Abstract
A regular daily meal regimen, as opposed to ad libitum consumption, enforces eating at a predefined time and within a short timeframe. Hence, it is important to study food intake regulation in animal feeding models that somewhat reflect this pattern. We investigated the effect of scheduled feeding on the intake of a palatable, high-sugar diet in rats and attempted to define central mechanisms – especially those related to opioid signaling - responsible for overeating sweet foods under such conditions. We found that scheduled access to food, even as challenging as 20 min per day, does not prevent overconsumption of a high-sucrose diet compared to a standard one. An opioid receptor antagonist, naloxone, at 0.3-1 mg/kg b. wt., decreased the intake of the sweet diet, whereas higher doses were required to reduce bland food consumption. Real-time PCR analysis revealed that expression of hypothalamic and brainstem genes encoding opioid peptides and receptors did not differ in sucrose versus regular diet-fed rats, which suggests that scheduled intake of sweet food produces only a transient change in the opioid tone. Intake of sugar was also associated with upregulation of orexin and oxytocin genes in the hypothalamus and NPY in the brainstem. We conclude that scheduled consumption of sugar diets is associated with activity of a complex network of neuroregulators involving opioids, orexin, oxytocin and NPY.
Keywords: restriction, diet, rats, deprivation, peptides, reward, obesity
Introduction
Humans and animals eat for a variety of reasons, including energy needs, reward, taste and time of day. The need to replenish lacking calories is the main factor driving basic feeding activity. However, a magnitude of consummatory response can be significantly increased when highly rewarding, palatable foods are presented. In fact, the amount of consumed palatable foods considerably exceeds that necessary to satisfy energy needs of the organism [22]. Several neuropeptidergic systems have been linked with the phenomenon of overconsumption of preferred tastants [11, 14]. Many investigators agree that opioids are an important component of the reward-related central circuitry that supports this process [3]. Sucrose and, to some extent, other sweet tastants appear to have an important relationship with the central opioid system. For example it has been shown that blockade of opioid receptors with antagonists, such as non-selective naltexone and naloxone or selective ones, decreases the intake of a sucrose solution, whereas agonists cause an opposite effect [12, 25, 28, 30]. In rats, chronic infusion of naltrexone suppresses consumption of chow accompanied by a 32% sucrose solution more effectively than of chow alone [17]. In line with those findings, mice that are genetically deficient in opioid receptors exhibit a lower preference for sweetened tastants than wild-type animals [39]. In addition, naloxone inhibits intake of a 10% sucrose solution in non-deprived sham-drinking rats, which suggests that this effect is not dependent on postabsorptive signals [28].
The majority of laboratory studies that elucidate reward-related regulation of ingestive behavior are conducted in free-feeding animals given either unrestricted or only somewhat limited access to “attractive” foods/solutions (in the latter case, a less palatable standard diet is available ad libitum, whereas a palatable one is rationed) [10]. However, this standardized ad libitum feeding protocol applied in rodent studies does not reflect a consumption pattern enforced by a regular meal regimen, i.e., by eating at a predefined time of day and completing a meal within a certain timeframe [7].
Noteworthy, relatively little is known about central mechanisms that govern scheduled intake of palatable tastants. Most attention has been directed towards processes that underlie anticipation-related and circadian aspects of this feeding regimen [6, 18, 19, 35]. However, studies have shown that animals offered time-restricted and scheduled access to a palatable diet eat more of such foods than of a regular “bland” diet [15]. It seems likely therefore that the reward system drives overconsumption of palatable ingestants also when feeding is a scheduled activity. There is evidence that repeated and excessive ingestion of sucrose leads to endogenous opioid dependence. Animals subjected to intermittent high-sugar meals display signs of opioid withdrawal when an opioid receptor antagonist is injected [4] and show enhanced responding for sucrose in the operant environment after a sugar deprivation period [1]. Levine et al. showed that naloxone reduces the intake of sweetened food presented for 2 hours per day, but it has no effect on the consumption of a regular diet [15]. Conversely, some authors have suggested that the hypophagic outcome of opioid receptor antagonism in sucrose consumption may be nutrient-independent and simply stem from the energy status of the organism. For example, it has been shown that ineffectiveness of the antagonist treatment on “bland” diet intake in a restricted feeding paradigm can be reversed by applying a less challenging consumption regimen [15, 37]. To add to the confusion, several early papers demonstrated that naloxone failed to decrease food or water intake under the scheduled paradigm or the effect was weak and visible only when very high doses were administered [2, 31].
Considering the aforementioned discrepancies and lack of consensus in data interpretation, the current set of experiments was designed to investigate whether opioids are involved in scheduled consumption of palatable, high-sugar food versus a standard, “bland” diet in rats. We examined the effect of naloxone injection on the intake of sugar and regular chow pellets offered for either 1 hour per day or as little as 20 minutes per day, hence, in schedules varying in restriction “challenge”. We compared the amount of food eaten when each diet was presented individually for a given period of time and we scored the amount of actual time spent on consummatory activity within each timeframe. Finally, we employed real-time PCR methodology to assess whether scheduled exposure to a sweet diet alters opioid system gene expression levels; we also determined mRNA levels of several orexigenic and anorexigenic genes that do not belong to the opioid family. Gene expression was studied in the hypothalamus and brainstem as these structures host hypo- and hyperphagic neuroactive components involved in the control of both energy- and reward-driven food intake.
Materials and Methods
Adult male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) were housed in individual wire-mesh cages in a temperature- (21-23°C) and humidity-controlled vivarium with a 12:12 LD cycle (lights on at 0700). Tap water was available ad libitum unless indicated otherwise. Experiments 1-4 and 5 utilized separate sets of animals.
All procedures described herein received prior approval from the Minneapolis Veterans’ Affairs Medical Center IACUC and they are compliant with the NIH Guide for the Care and Use of Laboratory Animals (NIH Publ., no. 80–23, rev. 1996).
Experiments 1 – 4: Scheduled intake of palatable sweet versus “bland” food: modifications with naloxone
Experiments 1 through 4 examined whether a highly palatable sweet diet is overconsumed compared to “bland” food when the length of consumption period is drastically shortened in the scheduled feeding paradigm. We also determined whether this scheduled overconsumption of sweet diet is sensitive to the opioid receptor antagonist, naloxone (Naloxone HCl, Research Biochemicals International, Natick, MA) delivered subcutaneously (SC).
All rats were initially fed standard Teklad rodent chow pellets (Teklad 8604) ad libitum for 7 days. The animals were then schedule-fed beginning with a period of 120 minutes (1000-1200) of feeding per 24 hours for 5 days. Since consumption was primarily occurring within the first hour of chow availability, feeding time was shortened over the following 5 days to 60 minutes. Rats were then divided into two groups of equal mean body weight (ca. 227 g): one group started receiving sweet-flavored rat chow (F0078, Bioserv Holton Industries) instead of the Teklad pellets, whereas the other remained on the standard rodent chow. F0078 diet contains 61.5% carbohydrate, 18.8% protein, 5.0% fat, 4.6% fiber and 4.4% ash and it is a mixture of sucrose, dextrose, casein, fiber, corn oil, corn syrup, choline bitartrate, mineral mix, vitamin mix and flowing agents. Caloric density of the two diet types is similar (standard chow = 3.93 kcal/gram, sweet chow = 3.75 kcal/gram). The amount of food eaten was measured and corrected for spillage. Throughout the course of the experiment, body weight of animals belonging to the two treatments increased to 289 +/- 10 g in the standard food group and to 302 +/- 8 g in the sucrose group; the difference was not statistically significant (t-test). In order to minimize the effect of individual animals’ body weights and, thus, different energy needs, on the amount of consumed food, feeding was analyzed as grams of food eaten per kilogram of body weight. Aside from the food intake measurements, we visually evaluated the approximate time spent on consumption of water and food by performing observations of each animal’s behavior every 2 minutes (ca. 1-2 sec per animal at each sampling point). The results were statistically analyzed using one-factor ANOVA with repeated measures followed by Fisher’s least-significance test. Two-group comparisons were performed with the Student’s t-test. Values were considered statistically different when P<0.05.
Experiment 1
Schedule-fed rats (n=20; 10 animals fed regular chow and 10 – sweet pellets), trained to feed within 1 hour per day (1000-1100) for 5 days, were injected subcutaneously (SC) on different days with naloxone at doses of 0.3, 1 or 3 mg/kg b. wt. diluted in 0.9% NaCl which served as a control solution. Injections were done 15 minutes prior to food presentation, and were given alternately in a counterbalanced fashion to every rat in a repeated measures paradigm (2-3 days elapsed between subsequent injections). Rats in two groups were fed either standard rat chow pellets (Teklad, #8604) or sweet pellets (Bioserv, F0021). The amount of food eaten was measured and corrected for spillage and ingestive behavior was observed.
Experiment 2
The two groups of rats described above were then subjected to scheduled feeding of 30 minutes per 24 hours (1000-1030; 4 days of the shortened schedule preceded the beginning of injections). On alternate days each rat was injected SC with either 1 mg/kg naloxone or saline 15 minutes prior to food presentation (2 days elapsed between subsequent injections). Rats in two groups were fed either standard rat chow (Teklad, #8604) or sweet pellets (Bioserv, F0021). The amount of food eaten was measured and corrected for spillage and ingestive behavior was observed.
Experiment 3
Since animals offered palatable sweet pellets for 30 minutes were still able to eat more than those in the “bland” chow-fed groups, we sought to elucidate whether the observed discrepancy in regular versus sweet chow consumption might be due to texture rather than the composition of the two diets (e.g., regular chow being more difficult to masticate). Hence the two groups of schedule-fed rats trained to feed within 30 minutes per day (1000-1030) were given ground chow (powder) instead of intact pellets. Rats were fed either standard ground pellets (Teklad, #8604) or sweet ground pellets (Bioserv, F0021) 3 days prior to the measurement. The amount of food eaten was measured and corrected for spillage.
Experiment 4
Schedule-fed rats were then trained for 3 days to feed within 20 minutes per 24 hours (1000-1020), however, they were given ground chow (powder). Rats in two groups were fed either standard ground pellets (Teklad, #8604) or sweet ground pellets (Bioserv, F0021). Rats were injected SC on alternate days with either 1 mg/kg naloxone or saline 15 minutes prior to food presentation (2 days elapsed between subsequent injections). The amount of food eaten was measured and corrected for spillage and ingestive behavior was observed.
Experiment 5: The effect of scheduled intake of regular versus sweet diet on gene expression levels in the hypothalamus and brainstem
Rats were schedule-fed as described in Experiment 1, i.e., 1 hour per day (1000 – 1100) and they were given either regular or sweet diet (n=8/group). On the 14th day of this feeding regimen, the animals were decapitated 2-4 hours after the end of the meal. The brains were excised, hypothalami and brainstems were dissected out with the guidance of the mouse brain atlas, immersed in the RNAlater solution (Ambion, USA), kept at room temperature for 2 hours and, thereafter, stored at −80°C until further processed. The opioid system mRNA levels and expression of other feeding-related genes were analyzed in these rats (Table 1).
Table 1.
Real-time PCR primers (all supplied by Thermo Scientific)
| Primer | Accession no. | Forward sequence | Reverse sequence |
|---|---|---|---|
| POMC | AF510391 | tgggtcacttccgctggg | tcctccgcacgcctctg |
| MOR | NM_013071 | aaagccctggatttccgtacc | gcagaccgatggcagaagag |
| KOR | NM_017167 | gctgtctactctgtggtgtttgtg | tgcggtcttcatctttgtgtatcg |
| DOR | NM_012617 | tggacgctggtggacatc | ggttgaggctgctgttgg |
| Dynorphin | NM_019374 | aagaaggctacacggcactg | tgagacgctggtaaggagttg |
| Oxytocin | NM_012996 | cggtggatctcggactgaac | tagcaggcggaggtcagag |
| Orexin | NM_013179 | ctccttcaggccaacggtaac | cagggcagggatatggctcta |
| CRH | NM_031019 | gttgagaaactgaagagaaaggg | actgttgttctgcgaggtac |
| AVP | NM_016992 | tgctcaacactacgctctctg | cctcctcttgggcagttctg |
| NPY | NM_012614 | cagaggcgcccagagcag | cagccccattcgtttgttacc |
| CART | NM_017110 | tgcttgtgaaggggtgacagc | ttaaagcggctccagggacaa |
| ENK | Y07503 | acctccaggaagacagaatgc | ccgagtgaaccagggatagc |
Aside from studying the tissue excised from schedule-fed rats, we also dissected the hypothalami from rats maintained on either regular or sweet diet (n=8/group) ad libitum for 14 days (no significant difference in body weight after the diet exposure; p=0.149). The opioid system genes were analyzed in this “control” set-up that did not involve restricted access to food.
RNA isolation and cDNA synthesis
Tissue samples were homogenized by sonication in TRIzol (Invitrogen, Sweden) using a Branson sonifier. Chloroform was added to the homogenate, which was then centrifuged at 10000 × g at 4°C for 15 min. The water phase was transferred to a new tube, and RNA was precipitated with isopropanol. The pellets were washed with 75% ethanol, air dried at room temperature, and dissolved in RNAse-free water. DNA was removed by treatment with DNAse I (Roche Diagnostics, Sweden) for 4 hours at 37°C, and the enzyme was thereafter inactivated by heating the samples at 75°C for 15 min. The absence of genomic DNA was determined by the PCR analysis with primers for the mouse RNA extractions with glyceraldehyde-3-phosphate dehydrogenase (GAPDH; NM_017008; forward TCC CTC AAG ATT GTC AGC AA; and reverse CAC CAC CTT CTT GAT GTC ATC) on the DNAse-treated RNA. RNA concentration was measured using a Nanodrop® ND-1000 Spectrophotometer (NanoDrop Technologies, Delaware, USA). cDNA was synthesized with MMLV reverse transcriptase (GE Healthcare, Sweden), using random hexamers as primers according to the manufacturer’s recommendations.
Real-time PCR
The cDNA was analyzed with a MyIQ thermal cycler (Bio-Rad Laboratories, Sweden). Each real-time PCR reaction with a total volume of 20 μl contained cDNA synthesized from 25 ng total RNA, 0.25 M each primer, 20 mM Tris/HCl (pH 8.4), 50 nM KCl, 4 mM MgCl2, 0.2 mM dNTP, SYBR Green (1:50000). A real-time PCR reaction was performed with 0.02 U/liter Taq DNA polymerase (Invitrogen, Sweden) under the following conditions: initial denaturation for 4 min at 95°C, followed by 50 cycles of 15 sec each at 95°C, 30 seconds at 55-62°C (i.e. at the optimal annealing temperature for each primer pair), and 30 sec at 72°C. This was followed by 1 min at 55-62°C (optimal annealing temperature) and a melting curve with 84 cycles of 10 sec at 55°C increased by 0.5°C per cycle. All experiments were performed in duplicates. The measurements where the threshold cycle (Ct) values between the duplicates had a difference of over 0.9 were repeated. A negative control for a given primer pair and a positive control with 25 ng of genomic DNA was included on each plate. The following housekeeping genes were used to define expression normalization factors: glyceraldehyde-3-phosphate-dehydrogenase (GAPDH), β-tubulin (TUB), ribosomal protein 19 (RPL19), histone H3 (H3), cyclophilin (CYCLO), β-actin (ACT) and succinate dehydrogenase complex, subunit B (SUCB). All primer sequences are listed in Table 1.
Data analysis and relative expression calculations
The MyiQ software version 1.04 (Bio-Rad Laboratories, Sweden) was used to analyze qPCR data and derive threshold cycle (Ct) values. Melting curves were analyzed to confirm that only one product was amplified and that it was significantly shifted compared to the melting curve for the negative control. The sample Ct values were analyzed further if the difference between those and the negative control was greater than 3; otherwise, the transcript was considered not to be expressed. The GeNorm software was used on the HKGs to determine the most stable HKGs and calculate normalization factors for each sample to compensate for differences in cDNA amount. LinRegPCR was employed to calculate PCR efficiencies for each sample. Grubb’s test (GraphPad, USA) was used to identify and remove outliers and calculate average PCR efficiencies for each primer pair. Differences in gene expression were analyzed using one-way ANOVA (analysis of variance) and two-sample t-test. Statistics were performed using Minitab 14.2.
Results
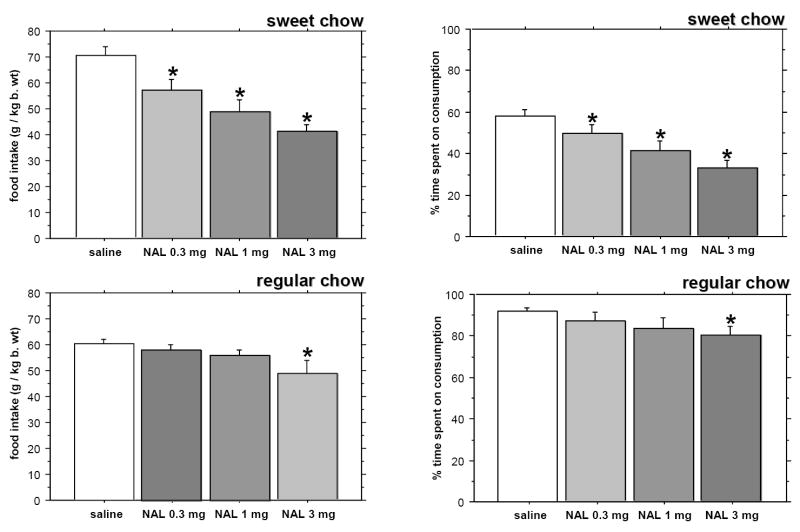
When subjected to the 1-h feeding schedule, control animals given sweet pellets ate significantly more than those presented with regular chow (P=0.0085). The amount of time spent on consummatory activity was lower in the sugar chow group (P<0.0001). Naloxone at a dose of just 0.3 mg/kg b. wt. reduced the amount of sweet chow eaten and time spent on consumption of the palatable diet. When regular chow was offered, naloxone was effective at inducing hypophagia, however the dose of the compound necessary to decrease food intake and the amount of time spent on consummatory activity had to be increased 10-fold to achieve a statistically significant difference in the two parameters (Fig. 1).
Fig. 1.
The effect of naloxone (NAL) at doses of 0.3, 1 and 3 mg/kg b. wt. on the intake of sweet versus regular chow (left panel) and on the percentage of time spent on consumption (right panel). Controls were injected with saline. Chow was available for 1 hour per day. * - p < 0.05
Reducing the period of food availability to 30 minutes per day did not eliminate the difference in the amount of ingested sweet versus regular chow (P=0.0009; Fig. 2) nor in the time spent on consumption (regular chow: 95.7 +/- 5.5, sweet chow: 79.2 +/- 2.9; P=0.0091) in saline controls. Naloxone at 1 mg/kg b. wt. was effective only at decreasing the intake of the more palatable diet (Fig. 2). Grinding the chow and presenting it in a powdered form did not affect the difference between sweet and regular diet consumption during the 30 minute feeding period: sweet flavored chow-fed rats still ate significantly more food and spent less time ingesting this more palatable food and drinking water (P=0.0010 and 0.0318, respectively; Fig. 3).
Fig. 2.
The effect of naloxone (NAL) on the intake of sweet versus regular chow in the 30-minute feeding period. Controls were injected with saline. * - p < 0.05
Fig. 3.
The effect of naloxone on the intake of sweet versus regular ground chow in the 30-minute feeding period. Controls were injected with saline. * - p < 0.05
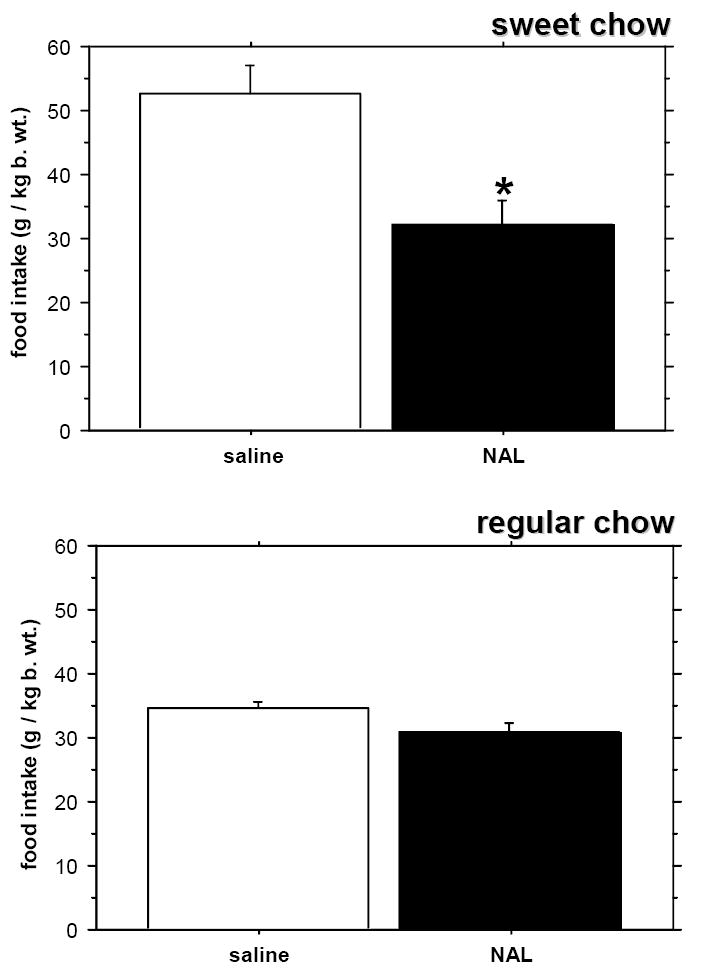
When the period of food availability was shortened even further to 20 minutes, saline-treated rats still ingested more ground sweet pellets than powdered regular chow (P=0.0089), and the time dedicated to consummatory activity differed between the groups (P=0.0134). Only sweet diet intake was sensitive to naloxone both in terms of the amount of ingested food and time spent on consumption (Fig. 4).
Fig. 4.
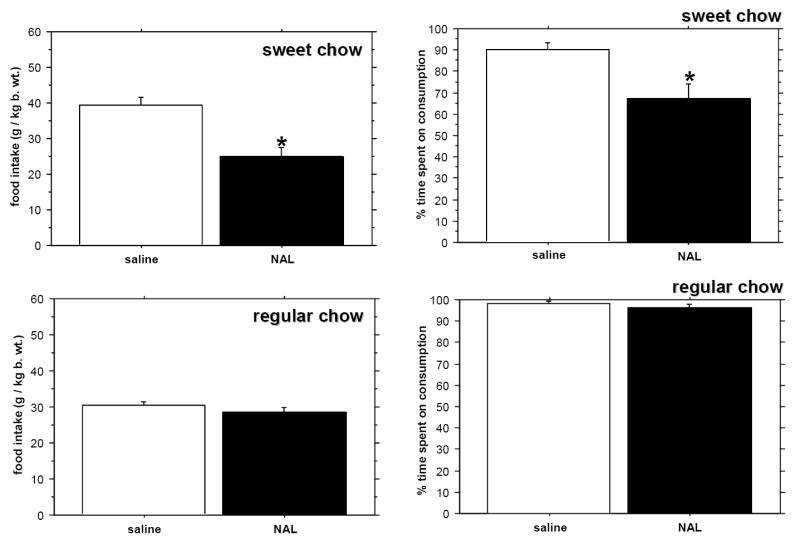
The effect of naloxone (NAL) on the intake of sweet versus regular chow (left panel) and on the percentage of time spent on consumption (right panel) in the 20-minute feeding period. Controls were injected with saline. * - p < 0.05
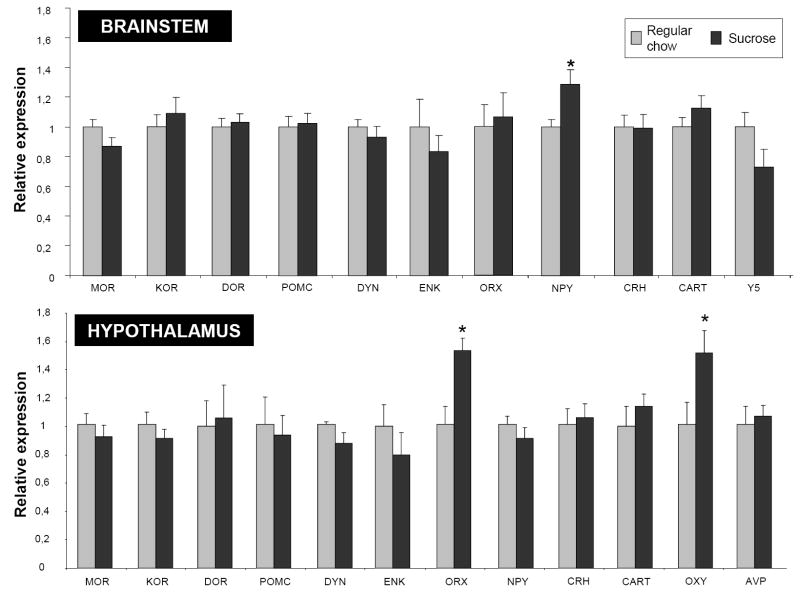
We did not observe any differences in the expression levels of genes encoding opioid peptides or receptors in animals consuming sweet versus regular food under the scheduled regimen (Fig. 5). However, in the rats consuming the diets ad libitum for 14 days, we found a significant change in the expression of the gene encoding the kappa receptor (P=0.024) and a clear trend in the mu receptor mRNA levels (P=0.087) (Fig. 6).
Fig. 5.
The effect of scheduled intake (1 h per day) of a high-sugar versus standard diet on the expression of hypothalamic and brainstem genes involved in feeding control. * - p < 0.05.
MOR, mu opioid receptor; KOR, kappa opioid receptor; DOR, delta opioid receptor; POMC, proopiomelanocortin; DYN, dynorphin; ENK, enkephalin; ORX, orexin; NPY, neuropeptide Y; CRH, corticotropin releasing hormone; CART, cocaine and amphetamine regulated transcript; Y5, Y5 receptor; OXY, oxytocin; AVP, vasopressin
Fig. 6.
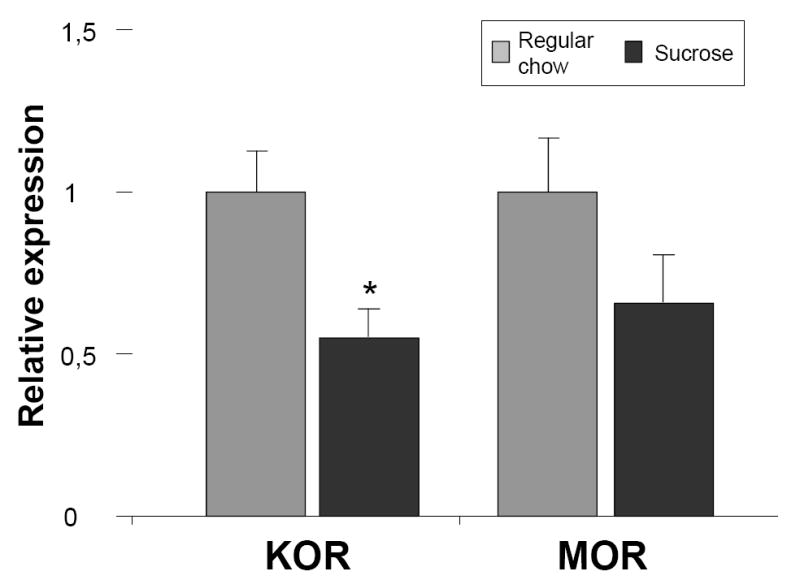
The effect of ad libitum of a high-sugar versus standard diet for 14 days on the expression of genes encoding the mu and kappa opioid receptors in the hypothalamus. * - p < 0.05. MOR, mu opioid receptor; KOR, kappa opioid receptor; grey bars – regular chow; black bars - sucrose
Interestingly, other feeding-related genes were sensitive to diet manipulation in the scheduled feeding paradigm: in the sweet diet group, a significant increase in the hypothalamic orexin (ORX) and oxytocin (OXY) as well as in the brainstem NPY mRNA levels was observed (Fig. 5).
Discussion
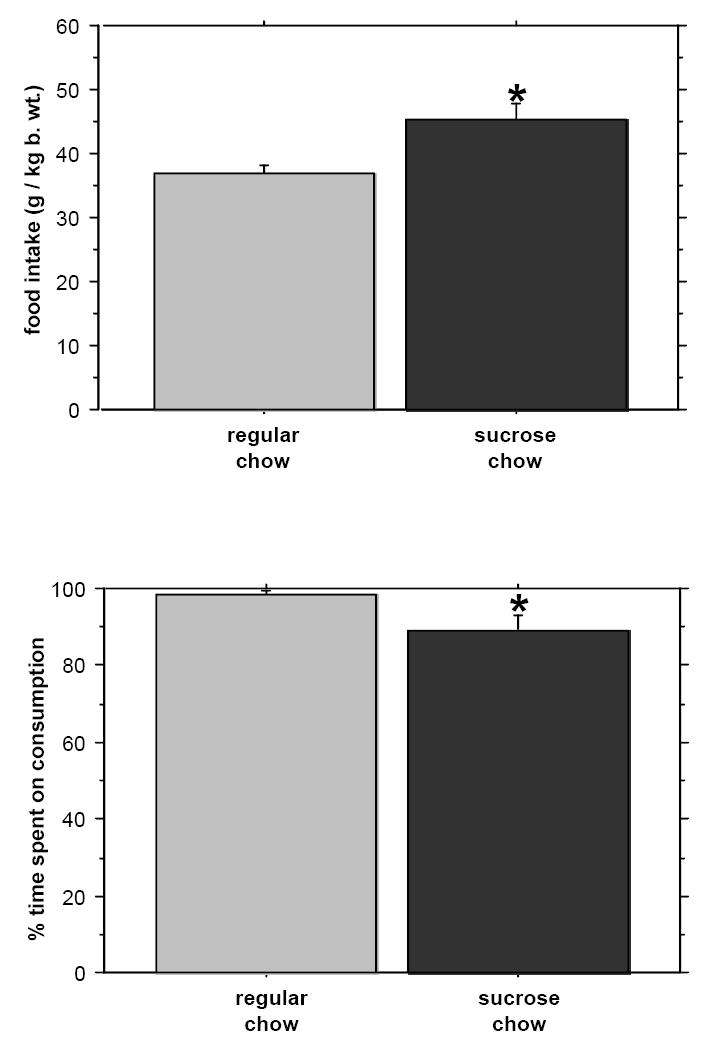
Overconsumption of preferred foods is a well established phenomenon and sucrose is one of the most palatable nutrients driving excessive food intake [22]. The current set of experiments confirms that under the scheduled feeding regimen, rats eat more sweet food than the standard “bland” diet. It seems particularly interesting that this elevated level of intake could be observed not only when high-sugar chow was available for 1 hour per day, but also when the daily period of availability was shortened to merely 20 minutes. Regardless of the length of access to food, all saline-treated and untreated animals offered regular or sweet chow consumed at least the minimum daily caloric requirement of 0.3 kcal/g b. wt., i.e., enough to maintain all physiological processes and prevent weight loss [29]. It was evident though that a shorter meal was associated with lower energy intake. However, even a drastically reduced, 20-minute meal timeframe that forced the regular chow-fed rats to carry on their consummatory activity almost throughout the entire period of food presentation, was insufficient to prevent a more voracious consumption of the high-sugar diet. Interestingly, in the latter case, the animals needed less time to acquire more sugar-chow calories than their standard chow-given counterparts. In fact, this pattern of more efficient ingestion of the sugar diet occurred in all three schedules, which indirectly supports previous conclusions from studies employing operant behavior progressive ratio paradigm that animals are much more motivated to consume sweet tastants than “bland” foods [3, 23]. The difference in the amount of consumed diets does not seem to stem from their texture rather than composition, because rats maintained higher level of sweet chow intake regardless of whether intact or powdered pellets were used. To some extent, the higher level of sweet diet consumption seen in this experimental model is reminiscent of binge-type eating. As shown by Dr. Corwin’s group, limited access to palatable food (both fat and sucrose) promotes binge-type behavior, and the magnitude of a consummatory response is independent from energy intake on the previous day [5, 38]. The current set of data suggests that reducing the length of a period when sweet food is available does not eliminate this binge-type of consummatory activity.
Opioid receptor antagonists have been shown to inhibit the intake of palatable foods and solutions, including those that contain sugar. Importantly, when animals are non-deprived naloxone and naltrexone decrease the intake of tastants regardless of palatability, although the effect is much more pronounced when preferred foods are offered [9] and it seems proportional to the relative “attractiveness” of a diet dependent on an individual preference profile [8]. Some authors have shown that under the conditions of restricted access to food, naloxone’s inhibitory effects on preferred diet consumption are very powerful, whereas the intake of “bland” food remains unchanged [15, 37]. In the current study, we focused on the influence of naloxone on scheduled consumption of high-sugar versus regular chow. In agreement with the presumed role of opioids in rewarding aspects of consummatory behavior, naloxone was much more effective at reducing the intake of the sweet chow, whereas its ability to decrease consumption of the regular diet was limited. The minimum dose of naloxone necessary to suppress standard chow intake was 10-fold higher than the dose affecting sugar diet consumption. In fact, scheduled intake of sweet tastants appears to be as sensitive to the opioid tone modulation as feeding models that utilize unrestricted access paradigms: the 0.1-1 mg/kg b. wt. dose range has been typically found effective in calorie/time-unrestricted feeding [10, 24]. Our data suggest that the hedonic aspects of sucrose consumption – as evidenced by its sensitivity to opioid receptor blockade - are present even upon scheduling very time-restricted meals. In addition, since in the current study, meals were presented during the daytime, it would be of interest to examine in a follow-up experiment whether sensitivity of hedonic and energy-related aspects of scheduled consumption is affected by the phase of the LD cycle.
Obviously, taking into consideration anorexigenic actions of naloxone in standard chow-fed groups, a question arises as to whether opioid receptor antagonism affects only reward-related feeding or also energy-driven ingestive behavior. One should note, however, that regardless of the diet and meal timeframe used, the antagonist treatment was unable to decrease consumption below the minimum daily caloric requirement [29]. In addition, feeding-related reward appears to exhibit a high degree of “plasticity”, i.e., when one is hungry, bland food is rewarding, whereas when the need to replenish energy is low, typically only those tastants that contain a specific and preferred macronutrient (or a combination of macronutrients) are perceived as palatable [14]. This relatively unclear demarcation line between feeding driven by palatability versus hunger is reflected by, e.g., the fact that expression of genes thought to be involved in reward is influenced also by chronic and temporary deprivation [13]. Another issue that needs to be addressed in future studies is whether similar responses to naloxone can be induced by chronic infusions (either through injections or an implanted pump) of this compound. One should note that in this project the opioid receptor antagonist was administered several times in each animal throughout the course of the experiment. Naloxone’s feeding inhibitory effects were maintained even after several injection trials had been performed and the animals did not seem to have built “tolerance” to the compound.
An acute decrease in scheduled sucrose consumption in response to naloxone administration suggests that sugar intake is associated with the elevated opioid tone. However, the results of injection studies do not allow one to speculate whether this increase in opioid signaling is a transient event occurring just around the time of actual feeding activity or whether the long-term scheduled intake of the preferred sweet diet leads to an increase in opioid or opioid receptor gene expression. Since intermittent, excessive sugar intake has been found to create dependency, and withdrawal signs can be precipitated by naloxone administration or palatable diet permanent removal [4], a change in the opioid gene expression has been hypothesized as the likely basis of this phenomenon. Previous studies on the relationship between opioid gene expression and sugar intake have produced interesting yet still incomplete data. For example, Welch et al. showed that rats receiving a diet rich in sucrose and fat had increased prodynorphin mRNA levels in the hypothalamic arcuate nucleus (ARC), but there was no modification in the expression of the remaining genes that belong to the opioid family in the ARC nor in the paraventricular nucleus (PVN) [36]. Spangler and coworkers detected alterations in opioid mRNA levels in the ventral striatum [32]. The opioid system forms a widespread network and individual genes belonging to this family seem to respond differently to palatability depending on their localization in the brain. In the current study, we did not see any change in the expression of opioid system genes in the hypothalamus or brainstem in response to the scheduled intake of the high-sugar diet. This seems, to some extent, surprising as elevated prodynorphin mRNA in the ARC has been previously detected with ad libitum exposure to palatable foods [36]. Apparently though scheduled feeding engages a different subset of molecular mechanisms than unrestricted access to foods does. This seems particularly likely considering the fact that genes encoding opioid receptors are affected (kappa – significantly, and mu – displaying a clear trend) by the intake of a sweet diet in an ad libitum feeding paradigm employed as a control set-up in our study. Obviously, one cannot exclude a possibility that there was a change in opioid gene expression outside the hypothalamus and brainstem that host both energy- and reward-related neuroactive substances [14].
Although mRNA content for opioid peptides and receptors was unchanged, sucrose consumption affected expression of several other feeding-related genes. These results open new avenues in understanding the molecular mechanisms responsible for overconsumption of sugar in the scheduled intake model. One such hypothalamic gene codes for orexin/hypocretin (hereafter: orexin). Recently, orexin has been proposed to play a role in the control of sugar consumption. Richards et al. reported that antagonism of the orexin receptor in rats impairs these animals’ drive to seek sucrose [26]. Thorpe and colleagues found that central orexin injections increased motivation for sweet foods in the rat [33]. Noteworthy, orexin’s ability to increase food intake is augmented by caloric challenge, i.e., food deprivation [34]. The fact that orexin signaling may be increased during nutritional duress in conjunction with this peptide’s influence on sucrose appetite provides the initial explanation of orexin’s involvement in scheduled consumption of high-sugar foods as our paradigm combines both caloric challenge and palatability.
The observed upregulation of oxytocin (OXY) gene expression in the sucrose-fed group is quite surprising in the light of recent experiments showing that OXY knock out animals ingest more sucrose than wild type ones [20]. On the other hand, however, it should be noted that OXY is part of the hypothalamic-pituitary-adrenal axis whose activity prepares the animal for any scheduled task or behavioral response, including a scheduled meal [21]. Hence, the change in OXY-dependent signaling may reflect entrainment processes that differ with palatable versus unpalatable diet presentation rather than the relationship with sugar consumption [18].
Finally, we observed an increase in NPY gene expression in the brainstem. Unlike the hypothalamic pool of this peptide, whose orexigenic properties have been proven beyond any reasonable doubt [14], the hindbrain population of NPY-synthesizing neurons still requires a better characterization in relation to their involvement in feeding control. It has been shown that many of the hindbrain NPY cells co-express norepinephrine or epinephrine. These neurons project to the hypothalamus and respond to glucose deficits induced by administration of the glycolytic inhibitor, 2-deoxy-d-glucose [16]. Glucoprivation increases NPY mRNA levels and induces c-Fos immunoreactivity in these cells [16, 27]. Our findings expand our understanding of the role of brainstem NPY as they suggest that upregulation of the NPY gene in this region may support scheduled overconsumption of sugar.
In sum, we found that a scheduled feeding regimen even with very short access to food does not prevent overconsumption of a high-sucrose diet. Opioids mediate rewarding aspects of consumption also in schedule-fed rats. Elevated intake of high-sugar foods during scheduled meals is associated with upregulation of genes encoding for orexin and OXY in the hypothalamus and NPY in the brainstem.
Acknowledgments
This research was supported in part by the National Institute of Drug Abuse (R01DA021280), the National Institute of Diabetes and Digestive and Kidney Diseases (P30 DK50456) and the Swedish Research Council and Åhlens foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Avena NM, Long KA, Hoebel BG. Sugar-dependent rats show enhanced responding for sugar after abstinence: evidence of a sugar deprivation effect. Physiol Behav. 2005;84:359–62. doi: 10.1016/j.physbeh.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 2.Brown DR, Holtzman SG. Suppression of drinking by naloxone in the rat: a further characterization. Eur J Pharmacol. 1981;69:331–40. doi: 10.1016/0014-2999(81)90479-9. [DOI] [PubMed] [Google Scholar]
- 3.Cleary J, Weldon DT, O’Hare E, Billington C, Levine AS. Naloxone effects on sucrose-motivated behavior. Psychopharmacology (Berl) 1996;126:110–4. doi: 10.1007/BF02246345. [DOI] [PubMed] [Google Scholar]
- 4.Colantuoni C, Rada P, McCarthy J, Patten C, Avena NM, Chadeayne A, et al. Evidence that intermittent, excessive sugar intake causes endogenous opioid dependence. Obes Res. 2002;10:478–88. doi: 10.1038/oby.2002.66. [DOI] [PubMed] [Google Scholar]
- 5.Corwin RL. Binge-type eating induced by limited access in rats does not require energy restriction on the previous day. Appetite. 2004;42:139–42. doi: 10.1016/j.appet.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 6.Escobar C, Martinez-Merlos MT, Angeles-Castellanos M, del Carmen Minana M, Buijs RM. Unpredictable feeding schedules unmask a system for daily resetting of behavioural and metabolic food entrainment. Eur J Neurosci. 2007;26:2804–14. doi: 10.1111/j.1460-9568.2007.05893.x. [DOI] [PubMed] [Google Scholar]
- 7.Frecka JM, Mattes RD. Possible entrainment of ghrelin to habitual meal patterns in humans. Am J Physiol Gastrointest Liver Physiol. 2008;294:G699–707. doi: 10.1152/ajpgi.00448.2007. [DOI] [PubMed] [Google Scholar]
- 8.Giraudo SQ, Grace MK, Welch CC, Billington CJ, Levine AS. Naloxone’s anorectic effect is dependent upon the relative palatability of food. Pharmacol Biochem Behav. 1993;46:917–21. doi: 10.1016/0091-3057(93)90222-f. [DOI] [PubMed] [Google Scholar]
- 9.Glass MJ, Grace M, Cleary JP, Billington CJ, Levine AS. Potency of naloxone’s anorectic effect in rats is dependent on diet preference. Am J Physiol. 1996;271:R217–21. doi: 10.1152/ajpregu.1996.271.1.R217. [DOI] [PubMed] [Google Scholar]
- 10.Glass MJ, Grace MK, Cleary JP, Billington CJ, Levine AS. Naloxone’s effect on meal microstructure of sucrose and cornstarch diets. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1605–12. doi: 10.1152/ajpregu.2001.281.5.R1605. [DOI] [PubMed] [Google Scholar]
- 11.Kelley AE, Baldo BA, Pratt WE, Will MJ. Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol Behav. 2005;86:773–95. doi: 10.1016/j.physbeh.2005.08.066. [DOI] [PubMed] [Google Scholar]
- 12.Kelley AE, Bless EP, Swanson CJ. Investigation of the effects of opiate antagonists infused into the nucleus accumbens on feeding and sucrose drinking in rats. J Pharmacol Exp Ther. 1996;278:1499–507. [PubMed] [Google Scholar]
- 13.Kim EM, Welch CC, Grace MK, Billington CJ, Levine AS. Chronic food restriction and acute food deprivation decrease mRNA levels of opioid peptides in arcuate nucleus. Am J Physiol. 1996;270:R1019–24. doi: 10.1152/ajpregu.1996.270.5.R1019. [DOI] [PubMed] [Google Scholar]
- 14.Levine AS, Billington CJ. Why do we eat? A neural systems approach. Annu Rev Nutr. 1997;17:597–619. doi: 10.1146/annurev.nutr.17.1.597. [DOI] [PubMed] [Google Scholar]
- 15.Levine AS, Weldon DT, Grace M, Cleary JP, Billington CJ. Naloxone blocks that portion of feeding driven by sweet taste in food-restricted rats. Am J Physiol. 1995;268:R248–52. doi: 10.1152/ajpregu.1995.268.1.R248. [DOI] [PubMed] [Google Scholar]
- 16.Li AJ, Ritter S. Glucoprivation increases expression of neuropeptide Y mRNA in hindbrain neurons that innervate the hypothalamus. Eur J Neurosci. 2004;19:2147–54. doi: 10.1111/j.1460-9568.2004.03287.x. [DOI] [PubMed] [Google Scholar]
- 17.Marks-Kaufman R, Balmagiya T, Gross E. Modifications in food intake and energy metabolism in rats as a function of chronic naltrexone infusions. Pharmacol Biochem Behav. 1984;20:911–6. doi: 10.1016/0091-3057(84)90016-9. [DOI] [PubMed] [Google Scholar]
- 18.Mendoza J, Angeles-Castellanos M, Escobar C. A daily palatable meal without food deprivation entrains the suprachiasmatic nucleus of rats. Eur J Neurosci. 2005;22:2855–62. doi: 10.1111/j.1460-9568.2005.04461.x. [DOI] [PubMed] [Google Scholar]
- 19.Mendoza J, Angeles-Castellanos M, Escobar C. Entrainment by a palatable meal induces food-anticipatory activity and c-Fos expression in reward-related areas of the brain. Neuroscience. 2005;133:293–303. doi: 10.1016/j.neuroscience.2005.01.064. [DOI] [PubMed] [Google Scholar]
- 20.Miedlar JA, Rinaman L, Vollmer RR, Amico JA. Oxytocin gene deletion mice overconsume palatable sucrose solution but not palatable lipid emulsions. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1063–8. doi: 10.1152/ajpregu.00228.2007. [DOI] [PubMed] [Google Scholar]
- 21.Olszewski PK, Bomberg EM, Martell A, Grace MK, Levine AS. Intraventricular ghrelin activates oxytocin neurons: implications in feeding behavior. Neuroreport. 2007;18:499–503. doi: 10.1097/WNR.0b013e328058684e. [DOI] [PubMed] [Google Scholar]
- 22.Olszewski PK, Levine AS. Central opioids and consumption of sweet tastants: when reward outweighs homeostasis. Physiol Behav. 2007;91:506–12. doi: 10.1016/j.physbeh.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 23.Papaleo F, Kieffer BL, Tabarin A, Contarino A. Decreased motivation to eat in mu-opioid receptor-deficient mice. Eur J Neurosci. 2007;25:3398–405. doi: 10.1111/j.1460-9568.2007.05595.x. [DOI] [PubMed] [Google Scholar]
- 24.Philopena J, Greenberg D, Smith GP. Naloxone decreases intake of 10% sucrose in preweanling rats. Pharmacol Biochem Behav. 1996;54:333–7. doi: 10.1016/0091-3057(95)02140-x. [DOI] [PubMed] [Google Scholar]
- 25.Ragnauth A, Ruegg H, Bodnar RJ. Evaluation of opioid receptor subtype antagonist effects in the ventral tegmental area upon food intake under deprivation, glucoprivic and palatable conditions. Brain Res. 1997;767:8–16. doi: 10.1016/s0006-8993(97)00539-8. [DOI] [PubMed] [Google Scholar]
- 26.Richards JK, Simms JA, Steensland P, Taha SA, Borgland SL, Bonci A, et al. Inhibition of orexin-1/hypocretin-1 receptors inhibits yohimbine-induced reinstatement of ethanol and sucrose seeking in Long-Evans rats. Psychopharmacology (Berl) 2008 doi: 10.1007/s00213-008-1136-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ritter S, Llewellyn-Smith I, Dinh TT. Subgroups of hindbrain catecholamine neurons are selectively activated by 2-deoxy-D-glucose induced metabolic challenge. Brain Res. 1998;805:41–54. doi: 10.1016/s0006-8993(98)00655-6. [DOI] [PubMed] [Google Scholar]
- 28.Rockwood GA, Reid LD. Naloxone modifies sugar-water intake in rats drinking with open gastric fistulas. Physiol Behav. 1982;29:1175–8. doi: 10.1016/0031-9384(82)90316-x. [DOI] [PubMed] [Google Scholar]
- 29.Rogers AE. Nutrition. Academic Press; Orlando: 1979. [Google Scholar]
- 30.Ruegg H, Yu WZ, Bodnar RJ. Opioid-receptor subtype agonist-induced enhancements of sucrose intake are dependent upon sucrose concentration. Physiol Behav. 1997;62:121–8. doi: 10.1016/s0031-9384(97)00151-0. [DOI] [PubMed] [Google Scholar]
- 31.Sanger DJ, McCarthy PS. The anorectic action of naloxone is attenuated by adaptation to a food-deprivation schedule. Psychopharmacology (Berl) 1982;77:336–8. doi: 10.1007/BF00432766. [DOI] [PubMed] [Google Scholar]
- 32.Spangler R, Wittkowski KM, Goddard NL, Avena NM, Hoebel BG, Leibowitz SF. Opiate-like effects of sugar on gene expression in reward areas of the rat brain. Brain Res Mol Brain Res. 2004;124:134–42. doi: 10.1016/j.molbrainres.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 33.Thorpe AJ, Cleary JP, Levine AS, Kotz CM. Centrally administered orexin A increases motivation for sweet pellets in rats. Psychopharmacology (Berl) 2005;182:75–83. doi: 10.1007/s00213-005-0040-5. [DOI] [PubMed] [Google Scholar]
- 34.Thorpe AJ, Teske JA, Kotz CM. Orexin A-induced feeding is augmented by caloric challenge. Am J Physiol Regul Integr Comp Physiol. 2005;289:R367–R72. doi: 10.1152/ajpregu.00737.2004. [DOI] [PubMed] [Google Scholar]
- 35.Verwey M, Khoja Z, Stewart J, Amir S. Differential regulation of the expression of Period2 protein in the limbic forebrain and dorsomedial hypothalamus by daily limited access to highly palatable food in food-deprived and free-fed rats. Neuroscience. 2007;147:277–85. doi: 10.1016/j.neuroscience.2007.04.044. [DOI] [PubMed] [Google Scholar]
- 36.Welch CC, Kim EM, Grace MK, Billington CJ, Levine AS. Palatability-induced hyperphagia increases hypothalamic Dynorphin peptide and mRNA levels. Brain Res. 1996;721:126–31. doi: 10.1016/0006-8993(96)00151-5. [DOI] [PubMed] [Google Scholar]
- 37.Weldon DT, O’Hare E, Cleary J, Billington CJ, Levine AS. Effect of naloxone on intake of cornstarch, sucrose, and polycose diets in restricted and nonrestricted rats. Am J Physiol. 1996;270:R1183–8. doi: 10.1152/ajpregu.1996.270.6.R1183. [DOI] [PubMed] [Google Scholar]
- 38.Wojnicki FH, Stine JG, Corwin RL. Liquid sucrose bingeing in rats depends on the access schedule, concentration and delivery system. Physiol Behav. 2007;92:566–74. doi: 10.1016/j.physbeh.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 39.Yirmiya R, Lieblich I, Liebeskind JC. Reduced saccharin preference in CXBK (opioid receptor-deficient) mice. Brain Res. 1988;438:339–42. doi: 10.1016/0006-8993(88)91360-1. [DOI] [PubMed] [Google Scholar]