Abstract
Purpose: To study magnetic resonance (MR) angiography findings in patients with acute stroke treated with intravenous tissue plasminogen activator (tPA) in relationship to perfusion- and diffusion-weighted imaging changes and clinical outcome.
Materials and Methods: Patients treated with intravenous tPA 3–6 hours after stroke onset (with informed consent) were evaluated in a HIPAA-compliant multicenter prospective study approved by all institutional review boards. MR imaging and MR angiography studies were performed before and 3–6 hours after treatment. MR angiography studies that were technically adequate at both time points were evaluated for occlusion, decreased flow, any early recanalization, and degree of recanalization. These results were compared with favorable clinical response (an improvement in National Institutes of Health Stroke Scale score of ≥8 points at 30 days or a modified Rankin scale score of 0 or 1 at 30 days) in patients with and those without mismatch between perfusion- and diffusion-weighted imaging at baseline.
Results: Seventy-four patients were enrolled in the initial investigation; pre- and posttreatment MR angiography studies were both technically adequate in 62 patients. MR angiography demonstrated occlusion or decreased flow in 46 patients. Patients with isolated middle cerebral artery (MCA) occlusion and early recanalization at MR angiography had higher rates of favorable clinical response than those with tandem internal carotid artery–MCA occlusion and early recanalization (P = .05). Any early recanalization was not associated with favorable clinical response, but degree of recanalization did correlate with favorable clinical response (P = .048). Favorable clinical response was more frequently seen in patients with mismatch between perfusion- and diffusion-weighted imaging findings at baseline who experienced early recanalization than in those who did not have early recanalization (odds ratio = 6.2; 95% confidence interval: 1.3, 30.2; P = .021). No relationship between early recanalization and favorable clinical response was seen in patients without mismatch.
Conclusion: Early recanalization seen at MR angiography before and after treatment coupled with diffusion- and perfusion-weighted imaging data may predict clinical outcome in patients with stroke treated with tPA 3–6 hours after symptom onset.
© RSNA, 2008
Currently, intravenous tissue plasminogen activator (tPA) is approved for the treatment of acute ischemic stroke in a 3-hour window after symptom onset (1). Trials based on unenhanced computed tomography (CT) studies that evaluated the use of this therapy in an expanded time window have not demonstrated a benefit (2–4). Magnetic resonance (MR) imaging with diffusion- and perfusion-weighted imaging may help identify patients likely to benefit from reperfusion therapy in an extended time window (5–7). It has been suggested that patients with mismatch between perfusion- and diffusion-weighted imaging findings will benefit from reperfusion therapy. The mismatch between perfusion- and diffusion-weighted imaging findings is considered a marker for the presence of an ischemic penumbra. Results of multiple animal and human studies suggest that within hypoperfused brain regions, the ischemic penumbra can be salvaged with recanalization of occluded brain vessels. In this study, mismatch was defined as a perfusion-weighted imaging volume that was 10 mL or more and 120% or more of the diffusion-weighted imaging lesion.
In a multicenter trial involving pretreatment MR imaging, the Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution (DEFUSE) study, it was hypothesized that patients treated in the 3–6-hour window with mismatch between perfusion- and diffusion-weighted imaging findings would have a more favorable clinical response after reperfusion compared with patients without a mismatch (8). Eligible patients were 18 years of age or older, had been given a clinical diagnosis of ischemic stroke, had a National Institutes of Health Stroke Scale (NIHSS) score greater than 5, and could be treated with tPA within 3–6-hours after symptom onset. Patients were excluded if they had evidence of acute hemorrhage or clearly identifiable hypoattenuation involving more than one-third of the middle cerebral artery (MCA) territory at baseline unenhanced brain CT.
In the present study, we utilized the DEFUSE study patient population to evaluate pretreatment and early posttreatment MR angiography findings, along with profiles of baseline mismatch between perfusion- and diffusion-weighted imaging findings and nonmismatch, to determine if the location of thrombosis or the degree of early recanalization correlated with clinical outcome. The analysis presented here differs from the analysis presented in our prior publication (8) in several ways. In this study, more detailed analysis of the specific MR angiography lesions was undertaken by two observers. MR angiography lesion location and recanalization rates were correlated with perfusion-weighted imaging changes and clinical outcomes. In addition, relationships between MR angiography results and perfusion-weighted imaging findings were analyzed in more detail. The purpose of this study was to correlate MR angiography findings in patients with acute stroke treated with intravenous tPA with diffusion- and perfusion-weighted imaging changes and clinical outcome.
MATERIALS AND METHODS
Patients were enrolled in DEFUSE, a prospective multicenter study. The primary results of that study have been previously reported (8). The local institutional review board at each site approved the study. The study was conducted under Health Insurance Portability and Accountability Act guidelines, and patients enrolled in the study gave informed consent. Patients included in the study had acute ischemic stroke with an NIHSS score greater than 5 and could be treated with intravenous tPA in a 3–6-hour window after symptom onset. The NIHSS is a quantitative clinical scale that measures the severity of neurologic impairment. Increasing values on the NIHSS indicate more severe neurologic impairment. Patients underwent MR angiography of the brain and were then treated with 0.9 mg intravenous tPA per kilogram of body weight administered as a 10% bolus over 1 minute, followed by continuous infusion of the remainder of the dose over 60 minutes. Patients were treated regardless of the findings at diffusion- and perfusion-weighted imaging. All other antithrombotic therapies were excluded for the first 24 hours. Repeat MR angiography was performed 3–6 hours after the tPA bolus was administered and at 30 days. Neurologic deficits were evaluated before, 3–6 hours after, and at 30 and 90 days after tPA therapy.
MR Imaging Sequences
MR imaging sequences performed at all time points included diffusion-weighted imaging, dynamic susceptibility perfusion-weighted imaging, a gradient-echo pulse sequence, and three-dimensional time-of-flight flow-compensated MR angiography of the circle of Willis. MR imaging was performed with 1.5-T clinical imaging units. Total imaging time for the MR imaging protocol was 8 minutes 45 seconds. Diffusion-weighted imaging was performed with a b value of 1000 sec/m2.
MR Angiography
Time-of-flight MR angiography was performed prior to the administration of the gadolinium chelate bolus used for perfusion-weighted imaging. The MR angiography parameters were as follows: flip angle, 20°; repetition time msec/echo time msec, 34/minimum; number of signals acquired, one; field of view, 24 cm2; rectangular field of view, 75%; section thickness, 1 mm; number of sections, 116; acquisition matrix, 512 × 128 interpolated to 512 × 512; and receiver bandwidth, ±32 kHz. To increase blood-tissue contrast, improvement of the time-of-flight sequence was achieved by using magnetization-transfer prepulses. Further enhancement of the image contrast between blood and tissue through consideration of saturation effects from slow-flowing blood and multiple radiofrequency excitations was achieved by breaking the three-dimensional slab into two smaller, overlapping volumes and by using a ramped excitation pulse with increasing flip angle in a distal direction. The amount of overlap between the two volumes was 12 sections. Because of the inclusion of MR imaging units from different vendors, slight variations of the MR angiography protocol across centers were allowed.
Analysis of Diffusion-weighted Images
Perfusion and diffusion volumetric analyses were performed by using software written in-house in Interactive Data Language, version 6.1 (Research Software, Boulder, Colo).
Regions of interest were identified on diffusion-weighted imaging lesion volumes by using a semiautomated thresholding algorithm that identified regions of high signal intensity that exceeded equivalent areas in the contralateral hemisphere by more than 3 standard deviations. Apparent diffusion coefficient maps were also generated and were used to confirm that the areas of high signal intensity on diffusion-weighted images were indeed new ischemic lesions.
Analysis of Perfusion-weighted Images
Perfusion-weighted imaging maps were generated by using a previously described technique (9). Tmax maps were used for all assessments. These were generated through deconvolution of the tissue concentration–over-time curve by using an arterial input function from the contralateral MCA (10). The arterial input function describes the concentration of the gadolinium-based contrast agent over time as it enters the brain. This function is critical in determining hemodynamic parameters quantitatively with bolus-tracking MR imaging. In a previous pilot study (10), hypoperfusion volumes derived from an arterial input function from the contralateral MCA correlated best with final infarct volumes. Tmax demonstrates the degree to which the tissue response lags behind the arterial input into each voxel. Tmax can be considered to be a correction for the bolus arrival time, as it is affected by factors that would also influence the bolus (eg, injection timing and the patient's hemodynamic state). A 2-second delay for Tmax was used as the lower threshold indicating hypoperfused tissue (10). The same prespecified threshold was used for all patients, including patients with a proximal carotid stenosis or occlusion, for which more severe thresholds might be more appropriate. Our acute stroke protocol did not mandate assessment of the extracranial carotid artery for the sake of time.
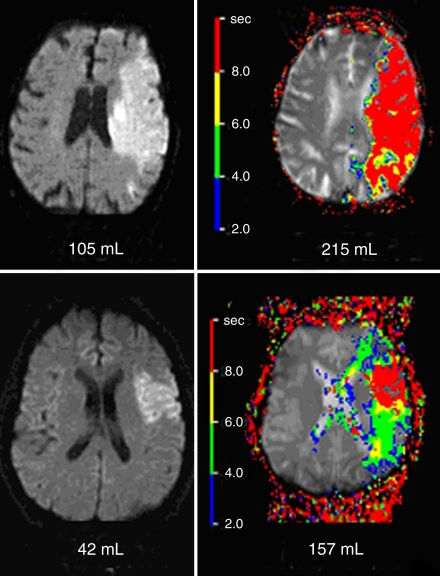
Lesion volume determination was performed by a single reader at the coordinating center. Mismatch between perfusion- and diffusion-weighted imaging findings was defined as a perfusion-weighted imaging lesion that was greater than 120% of the diffusion-weighted imaging lesion volume and greater than 10 mL. During the course of the original DEFUSE study, after 34 patients had been included, a preplanned interim analysis showed that a specific baseline MR imaging pattern (large baseline diffusion-weighted imaging lesions and large and severe perfusion-weighted imaging lesions) was associated with severe intracranial hemorrhage and poor outcome after reperfusion. This pattern was designated the “malignant profile.” Therefore, on the basis of this analysis, a “target mismatch” profile was defined as a mismatch where the diffusion-weighted imaging volume was 100 mL or less and the perfusion-weighted imaging lesion volume with a Tmax of 8 or more seconds was 100 mL or less (Fig 1).
Figure 1:
Example baseline MR images show malignant profile at diffusion-weighted imaging (top left) and perfusion-weighted imaging (top right) and target mismatch profile at diffusion-weighted imaging (bottom left) and perfusion-weighted imaging (bottom right). Color map data shows Tmax delay times in 2-second intervals. The malignant profile was defined as a baseline diffusion-weighted imaging volume of 100 mL or greater and/or a large and severe baseline perfusion abnormality (perfusion-weighted imaging volume with Tmax ≥ 8 seconds, ≥100 mL). The target mismatch profile was defined as a mismatch that did not satisfy the conditions of the malignant profile.
Analysis of MR Angiograms
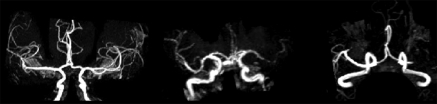
The vessels of the circle of Willis, including the supraclinoid internal carotid artery (ICA), the MCA (M1 segment), the anterior cerebral artery (A1 segment), and the posterior cerebral artery (P1 segment), were evaluated. A numeric scale was used to describe blood flow in the terminal ICA and the first branch (M1) of the MCA, with a score of 1 indicating normal flow; a score of 2, decreased flow; and a score of 3, occlusion (Fig 2). For the anterior cerebral artery, the posterior cerebral artery, and the second branch (M2) of the MCA, a score of 1 indicated normal flow and a score of 2, abnormal flow.
Figure 2:
Maximum intensity projection MR angiograms in three patients show normal MCA M1 flow (left), decreased flow in the left MCA M1 branch (middle), and occlusion in the left MCA M1 branch (right).
The segments that recanalized were scored as to the degree of recanalization by using a score of two points (occluded to normal) or one point (occluded to decreased flow or decreased flow to normal). M2 branches were also evaluated for recanalization in circumstances when the entire upstream vasculature (the supraclinoid, carotid, and MCA M1 segments) was normal. The MR angiography rating scale described above for the anterior cerebral artery, the posterior cerebral artery, and the second branch (M2) of the MCA, as well the fact that assessment was performed with a second reader, differ slightly from the approach previously reported (8). These changes in the MR angiography reading protocol resulted in four patients who were initially considered to have no MR angiography lesion being reclassified as follows: three were now considered to have MCA M2 lesions and one was considered to have a posterior cerebral artery lesion. All four of these patients had partial recanalization, and two of the four patients had a mismatch MR imaging profile. In addition, one patient with a mismatch profile who was initially classified as having no recanalization was reclassified as having complete recanalization.
Observers
Diffusion-weighted imaging lesion volumes were determined by a single observer (V.T., a stroke neurologist with 3 years of experience in analyzing diffusion- and perfusion-weighted imaging studies) at the coordinating center who was blinded to perfusion-weighted imaging and clinical information. In a separate session, arterial input function determination was performed and perfusion-weighted imaging lesion volume measurements were calculated by the same observer (V.T.), who was aware of the side of the ischemic lesion but was blinded to diffusion-weighted imaging findings and clinical information. Baseline perfusion-weighted images were interpreted before follow-up perfusion-weighted images in the same session. An analysis of source and maximum intensity projection images from the MR angiography studies was performed by a neuroradiologist (M.P.M., with 18 years of experience in interpreting MR angiography studies) and a stroke neurologist (J.M.O., with 10 years of experience), who were blinded to clinical data except the hemisphere of the brain that corresponded to the patient's stroke symptoms. The MR angiography studies at both baseline and 3–6 hours after tPA were directly compared at the time of reading. The MR angiography studies were initially evaluated independently by the two readers. Any discrepancies were reanalyzed jointly, and the scoring of the vessel in question was agreed to. Seven (11%) of 62 studies required a consensus reading for the presence of an initial lesion. Twelve (26%) of 46 studies required a consensus reading for the degree of recanalization.
Clinical End Points
A favorable clinical response was defined as an improvement in the NIHSS score of more than 8 points between baseline and 30 days or a modified Rankin scale score of 0 or 1 at 30 days. The modified Rankin scale is a disability scale. Patients with a Rankin scale score of 0 report no remaining symptoms after stroke, and patients with a score of 1 report only symptoms but have no remaining disability.
This end point was chosen on the basis of preliminary data that suggested that patients with a mismatch between perfusion- and diffusion-weighted imaging findings who had reperfusion after tPA therapy typically showed improvement in the NIHSS score of at least 8 points between baseline and day 30. In contrast, patients who did not have successful reperfusion rarely showed improvement in the NIHSS score of more than 7 points. The criterion of improvement of 8 or more points allowed patients who had had a severe stroke to be considered to have therapeutic success if they had a substantial improvement in NIHSS score, even if they were left with some disability. However, because patients with NIHSS scores of less than 5 were eligible for the DEFUSE study, a “compound” primary end point was chosen; patients with a baseline NIHSS score of less than 8 could achieve a favorable clinical response if they had a complete recovery (score of 0 or 1) on the NIHSS by day 30.
Statistics
Statistics were performed by using software (SPSS for Windows, version 10.0; SPSS, Chicago, Ill).
The Fisher exact test or the Pearson χ2 test was used to test associations between clinical outcome and MR angiography and MR imaging data. Recanalization was dichotomized into partial and complete recanalization versus no recanalization. The dichotomized comparisons of any recanalization versus no recanalization when compared with clinical outcome and lesion volumes were evaluated by using the Student t test. The analysis of variance test with a weighted linear trend or the nonparametric Spearman rank correlation test was used to evaluate the relationship between increasing degrees of recanalization and clinical and imaging parameters. The choice of statistical test depended on the distribution of the observed data. All statistical tests were two tailed, and P < .05 was considered to indicate a significant difference.
RESULTS
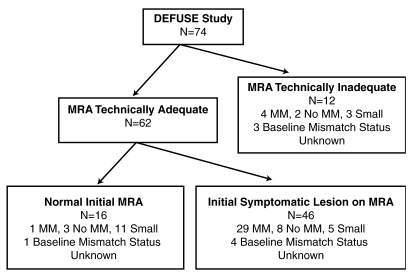
Seventy-four patients were enrolled in the initial DEFUSE study. MR angiography studies were technically adequate at both baseline and 3–6 hours after treatment in 62 (84%) of the patients; this group constitutes the study population for this analysis (Fig 3). Table 1 lists the baseline characteristics of these patients. At baseline, there was no significant difference between patients who experienced recanalization and those who did not in terms of age, NIHSS score, diffusion-weighted imaging volume, or perfusion-weighted imaging volume. Twenty-six (42%) of the 62 patients had a favorable clinical response.
Figure 3:
Flowchart of patients in study. MM = mismatch, MRA = MR angiography.
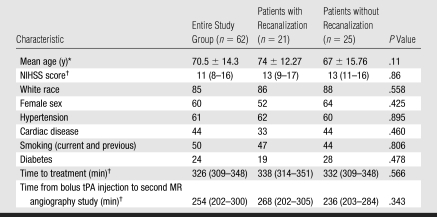
Table 1.
Patient Characteristics at Baseline
Note.—Unless otherwise specified, data are percentages of patients.
Data are means ± standard deviations.
Data are medians, with interquartile ranges in parentheses.
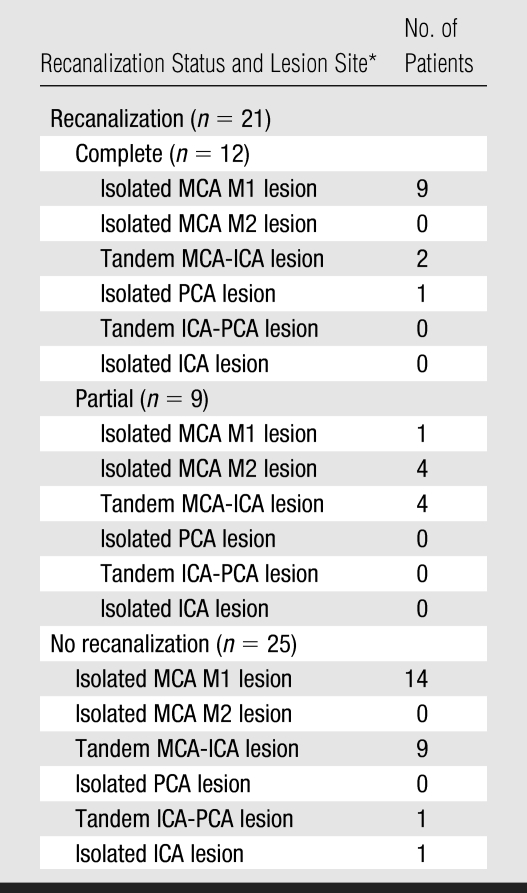
Median time to treatment was 326 minutes, and median time from bolus tPA injection to the second MR angiography study was 254 minutes. MR angiography demonstrated a symptomatic artery (occlusion or decreased flow) in 46 (74%) of the patients. Twenty-one (46%) of the 46 patients showed early recanalization at initial follow-up MR angiography performed 3–6 hours after injection of the tPA bolus, with 12 (26%) of these patients showing complete recanalization and nine (20%) showing partial recanalization. Table 2 shows the recanalization data according to the location of the abnormality at the baseline MR angiography study.
Table 2.
Early Recanalization according to Site of Arterial Lesion
PCA = posterior cerebral artery.
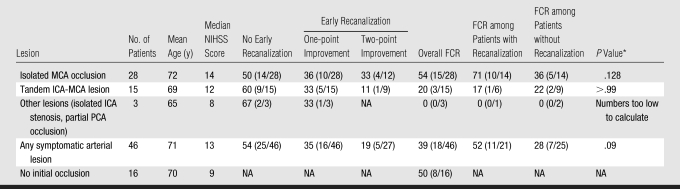
Table 3 displays the relationship between recanalization, favorable clinical response, and location of the lesion at baseline MR angiography. The overall rate of favorable clinical response in patients with a lesion at baseline MR angiography was 39%. The rate of favorable clinical response was 52% (11 of 21) in patients with early recanalization, compared with 28% (seven of 25) in patients with no early recanalization (P = .09, Pearson χ2 test). Among the 16 patients in whom a lesion was not identified at initial MR angiography, eight (50%) had a favorable clinical response.
Table 3.
Recanalization according to Location of Symptomatic Lesion at MR Angiography
Note.—Data are percentages, with numbers of patients in parentheses. FCR = favorable clinical response, NA = not applicable, PCA = posterior cerebral artery.
Calculated with Fisher exact test or χ2 test.
Twenty-eight patients had an infarct from an isolated MCA lesion, 14 (50%) of whom experienced early recanalization. Fifteen patients had tandem ICA-MCA lesions, six (40%) of whom experienced early recanalization. Nine (32%) of the patients with isolated MCA occlusion experienced complete recanalization, while only two (13%) of the patients with tandem ICA-MCA lesions experienced complete recanalization. The rate of favorable clinical response in patients with isolated MCA occlusion and recanalization (71%, 10 of 14) was significantly higher than the rate of favorable clinical response observed among patients with tandem ICA-MCA lesions (17%, one of six) (P = .05, Fisher exact test).
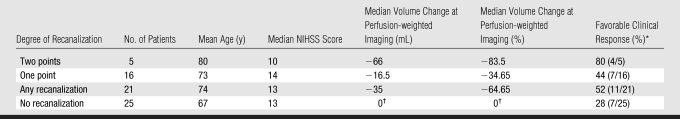
Table 4 shows the relationship between early recanalization, change in perfusion-weighted imaging volume, and favorable clinical response. There was no significant association between any degree of recanalization (both one- and two-point recanalization) and favorable clinical response (odds ratio = 2.8; 95% confidence interval: 0.8, 9.6; P = .132), but any recanalization was associated with a significant reduction in perfusion-weighted imaging volume (P = .03, Student t test). There was, however, an association between favorable clinical response and the degree of recanalization. Eighty percent of the patients with two-point recanalization had favorable clinical response, compared with 44% of the patients with one-point recanalization and 28% of the patients with no recanalization (P = .048). An analysis of variance test with weighted linear trend also revealed a significant relationship between degree of recanalization and perfusion-weighted imaging volume reduction (two-point recanalization: −66 mL; one-point recanalization: −16.5 mL; no recanalization: 0 mL; P = .001).
Table 4.
Relationship between Recanalization and Clinical Outcome
Data in parentheses are numbers of patients.
Six of the patients with no recanalization had technical failure of the initial or first follow-up perfusion-weighted imaging examination.
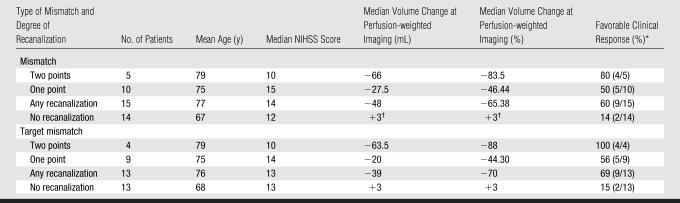
Table 5 shows the relationship between early recanalization, clinical outcome, and change in perfusion-weighted imaging volume in those patients with a mismatch between perfusion- and diffusion-weighted imaging findings or a target mismatch at baseline imaging. Twenty-nine patients with an initial symptomatic artery lesion exhibited a mismatch MR imaging profile, 15 (52%) of whom experienced early recanalization. Nine (60%) of these 15 patients had a favorable clinical response, compared with only two (14%) of the 14 patients who did not experience recanalization (odds ratio = 6.2; 95% confidence interval: 1.3, 30.2; P = .021). Increasing degrees of recanalization were also associated with changes in NIHSS score between baseline and 30 days (P = .011) and with percentage volume reduction of the perfusion-weighted imaging deficit (P = .001, Student t test). Thirteen of the study patients did not have mismatch (four patients were not included in the mismatch analysis because of technically inadequate perfusion-weighted imaging studies at one of the time points). In this group without mismatch, six (46%) experienced early recanalization. The group of patients without mismatch showed no association with early recanalization and favorable clinical response. Two (33%) of six patients in this group with early recanalization had a favorable clinical response, while three (43%) of seven patients without early recanalization also had a favorable clinical response (P > .99).
Table 5.
Recanalization and Clinical Outcome in Patients with Mismatch or Target Mismatch
Data in parentheses are numbers of patients.
One follow-up perfusion-weighted imaging examination was technically inadequate.
Twenty-six patients with an initial symptomatic artery lesion exhibited a target mismatch MR imaging profile. Thirteen (50%) experienced early recanalization. Nine (69%) of these 13 patients had a favorable clinical response, compared with only two (15%) of the 13 patients with target mismatch and no recanalization who had a favorable clinical response. The target mismatch profile with early recanalization was significantly associated with favorable clinical response (P = .013). The odds ratio of a favorable clinical response among patients with target mismatch and early recanalization was 13.5 (95% confidence interval: 2.0, 90.69). Increasing degree of recanalization was also significantly associated with a modified Rankin scale score of 0–2 at 30 days (P = .011), a modified Rankin scale score of 0–2 at 90 days (P = .043), a change in NIHSS score from baseline to first follow-up (P = .003), a change in NIHSS score from baseline to 30 days (P = .006), percentage volume reduction at perfusion-weighted imaging (P = .001), and volume reduction at perfusion-weighted imaging (P = .017).
Sixteen patients did not have a target mismatch MR imaging profile. Eight of the patients without a target mismatch profile experienced early recanalization. Two (25%) of these eight patients had a favorable clinical response, compared with five (62%) of the eight patients who did not experience early recanalization. There was a significant difference between the odds ratio for favorable clinical response observed among the patients with target mismatch and recanalization and that observed among the patients without target mismatch and with recanalization (P = .012).
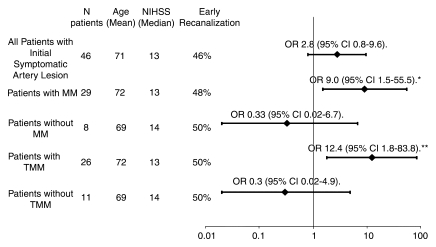
Figure 4 provides a summary of odds ratios for achieving a favorable clinical response for subgroups of patients with recanalization compared with those in patients in the same subgroup who did not experience recanalization. Significant differences in terms of favorable clinical response were identified in patients with mismatch or target mismatch who showed early recanalization (P = .021 and P = .015, respectively). No relationship between favorable clinical response and early recanalization was seen in patients without mismatch, patients without target mismatch, or for the entire study group.
Figure 4:
Graph shows odds ratio (OR) for favorable clinical response among patients who experienced recanalization compared with patients without recanalization in the same group. Values to the right of the vertical line indicate a more favorable clinical response, while values to the left indicate a less favorable response. An odds ratio greater than 1.0 indicates increased odds of a favorable clinical response. For the odds ratio for favorable clinical response among patients with mismatch versus patients without mismatch, P = .026. For the odds ratio for favorable clinical response among patients with target mismatch versus patients without target mismatch, P = .008. CI = confidence interval, MM = mismatch, TMM = target mismatch. *P = .021. **P = .015.
DISCUSSION
These results demonstrate an association between early recanalization at MR angiography and a favorable clinical response in patients with a mismatch between baseline perfusion-and diffusion-weighted imaging findings when they are treated with intravenous tPA in a 3–6-hour window. Early recanalization at MR angiography was also associated with a reduction in perfusion-weighted imaging volume at immediate follow-up imaging. In addition, patients with recanalization of an MCA occlusion were more likely to have a favorable outcome compared with patients who had recanalization of a tandem ICA-MCA occlusion. Moreover, there was a significant difference between the odds ratio of favorable clinical response observed among the patients with target mismatch and recanalization and that observed among patients without target mismatch and with recanalization. A mismatch at baseline imaging between diffusion- and perfusion-weighted imaging volumes has been used as an imaging characteristic that is thought to identify patients who are likely to benefit from thrombolytic therapy (5–7). This mismatch pattern has been shown to correlate with MR angiography–based findings at initial imaging that show absent flow in the MCA (11). These results suggest that the combined use of the MR angiography findings and mismatch between diffusion- and perfusion-weighted imaging findings at baseline can help predict both clinical and imaging outcomes in patients with acute stroke treated with intravenous tPA 3–6 hours after symptom onset.
To our knowledge, this is the first study to use a prospectively obtained early time window to evaluate changes at MR angiography and diffusion- and perfusion-weighted imaging compared with those at pretreatment imaging. Early recanalization was seen in 46% of the patients examined with MR angiography 3–6 hours after treatment. In a recent meta-analysis of 2066 patients (12), recanalization rates measured with various imaging techniques after administration of intravenous tPA were only 46.2%, compared with 63.2% after administration of intraarterial tPA and 83.6% after mechanical thrombolysis. Direct comparisons of these treatment approaches have not yet been performed.
Only ultrasonography (US) and conventional angiography have been used to assess recanalization within a few hours after treatment (13,14). US revealed a 33.5% rate of recanalization in the first 2 hours after intravenous treatment (14). In addition, US revealed higher rates of recanalization in patients with MCA occlusion alone compared with patients with tandem ICA-MCA occlusion. The rate of recanalization at US varied from 44.2% in the distal MCA to 27% for tandem ICA-MCA lesions (14). A separate US-based analysis also found tandem ICA-MCA occlusion to be a predictor of absence of recanalization (15). Our study showed higher rates of complete recanalization in patients with isolated MCA occlusion. In addition, our results also demonstrate that the rate of favorable clinical response was higher in patients with isolated MCA occlusion and recanalization than in those with tandem ICA-MCA occlusion and recanalization.
Rather than rate the degree of stenosis, we used a simplified scale for the MR angiography evaluations. We used a rating of occlusion, decreased flow, or normal flow in the ICA and the M1 segment of the MCA. We chose this three-point scale for the ICA and MCA and a two-point scale (normal or abnormal) for the other arteries of the circle of Willis, as compared with the four-point Thrombolysis in Myocardial Infarction and Thrombolysis in Cerebral Ischemia scales. This was done because of the limitations of time-of-flight MR angiography. Perhaps with the increased resolution of contrast material–enhanced MR angiography or CT angiography, a more detailed scale could be utilized. The degree of recanalization influenced both the perfusion-weighted imaging volume change and the clinical outcome in patients with mismatch. Those patients with one-point recanalization had a smaller decrease in perfusion-weighted imaging volume, compared with an impressive volume change in the patients with two-point recanalization. Favorable clinical response was seen in 80% of the patients with two-point recanalization and in 44% of the patients with one-point recanalization.
In the DEFUSE study, 1020 patients were screened to enroll 74 patients (7%). This enrollment rate is comparable to or greater than that achieved for previous neuroprotective and thrombolytic trials involving patients with stroke in this time window. Once the value of MR imaging for predicting the response to reperfusion therapy is clearly established, acute MR imaging availability and treatment rates are likely to increase.
This study had limitations. It is important to acknowledge that the study sample was small. Therefore, results of subgroup analyses in this study should be interpreted with caution. It may have been inappropriate to pool patients with partial recanalization and those with complete recanalization into a single response group. Similarly, the spatial resolution of the time-of-flight MR angiography performed in this study may have been inadequate to depict occlusions in smaller distal vessels. More sensitive MR angiography techniques may have depicted occlusions in some of our patients. For analysis of perfusion-weighted images, the status of the extracranial brain-supplying vessels was not taken into consideration. This might have biased the size of the perfusion-weighted imaging lesion volumes.
These results suggest that a patient with a target mismatch and MR angiography–proved occlusion of the anterior circulation is likely to benefit from recanalization in the 3–6-hour time window with intravenous tPA. However, this study was not a randomized trial designed to demonstrate the efficacy of intravenous tPA in this time window. A randomized trial will be needed to confirm the efficacy of this therapeutic approach. The degree of recanalization seen in this study was associated with outcome. This fact, coupled with relatively low recanalization rates after intravenous tPA administration, suggests that more efficient therapeutic techniques such as intraarterial approaches may be more beneficial.
ADVANCES IN KNOWLEDGE
This study demonstrates MR angiography–based early recanalization rates after intravenous tissue plasminogen activator (tPA) therapy, including recanalization rates for individual vessels of the circle of Willis.
This study showed a higher rate of favorable clinical response in patients with isolated middle cerebral artery (MCA) occlusion who experienced recanalization after administration of intravenous tPA in the 3–6-hour window compared with that in patients with tandem internal carotid artery–MCA lesions at pretreatment MR angiography.
Increasing degrees of recanalization seen at posttreatment MR angiography were associated with National Institutes of Health Stroke Scale score change from baseline to 30 days and with percentage volume reduction of the perfusion-weighted imaging deficit; an association between favorable clinical response and the degree of recanalization (seen at MR angiography) was also demonstrated in this study.
IMPLICATION FOR PATIENT CARE
Pre- and posttreatment MR angiography coupled with perfusion- and diffusion-weighted imaging information may provide valuable information for treatment decisions and prognosis in the patient with acute stroke considered for reperfusion treatment 3–6 hours after symptom onset.
Abbreviations
DEFUSE = Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution
ICA = internal carotid artery
MCA = middle cerebral artery
NIHSS = National Institutes of Health Stroke Scale
tPA = tissue plasminogen activator
Author contributions: Guarantors of integrity of entire study, M.P.M., G.W.A., V.T.; study concepts/study design or data acquisition or data analysis/interpretation, all authors; manuscript drafting or manuscript revision for important intellectual content, all authors; manuscript final version approval, all authors; literature research, M.P.M., J.M.O., M.G.L., R.B., L.R.W., G.W.A., V.T.; clinical studies, M.P.M., J.M.O., S.K., M.G.L., R.B., G.W.A., V.T.; statistical analysis, M.G.L., L.R.W., V.T.; and manuscript editing, M.P.M., S.K., M.G.L., R.B., L.R.W., G.W.A., V.T.
Authors stated no financial relationship to disclose.
Funding: This research was funded by the National Institutes of Health (grant R01 NS39325).
References
- 1.Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med 1995;333:1581–1587. [DOI] [PubMed] [Google Scholar]
- 2.Clark WM, Wissman S, Albers GW, Jhamandas JH, Madden KP, Hamilton S. Recombinant tissue-type plasminogen activator (Alteplase) for ischemic stroke 3 to 5 hours after symptom onset: the ATLANTIS study—a randomized controlled trial. Alteplase thrombolysis for acute noninterventional therapy in ischemic stroke. JAMA 1999;282:2019–2026. [DOI] [PubMed] [Google Scholar]
- 3.Hacke W, Kaste M, Fieschi C, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS). JAMA 1995;274:1017–1025. [PubMed] [Google Scholar]
- 4.Hacke W, Kaste M, Fieschi C, et al. Randomized double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischemic stroke. (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet 1998;352:1245–1251. [DOI] [PubMed] [Google Scholar]
- 5.Parsons MW, Barber PA, Chalk J, et al. Diffusion- and perfusion-weighted MRI response to thrombolysis in stroke. Ann Neurol 2002;51:28–37. [DOI] [PubMed] [Google Scholar]
- 6.Butcher KS, Parsons M, MacGregor L, et al. Refining the perfusion-diffusion mismatch hypothesis. Stroke 2005;36:1153–1159. [DOI] [PubMed] [Google Scholar]
- 7.Rother J, Schellinger PD, Gass A, et al. Effect of intravenous thrombolysis on MRI parameters and functional outcome in acute stroke <6 hours. Stroke 2002;33:2438–2445. [DOI] [PubMed] [Google Scholar]
- 8.Albers GW, Thijs VN, Wechsler L, et al. Magnetic resonance imaging profiles predict clinical response to early reperfusion: the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Ann Neurol 2006;60:508–517. [DOI] [PubMed] [Google Scholar]
- 9.Shih LC, Saver JL, Alger JR, et al. Perfusion-weighted magnetic resonance imaging thresholds identifying core, irreversibly infarcted tissue. Stroke 2003;34:1425–1430. [DOI] [PubMed] [Google Scholar]
- 10.Thijs VN, Somford DM, Bammer R, Robberecht W, Moseley ME, Albers GW. Influence of arterial input function on hypoperfusion volumes measured with perfusion-weighted imaging. Stroke 2004;35:94–98. [DOI] [PubMed] [Google Scholar]
- 11.Barber PA, Davis SM, Darby DG, et al. Absent middle cerebral artery flow predicts the presence of the ischemic penumbra. Neurology 1999;52:1125–1132. [DOI] [PubMed] [Google Scholar]
- 12.Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke 2007;38:967–973. [DOI] [PubMed] [Google Scholar]
- 13.Lee KY, Han SW, Kim SH, et al. Early recanalization after intravenous administration of recombinant tissue plasminogen activator as assessed by pre- and post- thrombolytic angiography in acute ischemic stroke patients. Stroke 2007;38:192–193. [DOI] [PubMed] [Google Scholar]
- 14.Saqqur M, Uchino K, Demchuk AM, et al. Site of arterial occlusion identified by transcranial doppler predicts the response to intravenous thrombolysis for stroke. Stroke 2007;38:948–954. [DOI] [PubMed] [Google Scholar]
- 15.Rubiera M, Ribo M, Delgado-Mederos R, et al. Tandem internal carotid artery/middle cerebral artery occlusion. Stroke 2006;37:2301–2305. [DOI] [PubMed] [Google Scholar]