±222 | 2323±718 | 2633±886 | *** | *** | |
| EGFR | 8548±344 | 7233±397 | 7552±636 | *** | ** | |
| FSH2 | 37487±5344 | 24744±5182 | 36471±7751 | ** | ||
| HE4 | 5476±4357 | 74816±42866 | 43857±18512 | *** | *** | |
| IGFBP-1 | 10178±1891 | 15509±3660 | 9734±3536 | * | ||
| IL-10 | 15.67±2.63 | 32.48±10.78 | 20.79±4.31 | *** | ** | |
| IL-2R | 355.7±57.2 | 541.6±105.2 | 651.6±169.8 | *** | *** | |
| IL-6 | 19.67±3.4 | 31.55±5.87 | 27.07±9.01 | *** | ||
| IL-7 | 8.5±.864 | 11.6±1.85 | 10.2±1.69 | *** | * | |
| IL-8 | 15±3.91 | 24.8±15.17 | 16.9±5.24 | ** | * | |
| IP-10 | 49.86±5.35 | 42.4±5.71 | 72.53±15.54 | * | *** | *** |
| LH2 | 19118±2435 | 15767±2982 | 22441±4496 | ** | ** | |
| MMP-2 | 150963±8262 | 131903±9126 | 137652±14133 | ** | ||
| MMP-9 | 212302±34252 | 361810±62411 | 250084±64652 | *** | ** | |
| MPO | 91818±21710 | 123134±30713 | 80550±30403 | * | ** | |
| SMRP3 | 44227±19250 | 43804±15896 | 117660±49778 | *** | *** | |
| sVCAM-1 | 876645±68143 | 772258±66047 | 796917±70051 | ** | ||
| TgII4 | 9.44±1.38 | 14.34±1.61 | 14.95±3.09 | *** | *** | |
| TNF-R2 | 1515±137 | 1798±186 | 1836±228 | * | ** | |
| tPAI-1 | 35876±3432 | 47987±4514 | 36723±4949 | *** | ** | |
U/ml,
IU/ml,
pM,
mU/ml CEM: clear cell, endometrioid, mucinous carcinoma
-p<0.05,
-p<0.01,
-p<0.001
When the benign group was compared to the CEM carcinoma group, a number of significant serum biomarker level differences were observed. Among the cancer antigens and oncogenes assayed, CA 125, CA 72-4, Cyfra 21-1, and HE4 were all elevated in the CEM carcinoma group while levels of EGFR were reduced in the same group. The CEM carcinomas demonstrated higher levels of the cytokines IL-10, IL-2R, IL-6, IL-7, IL-8, and MPO as well as the cytokine receptor TNF-R2 in comparison to benign cases. IP-10 was decreased among CEM carcinomas. Levels of MMP-2 were decreased among the CEM carcinomas while levels of MMP-9 were increased. The CEM carcinomas also exhibited increased levels of tTG and tPAI-1 and decreased levels of LH and sVCAM-1 when compared to the benign group.
Serum samples from the serous carcinoma group were compared with the benign group and several significant differences were identified. CA 125, Cyfra 21-1, HE4, and SMRP were elevated in serous carcinomas while levels of EGFR were reduced. Among cytokines and their receptors, IL-10, IL-2R, IL-7, IL-8, IP-10, and TNF-R2 were all found to be increased in serum samples from serous carcinoma patients in comparison to the benign group. Serum levels of LH and tTG were also increased in the serous carcinoma group.
The CEM carcinoma group was compared to the serous carcinoma group to identify serum biomarker level differences. Levels of CA 125 and SMRP were higher in the serous carcinoma group while CA 72-4 was higher in the CEM group. The CEM carcinomas demonstrated increased levels of CD40L and MPO and decreased levels of IP-10 when compared to the serous carcinomas. In the serous carcinomas, serum levels of MMP-9, FSH, and tPAI-1 were elevated while levels of IGFBP-1 were reduced in comparison to the CEM carcinoma group.
Discussion
Tumorigenesis is a complicated and multi-faceted process that involves unchecked proliferation, immune evasion, angiogensis, stroma formation, tumor cell invasion and migration, and implantation and growth within distant tissues. To accomplish each of these feats requires a balanced and precise genetic background and tumor microenvironment, the components of which remain largely unresolved by cancer researchers. Here we attempt to identify circulating factors associated with ovarian epithelial tumorigenesis through the comparison of a broad array of serum biomarkers in patients with distinct ovarian carcinoma histotypes.
The results of our analysis of serum biomarkers across OEC subtypes are outlined in Figure 1. We identified nine biomarkers that were elevated in the serum of patients diagnosed with ovarian carcinoma regardless of the disease subtype. These included the commonly used ovarian cancer biomarkers CA 125 and Cyfra 21-1 as well as the inflammatory cytokines IL-7, IL-8, and IL-10 and the receptors IL-2R and TNF-R2. These same cytokines, known to promote growth and inhibit apoptosis [32], have been previously found to be produced in vitro by ovarian cell lines and primary cells [33–35] and have also been observed to be elevated in the sera of ovarian cancer patients in comparison to healthy and benign controls [36]. Also increased in our ovarian cancer group were HE4 and tissue transglutaminase (tTG). HE4 is an 11kDa precursor to the epididymal secretory protein E4 and is an emerging biomarker for the detection of ovarian and endometrial cancer [37–39]. HE4 is overexpressed in ovarian carcinomas and demonstrates minimal gene expression and production in all tested normal tissues [40, 41]. tTG is highly expressed in ovarian tumors and has a proposed role in tumor invasion and migration by facilitating cell adhesion to fibronectin[42]. tTG overexpression was most recently reported to be an adverse prognostic factor in ovarian carcinoma [43]. Our analysis found that serum levels of soluble EGFR were lower in ovarian cancer patients in comparison to benign cases. Although cell surface EGFR is overexpressed in 35% to 70% of EOCs [44], it would appear from our investigation, and that of another group [45] that levels of the soluble form of EGFR present in serum are inversely correlated with ovarian cancer risk.
Figure 1. Serum Biomarkers Significant Across Ovarian Epithelial Carcinoma Subtypes.
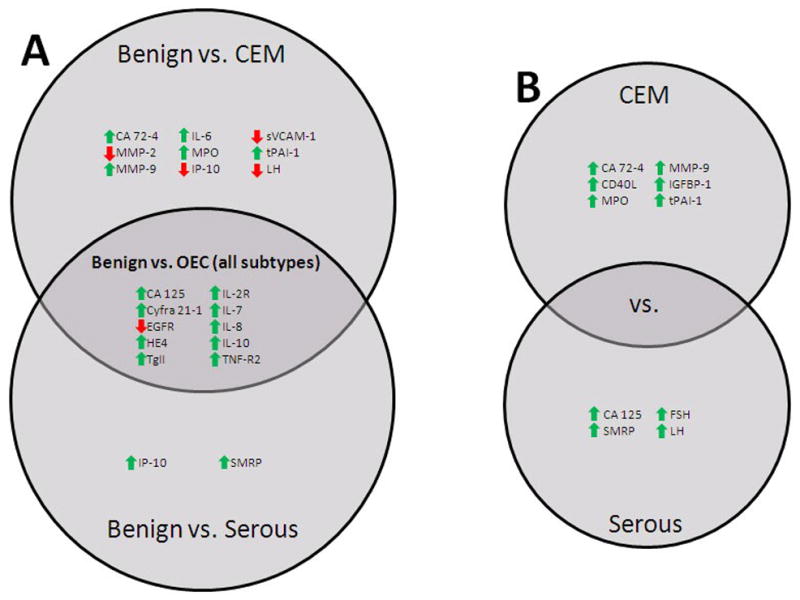
Findings presented in Table 3 are summarized. Listed biomarkers were found to differ significantly between comparison groups. Arrow preceding each biomarker name indicates increased or decreased serum concentrations in the cancer group. A. Comparison between benign cases and ovarian cancer subtypes. B. Comparison between clear cell, endometrial, and mucinous (CEM) carcinomas and serous carcinomas.
Our primary aim in this investigation was to identify biomarkers with distinct serum levels among presumed type I and type II OECs. In comparison to benign pelvic disease, OECs of the clear cell, endometrioid, and mucinous (CEM) subtypes demonstrated significant differences in nine serum biomarkers while serous carcinomas differed among only two. Among conventional tumor markers, our observations are in agreement with a previous study that found CA 72-4 to be highly specific for mucinous ovarian carcinoma while CA 125 was specific for the serous subtype [46]. Two additional emerging ovarian cancer biomarkers, IGFBP-1 and SMRP, were also found to be subtype specific, an observation not previously reported. Both of these markers have been implicated in ovarian cancer but remain uncharacterized [37, 47, 48]. Significant among the cytokines tested were IP-10 for serous carcinomas and IL-6 and sCD40L for CEM carcinomas. These differences in serum cytokine levels were not as robust as those observed for all OECs considered together, suggesting a relative uniformity in tumor behavior. Interestingly, the CEM carcinomas demonstrated higher levels of myeloperoxidase (MPO). MPO is the chief protein product of neutrophils and is believed to play a role in the production of ROS and the oxidative activation of environmental carcinogens [49, 50]. The results for the invasion, migration, and metastasis related molecules were somewhat mixed. The CEM carcinomas demonstrated relatively high serum levels of tPAI-1 and MMP-9 and relatively low levels of MMP-2 and sVCAM. Serous carcinomas did not differ significantly from the other groups for any of these markers. This is intriguing in light of the clinical observation that serous carcinomas are the most aggressive subtype of OEC and metastasize far more readily. Further investigation related to these observations would be justified. The serous carcinomas demonstrated higher serum levels of the gonadotropins LH and FSH. These hormones are important regulators of ovarian cell function and have been long implicated in the development of ovarian cancer, however the results from previous investigation concerning serum levels of the gonadotropins have been inconsistent [51]. The finding that LH and FSH play a greater role in the development of a particular histological subtype of ovarian carcinoma would be of great clinical significance.
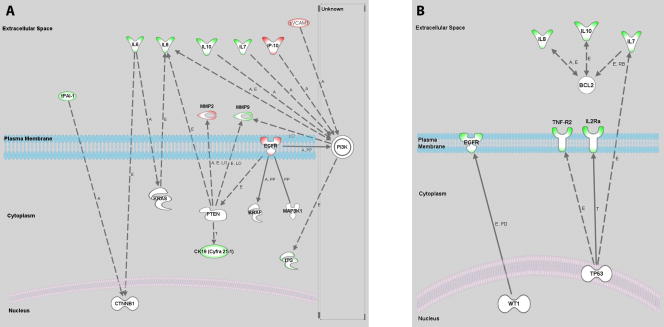
Circulating biomarkers found in the serum of ovarian cancer patients may represent factors involved in either the cause of or the systemic response to the malignancy. These factors may originate from a number of sources including the tumor itself, the surrounding stroma, or systemic tissues involved in the host response. It is crucial that ongoing work in the field of serum biomarkers is aimed at pinpointing the origins and functional roles of identified biomarkers. We sought to approach these questions by placing our findings within the broader context of genetic regulation of ovarian epithelial tumorigenesis. To that end, we utilized the Ingenuity Pathway Analysis (IPA) software package (Ingenuity Systems Inc., Redwood City, CA) to identify published relationships between the biomarkers we found to be informative and a consensus list of genetic markers currently under investigation in the field. A list of genes identified to be commonly mutated or overexpressed in CEM carcinomas includes: BRAF [10, 17], KRAS [10, 17], CTNNBI [19, 20], PTEN [19], MAP3K [52], and PI3K [52]. This list was entered into the software package along with the list of biomarkers we identified when comparing CEM carcinomas with benign cases. The IPA software identified relationships between molecules in the two groups as shown in Figure 2A. A similar analysis was performed for biomarkers we identified in our comparison of serous carcinomas with benign samples utilizing a list of genes including: AKT2 [26, 53], APOE2 [27], BCL2 [29], HLA-G [28], MK167 [29], TP53 [21–25], and WT1 [9]. The results of this analysis are shown in Figure 2B. Several of the genes examined are established players within molecular pathways widely considered to play a role in ovarian cancer. Among these are the RAS/RAF/MAP pathway and the PI-3 kinase/PTEN pathway, both of which have been implicated in type 1 ovarian carcinomas [2, 52], and the p53 pathway active in type II carcinomas[13]. The IPA analysis demonstrates that several of the serum biomarkers identified in this study have been reported to interact with members of these pathways and further study aimed at characterizing these relationships would be well warranted.
Figure 2. Ingenuity Pathway Analysis of Identified Serum Biomarkers and Reported Molecular Alterations.
The Ingenuity Pathway Analysis software package (Ingenuity Systems Inc., Redwood City, CA) was used to identify relationships between identified serum biomarkers and genetic markers associated with ovarian carcinoma subtypes. A. Interactions identified between CEM carcinoma associated serum biomarkers and the following genes: BRAF, KRAS, CTNNBI, PTEN, MAP3K, and PI3K. B. Interactions identified between serous carcinoma associated serum biomarkers and the following genes: AKT2, APOE2, BCL2, HLA-G, MK167, TP53, and WT1. Biomarker outlines: green - increased in the serum of cancer patients, red – decreased in the serum of cancer patients. Interaction labels: A – activation, E – expression, PD – protein-DNA interaction, T – transcription, PP – protein-protein interaction, LO – localization, RB – regulation of binding, solid line – direct relationship, dashed line – indirect relationship.
This investigation clearly illustrates the unique and informative role of serum profiling in advancing our understanding of ovarian tumorigenesis. Our findings suggest that several traditional and emerging tumor markers, factors involved in the host cytokine and hormonal response, and adhesion- and metastasis-related proteins may be differentially utilized among OEC histological subtypes. An improved characterization of the mechanisms and molecular interactions that characterize the emerging pathways of ovarian epithelial tumorigenesis will allow for the development of improved tools and methods to better identify and capture every clinical opportunity.
Footnotes
Conflict of Interest Statement The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.http//www.cancer.org/docroot/home/index.asp
- 2.Shih Ie M, Kurman RJ. Ovarian tumorigenesis: a proposed model based on morphological and molecular genetic analysis. Am J Pathol. 2004;164(5):1511–1518. doi: 10.1016/s0002-9440(10)63708-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Auersperg N, Wong AS, Choi KC, Kang SK, Leung PC. Ovarian surface epithelium: biology, endocrinology, and pathology. Endocr Rev. 2001;22(2):255–288. doi: 10.1210/edrv.22.2.0422. [DOI] [PubMed] [Google Scholar]
- 4.Scully R. International Hisological Classification of Tumors: Histological Typing of Ovarian Tumors. World Health Organization; Geneva: 1999. [Google Scholar]
- 5.Scully R. World Health Organization International Classification of Tumours. Springer; 1999. 1999. [Google Scholar]
- 6.Seidman JD, Horkayne-Szakaly I, Haiba M, Boice CR, Kurman RJ, Ronnett BM. The histologic type and stage distribution of ovarian carcinomas of surface epithelial origin. Int J Gynecol Pathol. 2004;23(1):41–44. doi: 10.1097/01.pgp.0000101080.35393.16. [DOI] [PubMed] [Google Scholar]
- 7.Young RH, Scully RE. Differential diagnosis of ovarian tumors based primarily on their patterns and cell types. Semin Diagn Pathol. 2001;18(3):161–235. [PubMed] [Google Scholar]
- 8.Kaku T, Ogawa S, Kawano Y, Ohishi Y, Kobayashi H, Hirakawa T, Nakano H. Histological classification of ovarian cancer. Med Electron Microsc. 2003;36(1):9–17. doi: 10.1007/s007950300002. [DOI] [PubMed] [Google Scholar]
- 9.Tan DS, Kaye S. Ovarian clear cell adenocarcinoma: a continuing enigma. J Clin Pathol. 2007;60(4):355–360. doi: 10.1136/jcp.2006.040030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singer G, Kurman RJ, Chang HW, Cho SK, Shih Ie M. Diverse tumorigenic pathways in ovarian serous carcinoma. Am J Pathol. 2002;160(4):1223–1228. doi: 10.1016/s0002-9440(10)62549-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seidman JD, Kurman RJ. Subclassification of serous borderline tumors of the ovary into benign and malignant types. A clinicopathologic study of 65 advanced stage cases. Am J Surg Pathol. 1996;20(11):1331–1345. doi: 10.1097/00000478-199611000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Smith Sehdev AE, Sehdev PS, Kurman RJ. Noninvasive and invasive micropapillary (low-grade) serous carcinoma of the ovary: a clinicopathologic analysis of 135 cases. Am J Surg Pathol. 2003;27(6):725–736. doi: 10.1097/00000478-200306000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Kurman RJ, Visvanathan K, Roden R, Wu TC, Shih Ie M. Early detection and treatment of ovarian cancer: shifting from early stage to minimal volume of disease based on a new model of carcinogenesis. Am J Obstet Gynecol. 2008;198(4):351–356. doi: 10.1016/j.ajog.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bell DA, Scully RE. Early de novo ovarian carcinoma. A study of fourteen cases Cancer. 1994;73(7):1859–1864. doi: 10.1002/1097-0142(19940401)73:7<1859::aid-cncr2820730714>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 15.Peyssonnaux C, Eychene A. The Raf/MEK/ERK pathway: new concepts of activation. Biol Cell. 2001;93(1–2):53–62. doi: 10.1016/s0248-4900(01)01125-x. [DOI] [PubMed] [Google Scholar]
- 16.Gilks CB. Subclassification of ovarian surface epithelial tumors based on correlation of histologic and molecular pathologic data. Int J Gynecol Pathol. 2004;23(3):200–205. doi: 10.1097/01.pgp.0000130446.84670.93. [DOI] [PubMed] [Google Scholar]
- 17.Singer G, Oldt R, 3rd, Cohen Y, Wang BG, Sidransky D, Kurman RJ, Shih Ie M. Mutations in BRAF and KRAS characterize the development of low-grade ovarian serous carcinoma. J Natl Cancer Inst. 2003;95(6):484–486. doi: 10.1093/jnci/95.6.484. [DOI] [PubMed] [Google Scholar]
- 18.Mayr D, Hirschmann A, Lohrs U, Diebold J. KRAS and BRAF mutations in ovarian tumors: a comprehensive study of invasive carcinomas, borderline tumors and extraovarian implants. Gynecol Oncol. 2006;103(3):883–887. doi: 10.1016/j.ygyno.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 19.Obata K, Morland SJ, Watson RH, Hitchcock A, Chenevix-Trench G, Thomas EJ, Campbell IG. Frequent PTEN/MMAC mutations in endometrioid but not serous or mucinous epithelial ovarian tumors. Cancer Res. 1998;58(10):2095–2097. [PubMed] [Google Scholar]
- 20.Palacios J, Gamallo C. Mutations in the beta-catenin gene (CTNNB1) in endometrioid ovarian carcinomas. Cancer Res. 1998;58(7):1344–1347. [PubMed] [Google Scholar]
- 21.Chan WY, Cheung KK, Schorge JO, Huang LW, Welch WR, Bell DA, Berkowitz RS, Mok SC. Bcl-2 and p53 protein expression, apoptosis, and p53 mutation in human epithelial ovarian cancers. Am J Pathol. 2000;156(2):409–417. doi: 10.1016/S0002-9440(10)64744-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kohler MF, Marks JR, Wiseman RW, Jacobs IJ, Davidoff AM, Clarke-Pearson DL, Soper JT, Bast RC, Jr, Berchuck A. Spectrum of mutation and frequency of allelic deletion of the p53 gene in ovarian cancer. J Natl Cancer Inst. 1993;85(18):1513–1519. doi: 10.1093/jnci/85.18.1513. [DOI] [PubMed] [Google Scholar]
- 23.Kupryjanczyk J, Thor AD, Beauchamp R, Merritt V, Edgerton SM, Bell DA, Yandell DW. p53 gene mutations and protein accumulation in human ovarian cancer. Proc Natl Acad Sci U S A. 1993;90(11):4961–4965. doi: 10.1073/pnas.90.11.4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berchuck A, Carney M. Human ovarian cancer of the surface epithelium. Biochem Pharmacol. 1997;54(5):541–544. doi: 10.1016/s0006-2952(97)00061-0. [DOI] [PubMed] [Google Scholar]
- 25.Wen WH, Reles A, Runnebaum IB, Sullivan-Halley J, Bernstein L, Jones LA, Felix JC, Kreienberg R, el-Naggar A, Press MF. p53 mutations and expression in ovarian cancers: correlation with overall survival. Int J Gynecol Pathol. 1999;18(1):29–41. doi: 10.1097/00004347-199901000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Bellacosa A, de Feo D, Godwin AK, Bell DW, Cheng JQ, Altomare DA, Wan M, Dubeau L, Scambia G, Masciullo V, et al. Molecular alterations of the AKT2 oncogene in ovarian and breast carcinomas. Int J Cancer. 1995;64(4):280–285. doi: 10.1002/ijc.2910640412. [DOI] [PubMed] [Google Scholar]
- 27.Singer G, Rebmann V, Chen YC, Liu HT, Ali SZ, Reinsberg J, McMaster MT, Pfeiffer K, Chan DW, Wardelmann E, et al. HLA-G is a potential tumor marker in malignant ascites. Clin Cancer Res. 2003;9(12):4460–4464. [PubMed] [Google Scholar]
- 28.Chen YC, Pohl G, Wang TL, Morin PJ, Risberg B, Kristensen GB, Yu A, Davidson B, Shih Ie M. Apolipoprotein E is required for cell proliferation and survival in ovarian cancer. Cancer Res. 2005;65(1):331–337. [PubMed] [Google Scholar]
- 29.O’Neill CJ, Deavers MT, Malpica A, Foster H, McCluggage WG. An immunohistochemical comparison between low-grade and high-grade ovarian serous carcinomas: significantly higher expression of p53, MIB1, BCL2, HER-2/neu, and C-KIT in high-grade neoplasms. Am J Surg Pathol. 2005;29(8):1034–1041. [PubMed] [Google Scholar]
- 30.Gorelik E, Landsittel DP, Marrangoni AM, Modugno F, Velikokhatnaya L, Winans MT, Bigbee WL, Herberman RB, Lokshin AE. Multiplexed immunobead-based cytokine profiling for early detection of ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2005;14(4):981–987. doi: 10.1158/1055-9965.EPI-04-0404. [DOI] [PubMed] [Google Scholar]
- 31.University of Pittsburgh Cancer Institute Luminex Core Facility [http://www.upci.upmc.edu/facilities/luminex/sources.html]
- 32.Nash MA, Ferrandina G, Gordinier M, Loercher A, Freedman RS. The role of cytokines in both the normal and malignant ovary. Endocr Relat Cancer. 1999;6(1):93–107. doi: 10.1677/erc.0.0060093. [DOI] [PubMed] [Google Scholar]
- 33.Nilsson MB, Langley RR, Fidler IJ. Interleukin-6, secreted by human ovarian carcinoma cells, is a potent proangiogenic cytokine. Cancer Res. 2005;65(23):10794–10800. doi: 10.1158/0008-5472.CAN-05-0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsai JP, Chen HW, Cheng ML, Liu HK, Lee YP, Hsieh CL, Luh KT, Wu CW, Hsu LH, Chao TY, et al. Analysis of host versus tumor interaction in cancer patients: opposing role of transforming growth factor-beta1 and interleukin-6 in the development of in situ tumor immunity. Immunobiology. 2005;210(9):661–671. doi: 10.1016/j.imbio.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 35.Watson JM, Sensintaffar JL, Berek JS, Martinez-Maza O. Constitutive production of interleukin 6 by ovarian cancer cell lines and by primary ovarian tumor cultures. Cancer Res. 1990;50(21):6959–6965. [PubMed] [Google Scholar]
- 36.Lambeck AJ, Crijns AP, Leffers N, Sluiter WJ, ten Hoor KA, Braid M, van der Zee AG, Daemen T, Nijman HW, Kast WM. Serum cytokine profiling as a diagnostic and prognostic tool in ovarian cancer: a potential role for interleukin 7. Clin Cancer Res. 2007;13(8):2385–2391. doi: 10.1158/1078-0432.CCR-06-1828. [DOI] [PubMed] [Google Scholar]
- 37.Moore RG, Brown AK, Miller MC, Badgwell D, Lu Z, Allard WJ, Granai CO, Bast RC, Jr, Lu K. Utility of a novel serum tumor biomarker HE4 in patients with endometrioid adenocarcinoma of the uterus. Gynecol Oncol. 2008 doi: 10.1016/j.ygyno.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moore RG, Brown AK, Miller MC, Skates S, Allard WJ, Verch T, Steinhoff M, Messerlian G, DiSilvestro P, Granai CO, et al. The use of multiple novel tumor biomarkers for the detection of ovarian carcinoma in patients with a pelvic mass. Gynecol Oncol. 2008;108(2):402–408. doi: 10.1016/j.ygyno.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 39.Scholler N, Crawford M, Sato A, Drescher CW, O’Briant KC, Kiviat N, Anderson GL, Urban N. Bead-based ELISA for validation of ovarian cancer early detection markers. Clin Cancer Res. 2006;12(7 Pt 1):2117–2124. doi: 10.1158/1078-0432.CCR-05-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Drapkin R, von Horsten HH, Lin Y, Mok SC, Crum CP, Welch WR, Hecht JL. Human epididymis protein 4 (HE4) is a secreted glycoprotein that is overexpressed by serous and endometrioid ovarian carcinomas. Cancer Res. 2005;65(6):2162–2169. doi: 10.1158/0008-5472.CAN-04-3924. [DOI] [PubMed] [Google Scholar]
- 41.Wang K, Gan L, Jeffery E, Gayle M, Gown AM, Skelly M, Nelson PS, Ng WV, Schummer M, Hood L, et al. Monitoring gene expression profile changes in ovarian carcinomas using cDNA microarray. Gene. 1999;229(1–2):101–108. doi: 10.1016/s0378-1119(99)00035-9. [DOI] [PubMed] [Google Scholar]
- 42.Satpathy M, Cao L, Pincheira R, Emerson R, Bigsby R, Nakshatri H, Matei D. Enhanced peritoneal ovarian tumor dissemination by tissue transglutaminase. Cancer Res. 2007;67(15):7194–7202. doi: 10.1158/0008-5472.CAN-07-0307. [DOI] [PubMed] [Google Scholar]
- 43.Hwang JY, Mangala LS, Fok JY, Lin YG, Merritt WM, Spannuth WA, Nick AM, Fiterman DJ, Vivas-Mejia PE, Deavers MT, et al. Clinical and biological significance of tissue transglutaminase in ovarian carcinoma. Cancer Res. 2008;68(14):5849–5858. doi: 10.1158/0008-5472.CAN-07-6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bartlett JM, Langdon SP, Simpson BJ, Stewart M, Katsaros D, Sismondi P, Love S, Scott WN, Williams AR, Lessells AM, et al. The prognostic value of epidermal growth factor receptor mRNA expression in primary ovarian cancer. Br J Cancer. 1996;73 (3):301–306. doi: 10.1038/bjc.1996.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baron AT, Cora EM, Lafky JM, Boardman CH, Buenafe MC, Rademaker A, Liu D, Fishman DA, Podratz KC, Maihle NJ. Soluble epidermal growth factor receptor (sEGFR/sErbB1) as a potential risk, screening, and diagnostic serum biomarker of epithelial ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2003;12(2):103–113. [PubMed] [Google Scholar]
- 46.Hasholzner U, Baumgartner L, Stieber P, Meier W, Reiter W, Pahl H, Fateh-Moghadam A. Clinical significance of the tumour markers CA 125 II and CA 72-4 in ovarian carcinoma. Int J Cancer. 1996;69(4):329–334. doi: 10.1002/(SICI)1097-0215(19960822)69:4<329::AID-IJC16>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 47.Hofmann J, Wegmann B, Hackenberg R, Kunzmann R, Schulz KD, Havemann K. Production of insulin-like growth factor binding proteins by human ovarian carcinoma cells. J Cancer Res Clin Oncol. 1994;120(3):137–142. doi: 10.1007/BF01202191. [DOI] [PubMed] [Google Scholar]
- 48.Fowler DJ, Nicolaides KH, Miell JP. Insulin-like growth factor binding protein-1 (IGFBP-1): a multifunctional role in the human female reproductive tract. Hum Reprod Update. 2000;6(5):495–504. doi: 10.1093/humupd/6.5.495. [DOI] [PubMed] [Google Scholar]
- 49.Klebanoff SJ. Myeloperoxidase: friend and foe. J Leukoc Biol. 2005;77(5):598–625. doi: 10.1189/jlb.1204697. [DOI] [PubMed] [Google Scholar]
- 50.Knaapen AM, Gungor N, Schins RP, Borm PJ, Van Schooten FJ. Neutrophils and respiratory tract DNA damage and mutagenesis: a review. Mutagenesis. 2006;21 (4):225–236. doi: 10.1093/mutage/gel032. [DOI] [PubMed] [Google Scholar]
- 51.Choi JH, Wong AS, Huang HF, Leung PC. Gonadotropins and ovarian cancer. Endocr Rev. 2007;28(4):440–461. doi: 10.1210/er.2006-0036. [DOI] [PubMed] [Google Scholar]
- 52.Landen CN, Jr, Birrer MJ, Sood AK. Early events in the pathogenesis of epithelial ovarian cancer. J Clin Oncol. 2008;26(6):995–1005. doi: 10.1200/JCO.2006.07.9970. [DOI] [PubMed] [Google Scholar]
- 53.Cheng JQ, Godwin AK, Bellacosa A, Taguchi T, Franke TF, Hamilton TC, Tsichlis PN, Testa JR. AKT2, a putative oncogene encoding a member of a subfamily of protein-serine/threonine kinases, is amplified in human ovarian carcinomas. Proc Natl Acad Sci U S A. 1992;89(19):9267–9271. doi: 10.1073/pnas.89.19.9267. [DOI] [PMC free article] [PubMed] [Google Scholar]