Abstract
Purpose: To evaluate in vivo whole-body biodistribution of microbubbles (MBs) targeted to tumor angiogenesis–related vascular endothelial growth factor (VEGF) receptor 2 (VEGFR2) by using dynamic micro–positron emission tomography (PET) in living mice.
Materials and Methods: Animal protocols were approved by the Institutional Administrative Panel on Laboratory Animal Care. Lipid-shell perfluorocarbon-filled MBs, targeted to VEGFR2 via anti-VEGFR2 antibodies, were radiolabeled by conjugating the radiofluorination agent N-succinimidyl-4-[18F]fluorobenzoate (SFB) to the anti-VEGFR2 antibodies. These MBs were then injected intravenously into nude mice (n = 4) bearing angiosarcomas, and the whole-body biodistribution of these probes was assessed for 60 minutes by using dynamic micro-PET. Results were compared with ex vivo gamma counting (n = 6) and immunofluorescence staining (n = 6). Control studies in angiosarcoma-bearing mice were performed with injection of the radiolabeled antibodies alone (n = 3) or free SFB (n = 3). A mixed-effects regression of MB accumulation on fixed effects of time and tissue type (tumor or muscle) and random effect of animal was performed.
Results: VEGFR2-targeted MBs rapidly cleared from the blood circulation (50% blood clearance after approximately 3.5 minutes) and accumulated in the liver (mean, 33.4% injected dose [ID]/g ± 13.7 [standard deviation] at 60 minutes) and spleen (mean, 9.3% ID/g ± 6.5 at 60 minutes) on the basis of micro-PET imaging. These findings were confirmed with ex vivo gamma counting. Uptake of targeted MBs was significantly higher (P < .0001) in tumor than in adjacent skeletal muscle tissue. Immunofluorescence staining demonstrated accumulation of the targeted MBs within hepatic Kupffer cells and splenic macrophages. Biodistribution of the radiolabeled antibodies and free SFB differed from the distribution of the targeted MBs.
Conclusion: Dynamic micro-PET allows assessment of in vivo biodistribution of VEGFR2-targeted MBs.
© RSNA, 2008
Contrast material–enhanced ultrasonography (US) with targeted microbubbles (MBs) is rapidly emerging as a noninvasive and quantitative molecular imaging modality that combines the advantages of high spatial resolution, real-time imaging, and lack of ionizing radiation (1,2). These benefits have made contrast-enhanced US with targeted MBs an attractive imaging platform that is now rapidly finding its niche among other molecular imaging strategies for preclinical research. MBs are gas-filled echogenic US contrast agents that can be targeted to specific molecular markers by means of the attachment of appropriate ligands to the surface of the MBs. When these functionalized MBs are injected intravenously, they distribute throughout the whole body and attach at tissue sites expressing the targeted molecular marker, leading to a local increase of the US imaging signal (1–6).
Because of their size of several micrometers, MBs stay predominantly within the vascular system after intravenous administration. This factor makes them most appropriate for imaging events within the vascular compartment, such as inflammation, thrombus formation, and angiogenesis. Recently, tumor angiogenesis imaging with contrast-enhanced US has been explored with MBs targeted to αvβ3 integrin, endoglin, and vascular endothelial growth factor (VEGF) receptor 2 (VEGFR2) (3,4,6). Among other regulators, VEGFR2 is one of the major regulators of tumor angiogenesis, and activation of the VEGF/VEGFR2 axis triggers multiple signaling pathways that result in endothelial cell survival, mitogenesis, migration, differentiation, and alterations in vascular permeability (7). Because of this central role in tumor angiogenesis, a US strategy that can directly image VEGFR2 would be particularly helpful for tracking antiangiogenic tumoricidal treatments in the development of cancer therapies. Furthermore, with the addition of payloads, such as chemotherapeutic drugs or suicide genes in or onto these functionalized MBs, the role of these agents may be extended beyond imaging expression levels of molecular markers to providing target-specific therapeutic approaches at the site of these markers in living subjects (8).
Biodistribution studies of newer imaging or therapeutic agents are of paramount importance for assessment of their temporal and spatial pharmacokinetics, evaluation of their usefulness in imaging or treatment at specific target sites, the uncovering of possible side effects, as well as development of further improvements in their characteristics. Researchers in two studies (9,10) have evaluated biodistribution of nontargeted MBs in rats, pigs, and humans. To our knowledge, biodistribution of targeted MBs in living animals by using positron emission tomography (PET) has not been studied to date.
Small-animal PET imaging (micro-PET) for assessment of biodistribution has a number of advantages in comparison with traditional ex vivo biodistribution studies (which require sacrificing of large numbers of animals at several times) in that serial imaging of the same animal can be performed in vivo at theoretically unlimited times without euthanizing the animal (11). In addition, since counterparts of micro-PET are widely available for clinical purposes, use of micro-PET for pharmacokinetic studies early in the imaging probe and/or drug development process can be directly translated from small animals to large animals and finally to patients.
Thus, the purpose of this study was to evaluate the in vivo whole-body biodistribution of MBs targeted to the tumor angiogenesis–related receptor VEGFR2 by using dynamic micro-PET in living mice.
MATERIALS AND METHODS
VisualSonics (Toronto, Canada) provided the MBs used in this study. All authors who are not consultants of VisualSonics had control of inclusion of any data and information that might present a conflict of interest for the author who is a consultant of VisualSonics (S.S.G.).
Preparation of Targeted MBs
Lipid-shell MBs containing perfluorocarbon (MicroMarker; Bracco Research, Geneva, Switzerland) were prepared according to the manufacturer's instructions. These MBs have a mean diameter of 1.5 μm (range, 1–2 μm) as assessed by a cell counter (Multisizer III Coulter; Beckman Coulter, Fullerton, Calif) and contain approximately 7600 molecules of streptavidin per square micrometer (6). MBs targeted to tumor angiogenesis, or anti-VEGFR2–targeted MBs, were prepared by attaching radiolabeled rat antimouse VEGFR2 monoclonal antibodies (Avas 12a1; eBioscience, San Diego, Calif) by using biotin-streptavidin interactions, resulting in approximately 6000 ligands per square micrometer of surface area (6). Excess unbound radiolabeled antibodies were removed by centrifuging at 1200 rpm for 2 minutes, retaining the MBs collected at the surface, and were reconstituted in phosphate-buffered saline. This washing procedure was repeated twice.
Radiolabeling of Targeted MBs
The radiofluorination agent N-succinimidyl-4-[18F]fluorobenzoate (SFB) was prepared as described elsewhere (12). SFB (specific activity, 200–250 GBq/μmol), dissolved in 100 μL of dimethyl sulfoxide, was added to the anti-VEGFR2 antibodies (300 μg), was dissolved in Na2HPO4 buffer (900 μL, pH 8.0), and reacted for 1 hour at 40°C. The reaction solution was then purified by using a desalting column (PD-10; GE Healthcare, Piscataway, NJ) to remove unconjugated SFB.
Cell Line and Tumor Model
Mouse angiosarcoma (SVR) cells (American Tissue Type Collection, Manassas, Va) were grown in high-glucose Dulbecco's modified Eagle's medium, 4.5 g/L glucose with l-glutamine (Invitrogen, Carlsbad, Calif), supplemented with 10% fetal bovine serum, penicillin (100 U/mL), and streptomycin (100 μg/mL). SVR cells (5 × 106) were harvested by using trypsinization at 80%–90% confluence, were resuspended in 50 μL of phosphate-buffered saline, and were subcutaneously injected into the flank or shoulder region of 22 6–8-week-old female nude mice (Charles River Laboratories, Wilmington, Mass). Tumors were allowed to grow to a mean maximum diameter of 10 mm (range, 8–11 mm). All animal protocols were approved by the Institutional Administrative Panel on Laboratory Animal Care.
Dynamic Micro-PET
Dynamic micro-PET was performed in a total of 10 tumor-bearing mice by using a micro-PET system (Concorde R4; Siemens Medical Solutions, Malvern, Pa). In animals, anesthesia was maintained with 2% isoflurane (Aerrane; Baxter, Deerfield, Ill) in 2 L/min oxygen, and imaging was performed in the prone position. Image acquisition was started immediately prior to injection of 2.1 MBq fluorine 18 (18F)-labeled anti-VEGFR2–targeted MBs (n = 4) into the tail vein. List-mode data acquired after injection were rebinned into a sequence of four 15-second, 10 1-minute, and then 10 5-minute data frames. Image reconstruction was performed by using the ordered-subsets expectation maximization algorithm with a spatial resolution of 1.66–1.85 mm (13). No attenuation or partial volume corrections were performed.
To confirm that radiolabeled antibodies remained on the MB shells for the time of the experiments, 3.7 MBq 18F-labeled antibodies alone (without MBs) was injected in an additional three mice. To further confirm that SFB was stably bound to the MB-antibody complex, 3.9 MBq SFB was injected in an additional three mice. Dynamic micro-PET in these control experiments was performed according to the protocol described previously.
Image Analysis of Micro-PET Images
Imaging data analysis was performed by using nonproprietary PET analysis software (Amide, version 8.2) (14). Three-dimensional regions of interest (mean diameter, 1.5 mm; range, 1–2.5 mm) were placed over the brain, lung, heart (to estimate activity in the blood pool), liver, spleen, kidneys, gut, muscle, and tumor on decay-corrected PET images. Percentage of injected dose per gram values were calculated by means of a calibration constant obtained from scanning a cylindrical phantom in the small-animal PET scanner, with assumption of a tissue density of 1 g/mL, and dividing by the injected dose that was decay corrected to the time of scanning.
Traditional ex Vivo Biodistribution
To confirm findings from in vivo micro-PET, an additional six tumor-bearing mice were euthanized at two times after injection of radiolabeled MBs (n = 3 at 4 minutes and n = 3 at 60 minutes). The following were collected in all animals: brain, lung, heart, liver, spleen, kidneys, small bowel, large bowel, muscle, bone (femur), and tumor tissue and blood. These tissues and blood were weighed and radioactivity was counted for 1 minute in a well gamma counter (Cobra II; Packard/Perkin Elmer, Wellesley, Mass). Results were expressed as percentage of injected radioactivity dose per gram of tissue.
Immunofluorescence Staining
To further confirm findings from in vivo micro-PET, an additional six tumor-bearing mice received an injection of radiolabeled targeted MBs into the tail vein and were euthanized after 4 minutes (n = 3) and 60 minutes (n = 3). The brain, lung, heart, liver, spleen, kidneys, small bowel, large bowel, muscle, and tumor tissues were harvested, embedded in optical cutting temperature compound (Sakura Finetek, Torrance, Calif), and flash frozen in chilled 2-methyl butane. Frozen blocks were sectioned at 10 μm and mounted on glass slides for immunofluorescence staining. MBs were visualized by using Texas Red–conjugated rabbit antistreptavidin primary antibody (Abcam, Cambridge, Mass), in a dilution of 1:100, that recognized streptavidin on the shell of MBs. A double-staining procedure was employed to demonstrate the location of MBs relative to Kupffer cells and macrophages in liver and spleen, respectively, by using a primary monoclonal rat antimouse F4/80 antibody (isotype IgG2b; AbD Serotec, Raleigh, NC), in a dilution of 1:100, and a secondary fluorescein isothiocyanate–labeled, polyclonal goat antirat IgG2b antibody (AbD Serotec), in a dilution of 1:100. Microscopy (Axiophot; Carl Zeiss, Thornwood, NY) was performed, and fluorescent images were acquired with a digital camera (AxioCam; Carl Zeiss, Bernried, Germany).
Statistical Analysis
Data are given as the mean ± standard deviation. A mixed-effects regression of MB accumulation on fixed effects of time and tissue type (tumor or muscle) and random effect of animal was performed. Only data after the 1st minute were used, to eliminate the transient effect of the initial uptake. All statistical analyses were performed with software (Stata, release 9.2; Stata, College Station, Tex), and a difference with P < .05 was considered significant.
RESULTS
Dynamic Micro-PET of Targeted MBs in Living Mice
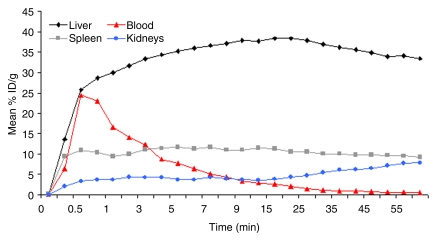
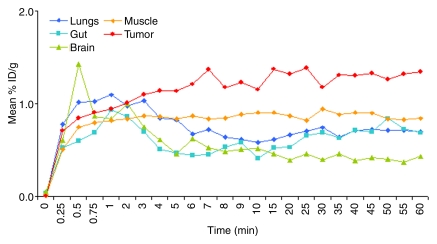
MBs rapidly accumulated in both the liver and spleen, and uptake values in both organs remained almost constant for the entire imaging period (Fig 1). In the liver, mean uptake values were 34.4% injected dose (ID)/g ± 7.3 at 4 minutes and 33.4% ID/g ± 13.7 at 60 minutes. Mean uptake values in the spleen were 11.4% ID/g ± 10.1 at 4 minutes and 9.3% ID/g ± 6.5 at 60 minutes. Radiolabeled MBs rapidly cleared from the blood circulation (50% blood clearance after approximately 3.5 minutes). After an initial mean peak of 24.5% ID/g ± 1.1 in the blood after 30 seconds, radioactivity decreased to a mean of 8.8% ID/g ± 0.4 at 4 minutes and further decreased to 0.5% ID/g ± 0.3 at 60 minutes after intravenous administration (Fig 1). In the kidneys, uptake increased from a mean of 4.3% ID/g ± 3.8 at 4 minutes to 7.8% ID/g ± 3.9 at 60 minutes (Fig 1). In the lungs (mean, 0.85% ID/g ± 0.1 at 4 minutes and 0.7% ID/g ± 0.2 at 60 minutes), gut (mean, 0.51% ID/g ± 0.16 at 4 minutes and 0.69% ID/g ± 0.08 at 60 minutes), and brain (mean, 0.61% ID/g ± 0.15 at 4 minutes and 0.44% ID/g ± 0.24 at 60 minutes), radioactivity minimally increased after administration of radiolabeled targeted MBs (Fig 2). At several minutes after administration of tumor angiogenesis–targeted MBs, uptake was significantly higher (P < .0001) in subcutaneous tumor tissue (mean, 1.14% ID/g ± 0.41 at 4 minutes and 1.35% ID/g ± 0.16 at 60 minutes) than in adjacent skeletal muscle tissue (mean, 0.84% ID/g ± 0.53 at 4 minutes and 0.84% ID/g ± 0.58 at 60 minutes) (Fig 2).
Figure 1:
In vivo biodistribution of intravenously injected 18F-labeled targeted MBs measured by using region-of-interest–based analysis of dynamic micro-PET data sets in nude mice (n = 4). Rapid uptake and retention of targeted MBs were observed in both liver and spleen. Targeted MBs cleared quickly from blood, with almost background levels observed 30 minutes after intravenous administration of imaging agent. Error bars were omitted for better visibility.
Figure 2:
In vivo biodistribution of intravenously injected 18F-labeled targeted MBs in lung, gut, brain, muscle, and tumor measured by using region-of-interest–based analysis of dynamic micro-PET data sets in nude mice (n = 4). Retention of tumor angiogenesis–targeted MBs was higher in tumor tissue than in surrounding muscle tissue. Error bars were omitted for better visibility.
To confirm stability of both the MB-antibody interaction and the radiolabeling with SFB in vivo, biodistribution of both radiolabeled antibodies alone and free SFB was assessed by using micro-PET. Highest mean uptake values of radiolabeled antibodies were found in the liver (29.3% ID/g ± 1.9 at 4 minutes and 30.2% ID/g ± 8.4 at 60 minutes) and in the blood (28.5% ID/g ± 2.7 at 4 minutes). Compared with radiolabeled targeted MBs, radiolabeled antibodies cleared slowly from the blood with high blood pool mean values persisting at 60 minutes after injection (20.8% ID/g ± 1.8) (Fig 3). Furthermore, there was high accumulation of radiolabeled antibodies in the kidneys (mean, 15.1% ID/g ± 4.1 at 4 minutes and 25.4 ± 7.7%ID/g at 60 minutes). Biodistribution patterns were also different after injection of free SFB. Free SFB rapidly cleared through the kidneys (mean, 47.9% ID/g ± 3.7 at 4 minutes and 72.7% ID/g ± 26.3 at 60 minutes after injection) (Fig 3). In contrast to both radiolabeled targeted MBs and antibodies alone, hepatic uptake of free SFB was low (mean, 6.9% ID/g ± 5.2 at 4 minutes and 5.2% ID/g ± 2.9 at 60 minutes).
Figure 3a:
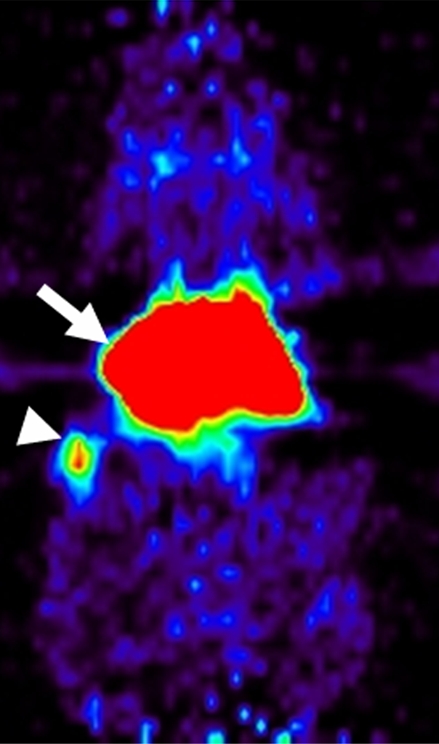
Coronal micro-PET images showed different whole-body biodistribution patterns among (a) radiolabeled targeted MBs, (b) radiolabeled antibodies alone, and (c) free SFB in living mouse. (a) Targeted MBs accumulated primarily in liver (arrow) and spleen (arrowhead) at 60 minutes after intravenous injection. (b) Radiolabeled antibodies alone showed high activity retained in blood pool (thin arrows) even after 60 minutes. Uptake in supraaortic vessels (arrowheads) and liver (thick arrow) were observed. (c) Free SFB cleared through kidneys (arrows).
Figure 3b:
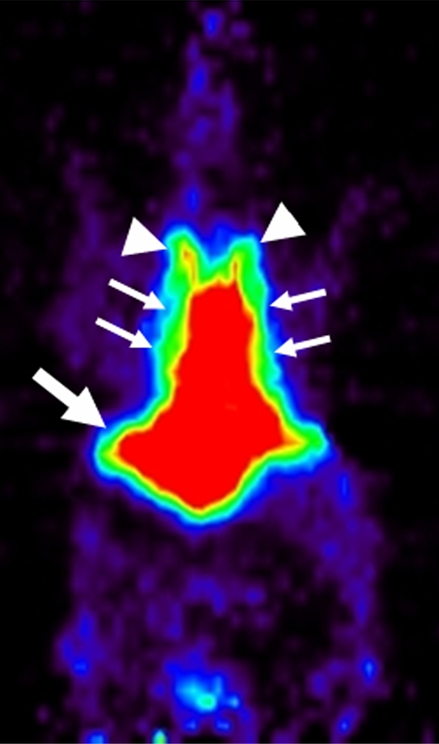
Coronal micro-PET images showed different whole-body biodistribution patterns among (a) radiolabeled targeted MBs, (b) radiolabeled antibodies alone, and (c) free SFB in living mouse. (a) Targeted MBs accumulated primarily in liver (arrow) and spleen (arrowhead) at 60 minutes after intravenous injection. (b) Radiolabeled antibodies alone showed high activity retained in blood pool (thin arrows) even after 60 minutes. Uptake in supraaortic vessels (arrowheads) and liver (thick arrow) were observed. (c) Free SFB cleared through kidneys (arrows).
Figure 3c:
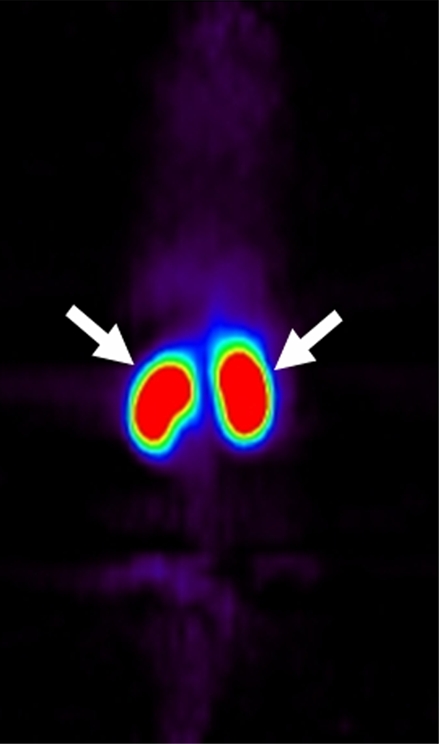
Coronal micro-PET images showed different whole-body biodistribution patterns among (a) radiolabeled targeted MBs, (b) radiolabeled antibodies alone, and (c) free SFB in living mouse. (a) Targeted MBs accumulated primarily in liver (arrow) and spleen (arrowhead) at 60 minutes after intravenous injection. (b) Radiolabeled antibodies alone showed high activity retained in blood pool (thin arrows) even after 60 minutes. Uptake in supraaortic vessels (arrowheads) and liver (thick arrow) were observed. (c) Free SFB cleared through kidneys (arrows).
Traditional ex Vivo Biodistribution Studies of Targeted MBs
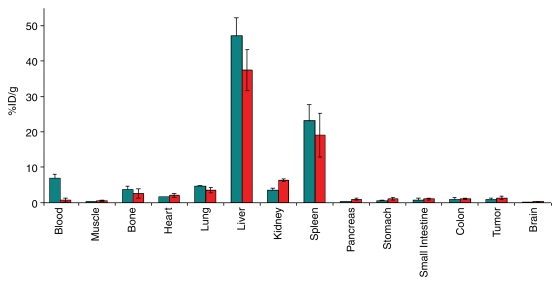
Traditional ex vivo biodistribution experiments confirmed the trend of in vivo biodistribution studies, with the highest retention of targeted MBs seen in both liver and spleen (Fig 4). In liver and spleen, mean values for radioactivity uptake were 47.3% ID/g ± 5.1 and 23.3% ID/g ± 4.3, respectively, at 4 minutes and 37.4% ID/g ± 5.7 and 19.1% ID/g ± 6.2, respectively, at 60 minutes. Uptake in kidneys increased from a mean of 3.5% ID/g ± 0.6 at 4 minutes to a mean of 6.3% ID/g ± 0.4 at 60 minutes. All other organs showed relatively low uptake at traditional ex vivo biodistribution studies at all times after targeted MB administration.
Figure 4:
Traditional ex vivo biodistribution studies in nude mice at 4 minutes (n = 3; teal bars) and 60 minutes (n = 3; red bars) after intravenous administration of targeted MBs. Ex vivo biodistribution confirmed trends seen in in vivo biodistribution study, with highest retention of targeted MBs in both liver and spleen. Bars are means; error bars are standard deviations.
Immunofluorescence Staining
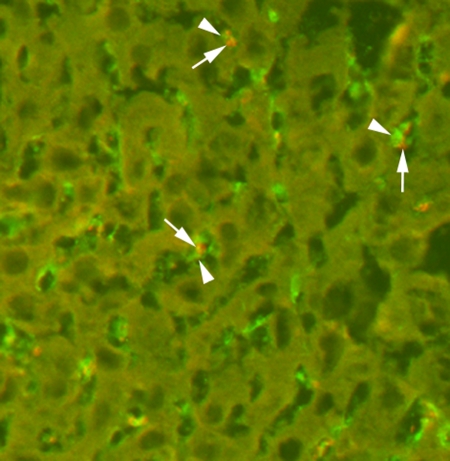
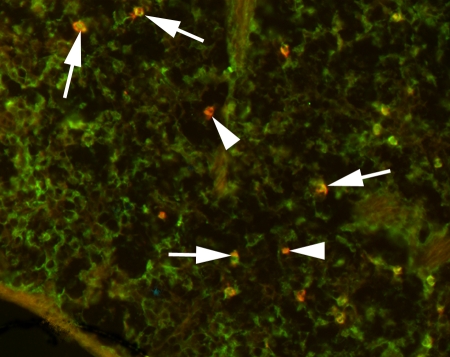
Targeted MBs were localized within Kupffer cells in the liver as soon as 4 minutes after intravenous injection (Fig 5) and were still visible in Kupffer cells after 60 minutes. In the spleen, MBs accumulated in splenic sinusoids and were also partially taken up by splenic macrophages (Fig 6). In all other organ tissues, no MBs or only isolated MBs were found.
Figure 5:
Immunofluorescence staining of liver tissue for MBs (arrows) and Kupffer cells (arrowheads). Targeted MBs were located within Kupffer cells as soon as 4 minutes after intravenous administration. For MB staining, slices were incubated with Texas red–conjugated rabbit antistreptavidin primary antibody (red). For Kupffer cell staining, slices were incubated with primary monoclonal rat antimouse F4/80 antibody and secondary fluorescein isothiocyanate–labeled polyclonal goat antirat IgG2b antibody (green). (Original magnification, ×400.)
Figure 6:
Immunofluorescence staining of spleen 4 minutes after intravenous administration of targeted MBs. Most of MBs (red areas, arrows) are localized in splenic macrophages (green areas); some MBs remain free in splenic sinusoids (arrowheads). MBs were stained with Texas red–conjugated rabbit antistreptavidin primary antibody and macrophages were visualized with primary monoclonal rat antimouse F4/80 antibody and secondary fluorescein isothiocyanate–labeled polyclonal goat antirat IgG2b antibody. (Original magnification, ×400.)
DISCUSSION
In this study, we evaluated biodistribution of targeted MBs in living mice by using dynamic micro-PET. Our results show fast clearance of targeted MBs from the blood and rapid uptake and retention of targeted MBs in both the liver and spleen. In the liver, targeted MBs are phagocytosed by Kupffer cells within several minutes after intravenous administration. In the spleen, targeted MBs are taken up by splenic macrophages.
Researchers in only a few studies have previously evaluated the biodistribution of MBs. Walday et al (10) used traditional ex vivo biodistribution techniques to study the organ distribution of first-generation, nontargeted air-filled iodine 125–labeled albumin microspheres (Albunex) in both rats and pigs up to 90 minutes after intravenous administration. Three minutes after administration in rats, about 60% of the radiolabeled albumin microspheres were recovered in the liver, 9% of them were recovered in the spleen, and 5% of them were recovered in the lungs, with only small amounts in other organs such as the brain, heart, and kidneys (10). In contrast, in pigs, more than 90% of the albumin microspheres were recovered in the lungs, most likely because of the presence of intravascular macrophages in the porcine lungs (10). In another study, the biodistribution of air-filled iodine 123–labeled human serum albumin microspheres (Quantison) was studied in humans for 58 hours by using scintigraphic imaging (9). One hour after intravenous administration, the greatest amount of radiolabeled microsphere uptake was in the liver (41.8%), followed by the spleen (11%) and the lungs (3.4%) (9). Small amounts of radiotracer were also visible in bone and heart tissue in that study (9). In the work presented here, we provide data in addition to data in these prior biodistribution studies by studying the biodistribution of newer-generation perfluorocarbon-filled lipid-shelled MBs targeted to the angiogenesis marker VEGFR2 with in vivo micro-PET.
After the MBs have been made “functional” (eg, by adding antibodies against VEGFR2 to target tumor angiogenesis, as done in our study), specific signal enhancement through accumulation of targeted MBs at sites of tumor angiogenesis can be measured by using dedicated US sequences (3,5,6). To quantify even subtle changes in the expression levels of these molecular markers by using targeted US, it is of paramount importance that the US imaging signal from MBs attached to the specific molecular markers can be clearly differentiated from background imaging signal in the surrounding normal tissue. Delineating subtle molecular signals from high background signals may be challenging in tissues with high levels of nonspecific MB accumulation. Therefore, accurate knowledge of the in vivo biodistribution of targeted MBs prior to designing targeted contrast-enhanced US strategies is crucial.
In our study we found that, on the basis of results with vivo micro-PET, on average, 34.4% ID/g of the radiolabeled targeted MBs were trapped in the liver as early as 4 minutes after tail vein administration, with the spleen retaining, on average, 11.4% ID/g of the targeted MBs at 4 minutes after injection. Uptake values from targeted MBs in other organs were relatively low in our study, suggesting that in organs such as the brain, intestine, pancreas, or muscle, tumor angiogenesis–specific imaging signal can be readily obtained by using targeted contrast-enhanced US because of the low background distribution levels of targeted MBs in these tissue types. In contrast, US-targeted imaging of tumor angiogenesis in the liver and spleen may be more challenging as a result of the high background accumulation levels of targeted MBs in these tissues.
In the kidneys, accumulation of the VEGFR2-targeted MBs was also relatively high, with an uptake value of 7.8% ID/g at micro-PET at 60 minutes after MB administration. Several studies have shown the presence of VEGFR2 expression on glomerular endothelial cells, peritubular capillaries, and before and after the glomerular vessels in murine kidneys (15,16). Furthermore, in a recent study, substantial uptake of radiolabeled VEGF121 in kidneys was related to the presence of renal VEGF receptor expression in normal kidneys of nude mice (17). Therefore, our results suggest that elevated uptake of VEGFR2-targeted MBs in normal kidneys may be due to VEGFR2 expression in normal renal tissue rather than nonspecific uptake mechanisms. However, the gradual increase of renal uptake over the course of the imaging experiments (Fig 1) could also be caused by some degree of cleavage of radiolabeled antibodies or free SFB, both of which accumulated in the kidneys in our control studies.
Accurate knowledge of the blood clearance time of targeted MBs is of practical value when planning targeted contrast-enhanced US experiments, since repeated injections of targeted MBs may be needed in the same animal in a single imaging session (3–6,18). Our study showed that 50% of targeted MBs cleared after 3.5 minutes and about 95% were cleared from the blood circulation after 30 minutes. Therefore, our results suggest that a delay time of about 30 minutes may be reasonable for repeated injections of targeted MB injections in the same mouse. These findings also agree with results from repeated in vivo targeted contrast-enhanced US; these results have shown that the US imaging signal in blood vessels returns to almost background levels several minutes after administration of targeted MBs (4,19). However, uptake values in the blood did not return to zero even after 60 minutes in our study, and this finding may be attributable to some degree of radiolabeled antibody cleavage from the MBs, as discussed later.
The results of the traditional ex vivo biodistribution experiments in our study showed trends of organ distribution for the targeted MBs that were similar to the trend observed from in vivo micro-PET imaging, with the highest levels of accumulation in the liver and spleen and with smaller accumulation values in all other organs. Absolute values, however, were somewhat different between the two methods. These values reflect different technical approaches of both methods, as well as interindividual differences between the different animals examined with both micro-PET and traditional ex vivo biodistribution studies. The in vivo micro-PET results were further confirmed with ex vivo histologic analysis of all tissues under consideration. At immunofluorescence staining, accumulation of MBs was found primarily in the liver and spleen. In the liver, targeted MBs were found in Kupffer cells as early as 4 minutes after administration. This finding is consistent with that in a study by Walday et al (10). In that study, the researchers reported that nontargeted albumin microspheres injected in rats were trapped primarily in Kupffer cells. Kupffer cells are specialized macrophages of the liver that are localized along the lining of the liver sinusoids. As part of the body's reticuloendothelial system, one of their primary functions includes phagocytosis of foreign particles. Kupffer cells have also been implicated in the clearance of various other imaging agents, including superparamagnetic iron oxide nanoparticles (used for magnetic resonance imaging) and quantum dots (used for optical imaging) (11,20). Rapid uptake of MBs by Kupffer cells is also corroborated by data in a study with electron microscopy in which researchers demonstrated the presence of nontargeted MBs within Kupffer cells as early as 10 minutes after administration in rats (21). In our study, uptake of targeted MBs into macrophages was slower in the spleen, with free MBs still present in the splenic sinusoids at 4 minutes after injection at immunofluorescence staining. In our study, targeted MBs in blood vessels other than the splenic sinusoids could not be visualized with the immunofluorescence technique. To directly demonstrate the attachment of targeted MBs to molecular markers on vascular endothelium, further studies with direct optical visualization by using intravital microscopy are warranted.
One limitation of our study was that we did not directly radiolabel the MBs. Because we studied commercially available MBs, we were not able to radiolabel components of the MBs themselves, such as phospholipids, during MB synthesis. In our preliminary experiments, the integrity of the gas-filled MBs could not be maintained during direct labeling, probably as a result of the reaction condition used for radiolabeling. Therefore, we used an indirect labeling approach by adding SFB to the biotinylated monoclonal antibodies prior to attachment of the biotinylated antibodies to the streptavidin-bearing MBs. Since streptavidin binds the small molecule biotin with femtomolar affinity (22), very stable binding of radiolabeled antibodies to MBs can be expected. Unbound antibodies during the labeling reaction were also removed by several washing steps to minimize administration of free radiolabeled antibodies during the biodistribution studies. However, to further assess how cleavage of the MB-antibody interaction would manifest during in vivo experiments, we performed control biodistribution studies of radiolabeled antibodies alone. As expected, blood clearance of the antibodies was substantially delayed compared with blood clearance of the targeted MBs; this finding suggests that a characteristic rapid blood clearance of radioactivity can be considered a useful internal control in our study for the stability of the MB-antibody interactions. In addition, we studied the biodistribution of free SFB to assess how SFB would manifest if it were to be released from the antibody in vivo. The biodistribution pattern of SFB was markedly different compared with the pattern of both the radiolabeled antibodies and the radiolabeled targeted MBs, with the predominant clearance of SFB occurring through the kidneys. Therefore, the differences in biodistribution among targeted MBs, antibodies alone, and free SFB suggest relatively good stability of the MB radiolabeling in our study. We acknowledge, however, that both control experiments cannot exclude some degree of radiolabeling cleavage from the MBs.
Another limitation relates to the limited spatial resolution of the micro-PET imaging system in our study. Without additional anatomic information, organs such as the small bowel, large bowel, and pancreas and the femur cannot be clearly delineated on the PET images in mice. Therefore, we did not differentiate between the small bowel and the large bowel on micro-PET images, and we did not include the pancreas or the femur in the micro-PET data analysis. However, a newer generation of combined PET and computed tomographic (CT) small-animal imaging systems is now available, and these systems allow accurate coregistration of anatomic CT information and molecular PET imaging data in a manner similar to that with clinical PET/CT imaging machines. Therefore, further studies are warranted to evaluate in vivo biodistribution studies by using combined small-animal PET/CT imaging systems.
In conclusion, the results of our study suggest that dynamic in vivo micro-PET allows noninvasive assessment of the whole-body biodistribution of targeted MBs in most tissues in mice. Targeted MBs quickly clear from the blood circulation and rapidly accumulate in the liver and spleen because of phagocytosis by the reticuloendothelial system.
Contrast-enhanced US by using MBs targeted to molecular markers is increasingly being used in preclinical research and is likely to be translated into clinical use in the near future. The results of our study give insights into the whole-body biodistribution of targeted MBs in living mice and have direct practical implications for planning and performing future molecular imaging experiments by using targeted MBs for contrast-enhanced US.
ADVANCES IN KNOWLEDGE
Dynamic micro-PET allows assessment of the whole-body biodistribution of targeted microbubbles (MBs) in living mice.
After intravenous administration, targeted MBs primarily accumulate within Kupffer cells in the liver and within macrophages in the spleen, as well as rapidly clear from the blood circulation.
IMPLICATION FOR PATIENT CARE
Our work in small animals provides groundwork information about whole-body in vivo biodistribution of targeted MBs before eventual future translation of this US approach for molecular imaging in patients.
Abbreviations
ID = injected dose
MB = microbubble
SFB = N-succinimidyl-4-[18F]fluorobenzoate
VEGF = vascular endothelial growth factor
VEGFR2 = VEGF receptor 2
Author contributions: Guarantors of integrity of entire study, J.K.W., S.S.G.; study concepts/study design or data acquisition or data analysis/interpretation, all authors; manuscript drafting or manuscript revision for important intellectual content, all authors; manuscript final version approval, all authors; literature research, J.K.W.; experimental studies, J.K.W., Z.C., C.D., A.M.L., M.L.S., C.H.N.; statistical analysis, J.K.W.; and manuscript editing, J.K.W., S.S.G.
See Materials and Methods for pertinent disclosures.
Funding: This research was supported by National Cancer Institute Small Animal Imaging Resource Program; National Heart, Lung, and Blood Institute (grant 1 R01 HL078632); and National Cancer Institute In Vivo Cellular and Molecular Imaging Center (grant no. CA114747 P50) and National Cancer Institute In Vivo Cellular and Molecular Imaging Center (grants CA1 272686).
References
- 1.Lindner JR. Microbubbles in medical imaging: current applications and future directions. Nat Rev Drug Discov 2004;3:527–532. [DOI] [PubMed] [Google Scholar]
- 2.Willmann JK, van Bruggen N, Dinkelborg LM, Gambhir SS. Molecular imaging in drug development. Nat Rev Drug Discov 2008;7:591–607. [DOI] [PubMed] [Google Scholar]
- 3.Ellegala DB, Leong-Poi H, Carpenter JE, et al. Imaging tumor angiogenesis with contrast ultrasound and microbubbles targeted to alpha(v)beta3. Circulation 2003;108:336–341. [DOI] [PubMed] [Google Scholar]
- 4.Korpanty G, Carbon JG, Grayburn PA, Fleming JB, Brekken RA. Monitoring response to anticancer therapy by targeting microbubbles to tumor vasculature. Clin Cancer Res 2007;13:323–330. [DOI] [PubMed] [Google Scholar]
- 5.Weller GE, Wong MK, Modzelewski RA, et al. Ultrasonic imaging of tumor angiogenesis using contrast microbubbles targeted via the tumor-binding peptide arginine-arginine-leucine. Cancer Res 2005;65:533–539. [PubMed] [Google Scholar]
- 6.Willmann JK, Paulmurugan R, Chen K, et al. US imaging of tumor angiogenesis with microbubbles targeted to vascular endothelial growth factor receptor type 2 in mice. Radiology 2008;246:508–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol 2005;23:1011–1027. [DOI] [PubMed] [Google Scholar]
- 8.Ferrara K, Pollard R, Borden M. Ultrasound microbubble contrast agents: fundamentals and application to gene and drug delivery. Annu Rev Biomed Eng 2007;9:415–447. [DOI] [PubMed] [Google Scholar]
- 9.Perkins AC, Frier M, Hindle AJ, et al. Human biodistribution of an ultrasound contrast agent (Quantison) by radiolabelling and gamma scintigraphy. Br J Radiol 1997;70:603–611. [DOI] [PubMed] [Google Scholar]
- 10.Walday P, Tolleshaug H, Gjoen T, et al. Biodistributions of air-filled albumin microspheres in rats and pigs. Biochem J 1994;299(pt 2):437–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schipper ML, Cheng Z, Lee SW, et al. MicroPET-based biodistribution of quantum dots in living mice. J Nucl Med 2007;48:1511–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen X, Park R, Shahinian AH, et al. 18F-labeled RGD peptide: initial evaluation for imaging brain tumor angiogenesis. Nucl Med Biol 2004;31:179–189. [DOI] [PubMed] [Google Scholar]
- 13.Knoess C, Siegel S, Smith A, et al. Performance evaluation of the microPET R4 PET scanner for rodents. Eur J Nucl Med Mol Imaging 2003;30:737–747. [DOI] [PubMed] [Google Scholar]
- 14.Loening AM, Gambhir SS. AMIDE: a free software tool for multimodality medical image analysis. Mol Imaging 2003;2:131–137. [DOI] [PubMed] [Google Scholar]
- 15.Feng D, Nagy JA, Brekken RA, et al. Ultrastructural localization of the vascular permeability factor/vascular endothelial growth factor (VPF/VEGF) receptor-2 (FLK-1, KDR) in normal mouse kidney and in the hyperpermeable vessels induced by VPF/VEGF-expressing tumors and adenoviral vectors. J Histochem Cytochem 2000;48:545–556. [DOI] [PubMed] [Google Scholar]
- 16.Ran S, Huang X, Downes A, Thorpe PE. Evaluation of novel antimouse VEGFR2 antibodies as potential antiangiogenic or vascular targeting agents for tumor therapy. Neoplasia 2003;5:297–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cai W, Chen K, Mohamedali KA, et al. PET of vascular endothelial growth factor receptor expression. J Nucl Med 2006;47:2048–2056. [PubMed] [Google Scholar]
- 18.Willmann JK, Lutz AM, Paulmurugan R, et al. Dual-targeted contrast agent for US assessment of tumor angiogenesis in vivo. Radiology 2008;248(3):936–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weller GE, Lu E, Csikari MM, et al. Ultrasound imaging of acute cardiac transplant rejection with microbubbles targeted to intercellular adhesion molecule-1. Circulation 2003;108:218–224. [DOI] [PubMed] [Google Scholar]
- 20.Thorek DL, Chen AK, Czupryna J, Tsourkas A. Superparamagnetic iron oxide nanoparticle probes for molecular imaging. Ann Biomed Eng 2006;34:23–38. [DOI] [PubMed] [Google Scholar]
- 21.Kindberg GM, Tolleshaug H, Roos N, Skotland T. Hepatic clearance of Sonazoid perfluorobutane microbubbles by Kupffer cells does not reduce the ability of liver to phagocytose or degrade albumin microspheres. Cell Tissue Res 2003;312:49–54. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez M, Bagatolli LA, Echabe I, et al. Interaction of biotin with streptavidin: thermostability and conformational changes upon binding. J Biol Chem 1997;272:11288–11294. [DOI] [PubMed] [Google Scholar]