Abstract
Mutational changes coupled with endocrine, paracrine, and/or autocrine signals regulate cell division during carcinogenesis. The hormone signals remain undefined, although the absolute requirement in vitro for fetal serum indicates the necessity for a fetal serum factor(s) in cell proliferation. Using prostatic cancer cell (PCC) lines as a model of cancer cell proliferation, we have identified the fetal serum component activin A and its signaling through the activin receptor type II (ActRII), as necessary, although not sufficient, for PCC proliferation. Activin A induced Smad2 phosphorylation and PCC proliferation, but only in the presence of fetal bovine serum (FBS). Conversely, activin A antibodies and inhibin A suppressed FBS-induced PCC proliferation confirming activin A as one of multiple serum components required for PCC proliferation. Basic fibroblast growth factor was subsequently shown to synergize activin A-induced PCC proliferation. Inhibition of ActRII signaling using a blocking antibody or antisense-P decreased mature ActRII expression, Smad2 phosphorylation, and the apparent viability of PCCs and neuroblastoma cells grown in FBS. Suppression of ActRII signaling in PCC and neuroblastoma cells did not induce apoptosis as indicated by the ratio of active/inactive caspase 3 but did correlate with increased cell detachment and ADAM-15 expression, a disintegrin whose expression is strongly correlated with prostatic metastasis. These findings indicate that ActRII signaling is required for PCC and neuroblastoma cell viability, with ActRII mediating cell fate via the regulation of cell adhesion. That ActRII signaling governs both cell viability and cell adhesion has important implications for developing therapeutic strategies to regulate cancer growth and metastasis.
Introduction
Mutations in tissue stem cells coupled with epigenetic changes promote carcinogenesis. However, despite the fact that these “hardwiring” changes are necessary for neoplasia, the cancer phenotype is environment-dependent, as illustrated by the fact that cancer cells can revert to a normal phenotype when injected into certain tissues. For example, hepatocyte carcinoma cells injected into the liver integrate into hepatic plates and differentiate into hepatocyte-like cells that are morphologically and functionally indistinguishable from mature hepatocytes, whereas those injected subcutaneously lead to death [1–3]. Likewise, injection of aggressive melanoma cells into zebrafish embryos [4–6] or epithelial cancer cells into mammary gland stroma [7] leads to normalization. These results indicate that “hardwiring”, although necessary for carcinogenesis, is insufficient for driving cancer growth and that signals from the immediate microenvironment regulate cell division.
Despite a vast amount of data indicating that various factors regulate cancer cell division, the exact tissue derived and circulating mitogenic and/or differentiation signals remain undefined, due in part to the ease of using fetal bovine serum (FBS) for in vitro cell growth. To address this question, we have focused our investigations on prostatic cancer cell (PCC) lines because PCC alter their responsiveness to hormones throughout the course of the disease. Early-stage prostate cancer is routinely treated with androgen-deprivation therapies, including the suppression of androgen production with gonadotropin-releasing hormone (GnRH) agonists and/or by peripheral blockage with nonsteroidal antiandrogens [8–10]. However, despite initial tumor regression, hormone-independent cells frequently emerge from such treatments and with a more aggressive phenotype [8,11,12]. These cells seem to undergo a transformation whereby there are alterations in androgen receptor signaling [13–17] and they develop metastatic properties [12]. Although these cells may be androgen-insensitive, they continue to divide in vivo, and when cultured in vitro, they only divide in the presence of serum, suggestive of neoplastic responsiveness to multiple mitogens.
Aside from sex steroid receptors, receptors for other hypothalamic-pituitary-gonadal (HPG) hormones have been reported in this reproductive tissue and associated cell lines, including GnRH receptor [18–20], luteinizing hormone (LH) receptor (Vadakkadath Meethal et al., unpublished data), follicle-stimulating hormone (FSH) receptor [21], and activin receptors [22–25]. Interpretation of the data generated from in vitro studies analyzing the effects of these hormones on PCC lines cultured in fetal serum [21,23,26–28] has been complicated by the complexity introduced by multiple serum factors (present in FBS). Because the incidence of prostate cancer is correlated with changes in these serum reproductive hormones [29], we examined the responsiveness of PCC to signaling through these known mitogen receptors using low serum conditions and strategies to antagonize (rather than stimulate) receptor signaling. Our results indicate that activin receptor type II (ActRII) signaling dictates PCC fate; sufficient ActRII signaling is permissive of proliferation whereas suppression of ActRII signaling leads to the up-regulation of ADAM-15 expression and cell detachment. Similar results were found for a neuroblastoma cell line. The centrality of ActRII-mediated ADAM-15 expression as a modulator of cancer cell adhesion and viability has obvious therapeutic implications.
Materials and Methods
Antibodies and Reagents
The antihuman ActRIIB affinity-purified mouse monoclonal antibody (A0856) was purchased from US Biological (Swampscott, MA) and recognizes the plasma membrane domain of the receptor. It does not cross-react with ActRIIA. The mouse antihuman activin recognizes human activin (US Biological). The antihuman phosphorylated-Smad2 affinity-purified rabbit polyclonal antibody (40-0800) recognizes Smad2 when it is dually phosphorylated at Ser465 and Ser467 (Zymed Laboratories, South San Francisco, CA). Antihuman ADAM-15 rabbit affinity-purified polyclonal antibody (Chemicon International, Millipore, Billerica, MA) recognizes the C-terminus amino acid sequence RPAPPPPAASSLYL. Antihuman caspase 3 (E-8) mouse monoclonal antibody was from Santa Cruz Biotechnology (Santa Cruz, CA). The glyceraldehyde-3-phosphate dehydrogenase (GAPDH) goat polyclonal antibody (V-18), the antihuman β-actin goat polyclonal antibody (C-11), and the horseradish peroxidase-linked goat antimouse, goat antirabbit, and donkey antigoat IgG were purchased from Santa Cruz Biotechnology.
Recombinant human activin A (βA, βA) peptide was purchased from R&D Systems (catalog no. 338-AC; Minneapolis, MN). Human pituitary FSH (hFSH) and human pituitary LH (hLH) were purchased from the National Hormone and Peptide Program, Harbor-UCLA Medical Center (Torrance, CA). Human chorionic gonadotropin was purchased from RayBiotech (Norcross, GA). GnRH1 peptide was purchased from Bachem California, Inc (Torrance, CA). Epidermal growth factor (EGF) and insulin-like growth factor 1 (IGF-1) were purchased from Sigma-Aldrich (St. Louis, MO). Basic fibroblast growth factor (bFGF) was purchased from Invitrogen (Carlsbad, CA). Low-weight prestained (∼20 to 110 kDa) molecular weight markers were from Bio-Rad Laboratories (Hercules, CA).
ActRII Antisense Oligonucleotides
For experiments using oligomers with phosphorothioate bonds (antisense-P; Integrated DNA Technology, Coralville, IA), oligomers were added to the medium (240 µl) that had been preincubated with lipofectamine (4 ng/µl; Invitrogen) for 5 minutes at room temperature. This mixture was then incubated at room temperature for 20 minutes before adding to the cells. Antisense-P was used at a final concentration of 0.4 µM. ActRIIA: antisense-P, 5′-TCCAGTTCAGAGTCCCATTTC-3′, 21nt, 49% G/C; sense, 5′-GAAATGGGACTCTGAACTGGA-3. ActRIIB: antisense-P, 5′-TCTCCCGTTCACTCTGCCAC-3′, 20nt, 60% G/C; sense, 5′-GTGGCAGAGTGAACGGGAGA-3′.
Cell Culture
PC-3 and LNCaP cells were graciously provided by Dr. Wade Bushman (University of Wisconsin, Madison, WI). PC-3 cells are androgen-insensitive cells derived from a grade 4 human prostate adenocarcinoma of epithelial origin [30,31]. Cells were maintained at 37°C in F-12 Nutrient Mixture (Ham; Gibco, Invitrogen) supplemented with 1% penicillin-streptomycin (P/S; Gibco, Invitrogen), 2 mM glutamine (Invitrogen), 0.4 mM sodium bicarbonate (Sigma), and 5% FBS (no. 26400-036; Gibco, Invitrogen). LNCaP cells are androgen-sensitive human prostate adenocarcinoma cells derived from a supraclavicular lymph node metastasis [32,33]. LNCaP cells were maintained at 37°C in RPMI 1640 medium (Cellgro, Manassas, VA) supplemented with 1% P/S, 2 mM glutamine, 18 mM sodium bicarbonate, 10 mM HEPES (Invitrogen), 1 mM sodium pyruvate (Invitrogen), 25 mM glucose (Sigma), and 10% FBS. Human M17 neuroblastoma cells were obtained from Dr. Robert Petersen (Case Western Reserve University, Cleveland, OH) and maintained at 37°C in OPTI-MEM medium (Invitrogen) containing 0.5% FBS and 1% P/S as previously described [34]. Fetal bovine serum concentrations used in each experiment are listed in the figure legends. For the MTS proliferation assay, cells were cultured in sterile 96-well plates (Falcon, Franklin Lakes, NJ); for cytology, cell number, and immunoblot analyses, cells were plated in sterile six-well plates (Nunc, Rochester, NY).
Determination of Cell Number
Cells were plated at serum levels (typically 1%) and confluence levels (typically 40%) that allowed unrestricted growth throughout the time (typically 1–3 days) of the experiment under our serum conditions. Cell number was assessed using the trypan blue (Invitrogen) staining technique in experiments where there were few treatment conditions. In experiments containing multiple (i.e., serum and/or hormone) treatments, cell proliferation was assayed using the Cell Titer 96 AQueous Non-Radioactive Cell Proliferation Assay (MTS tetrazolium assay; Promega, Madison, WI) as described. Briefly, cells were incubated with MTS/PMS solution, and the absorbance was read immediately (background control) and then again after 3 hours (final reading) at 490 nm using a 96-well plate reader (Spectramax Plus384; Molecular Devices Corporation, Sunnyvale, CA). Background absorbance values were subtracted from the final reading, and values were presented as a percentage of the absorbance value of the control group.
Cytology
The treated PC-3 cells were examined using a Zeiss Axiovert inverted microscope connected to a Fluo Arc light source, and images were captured with an Axio CamMRC-5 camera using Axio Vision 4.0 software (Zeiss Axiophot, Thornwood, NY).
Immunoblot Analysis
Cells grown in six-well plates were collected in 80 µl of lysis buffer (20 mM Tris, 150 mM NaCl, 1% SDS, 1 mM EDTA, and 1 mM EGTA, pH 7.6) containing protease inhibitors (10 µg/ml aprotinin and leupeptin, 1 µg/ml pepstatin A, 1 mM phenylmethanesulfonyl fluoride; Roche Diagnostics, Basel, Switzerland). Cells were directly sonicated using an ultrasonic processor continuously at a frequency of 20 Hz for 45 seconds followed by 1.5 minutes of cooling on ice. Three cycles were performed per sample. Protein was then determined using the bicinchoninic acid assay (Pierce, Rockford, IL), and equal amounts of protein were loaded onto 10% to 20% tricine gels (Novex, San Diego, CA). Samples were transferred onto polyvinylidine fluoride membranes (Bio-Rad Laboratories), fixed with 4% glutaraldehyde in Tris-buffered saline (TBS; 20 mM Tris, 150 mM NaCl, pH 7.6) containing Tween-20 (TBST), blocked with 10% milk in TBST for 2 hours, and incubated with primary antibody in 5% milk in TBST overnight at 4°C. On the second day, the primary antibody was removed, and the blot was washed in TBST and incubated with the appropriate secondary antibody for 2 hours at room temperature, washed again in TBST, and developed with Western Blotting Luminol Reagent (Santa Cruz Biotechnology) or ECL Plus (Amersham, Little Chalfont, England). The chemiluminescent signal was captured on film (Eastman Kodak Company, Aurora, IL). Additional immunoblot analyses were performed after the removal of antibodies from the blot using Restore stripping buffer (Pierce). Captured images were scanned, and the intensity of the autoradiograph signals (including a blank region) was determined using the NIH Image J software. Control and treatment values were corrected for blank values and normalized to their respective GAPDH or β-actin band intensity; the results were then expressed as either a fold or percentage change over control levels.
Statistical Analyses
Statistical analysis was performed using one-way and multivariate analysis of variance followed by pairwise comparisons with Fisher's protected least significant difference procedure to determine significant changes between treatment groups (Statview 5.0 and SuperAnova 3.0 programs; SAS Institute, Inc, Cary, NC).
Results
Activin A Is Required for PCC Proliferation
To examine potential mitogens of hormone-refractory PCCs, we treated androgen-insensitive PC-3 cells (a model of hormone-refractory prostate cancer) with FBS. As expected, increasing concentrations of FBS significantly increased PC-3 cell proliferation, whereas treatment of cells with heat-inactivated FBS (at 100°C for 5 minutes) suppressed PC-3 cell proliferation (Figure 1A). These results suggest that a serum protein factor(s) is required for the proliferation of androgen-insensitive PCCs.
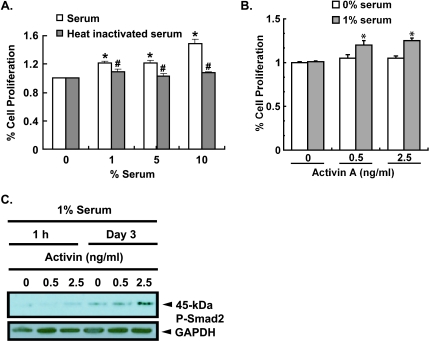
Figure 1.
Serum-dependent activin A-induced proliferation and signaling in PCCs. (A) PC-3 cells plated at 50% confluence for 24 hours in F12 medium without serum were then treated with medium containing increasing concentrations (0, 1%, 5%, and 10%) of FBS (S) or heat-inactivated FBS (HI). Proliferation was assessed using the MTS assay after 24 hours of treatment. Results are representative of three repetitions and presented as mean ± SEM; n = 8 (S) or 4 (HI) (*significantly different from 0% serum, P < .0001; #significantly different from serum treated cells, P < .05). (B) PC-3 cells were plated at 40% confluence in serum free or 1% FBS medium for 24 hours before being treated with and without activin A. Cells were treated with physiologically relevant concentrations of serum hormone as indicated on the figure and in [98]. Cell proliferation was assessed using the MTS assay after 3 days. Results are presented as mean ± SEM and are representative of five separate experiments (*significantly different from 0 and 1% serum alone, n = 4, P < .05). (C) PC-3 cells were plated at 50% confluence in medium containing 1% serum for 24 hours before being treated with activin A (0.5 or 2.5 ng/ml) in 1% FBS. Whole-cell lysates collected at 1 hour and 3 days after treatment were subjected to immunoblot analysis using a rabbit antihuman P-Smad2 antibody and goat antihuman GAPDH antibody was used as the loading control.
Because PC-3 cells are androgen-insensitive [31] and serum testosterone levels decline with aging (reviewed in [35]), we sought to examine other growth factors as mitogenic candidates for androgen-independent cells. Consequent to the decline in serum testosterone levels with aging and the loss of negative feedback on the hypothalamus and pituitary, there is a dramatic change in the synthesis, secretion, and serum concentrations of all HPG hormones [36]. Therefore, given that prostate cancer is an age-dependent disease, we tested whether other HPG hormones are mitogenic to androgen-insensitive PCCs. Of the hormones tested (LH, human chorionic gonadotropin, FSH, GnRH, activin A), only activin A at physiological concentrations (0.5–2.5 µg/L) [37] induced a significant increase in PC-3 cell proliferation in the presence of 1% serum after 24 hours (Figure 1B). At 1% serum, all hormone receptors of the HPG axis are highly expressed (Vadakkadath Meethal et al., unpublished results). No HPG hormones induced PCC proliferation in the absence of serum. Blocking activin A signaling with physiological concentrations of the antagonist inhibin A (60,000 mIU/ml) suppressed serum-induced PC-3 cell proliferation by 50% confirming that activin A present in serum induces PC-3 cell proliferation. Similarly, treatment of PC-3 cells with an activin A-specific antibody suppressed serum-induced PC-3 proliferation in a dose-dependent fashion by 20% and 31% with 20- and 50-µg/ml antibodies, respectively, compared with control (n = 4, P < .01). Together, these results suggest that activin A present in FBS [37] is permissive of PC-3 cell proliferation but that multiple different activins or other mitogenic ligands present in serum also promote proliferation independently or in synergism with activin A.
Activins signal through two classes of serine/threonine kinases, namely, type I (a.k.a. ALK) and type II (ActRII), and mediate their signals through a family of downstream transcription factors known as Smads that, on phosphorylation by the activin receptor complex, induce gene transcription [38–41]. To confirm that activin A was signaling through activin receptors previously reported on normal and transformed PCCs [22–25], we treated PC-3 cells with activin A and measured the phosphorylation of the transcription factor Smad2. Immunoblot analysis demonstrated a dose- and time-dependent increase in activin A-induced Smad2 phosphorylation (45 kDa) such that by 3 days of treatment, P-Smad2 expression (normalized to GAPDH expression) was increased 81% with 2.5 ng/ml activin A, compared with that of control (Figure 1C). Taken together, these results implicate activin A-induced receptor activation and MH2 domain phosphorylation [39,42–44] in activin A signaling in PC-3 cells.
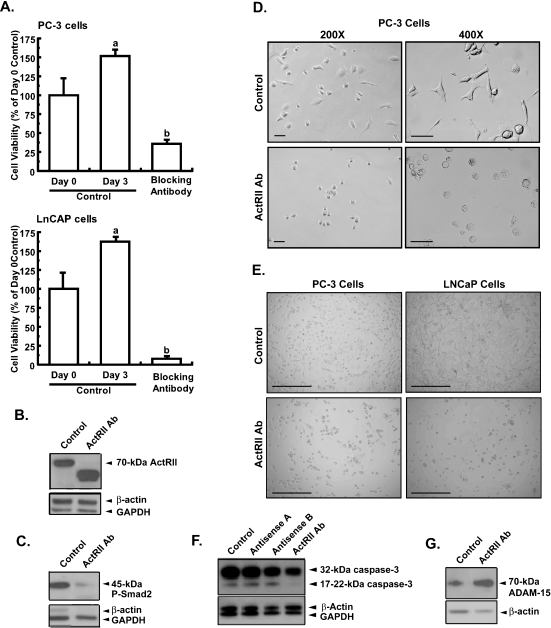
To confirm that activin signaling is essential for androgen-insensitive PCC proliferation, we inhibited activin A signaling through its cognate receptor using a blocking antibody specific to the plasma membrane domain of both ActRIIA and ActRIIB. This antibody not only blocked further cell growth but also significantly decreased PC-3 cell viability by 64% compared with the day 0 control (Figure 2A). Addition of activin A (2.5 ng/ml) to ActRII antibody-treated PC-3 cells did not significantly increase PC-3 cell proliferation (40 ± 11% activin A + ActRII antibody treated vs 24 ± 3% ActRII antibody treated only; mean ± SEM, n = 4, NS). Because blocking ActRII signaling reduced cell viability well below day 0 control levels, these results suggest that ActRII signaling is a basic cell requirement.
Figure 2.
Blocking antibody suppression of ActRII signaling inhibits PCC adhesion and viability. PC-3 or LNCaP cells were plated in 10% serum at 40% confluence for 24 hours, after which cells were either collected (day 0 control) or treated every day for 3 days with 1) medium containing 5% serum or with 2) medium containing 5% serum plus ActRII monoclonal antibody (25 µg/ml), before collection. (A) PC-3 (upper panel) and LNCaP (lower panel) cell viability was measured using the trypan blue staining assay. Results are presented as a percentage change from the untreated day 3 control (mean ± SEM; n = 4, P < .01; different letters indicate significant differences between treatments; experiments were repeated five times). (B) Immunoblot analysis of PC-3 cells using a mouse monoclonal antibody against human ActRII (70 kDa) and normalization against GAPDH (37 kDa). (C) Immunoblot analysis of PC-3 cells using an affinity purified rabbit polyclonal antibody against human phosphorylated Smad2 (45 kDa) and normalization against GAPDH (37 kDa). (D) PC-3 cells were cultured in F12 medium with 5% serum for 1 day before treatment ± antihuman ActRII antibody (20 µg/ml) for 3 days. Morphology was then assessed using a Zeiss Axiophot inverted microscope. Magnification is given on the figure. Scale, 50 µm. (E) PC-3 cells (left panels) were cultured in F12 medium with 5% serum, and LNCaP cells (right panels) were cultured in RPMI-1640 medium with 5% serum for 1 day before treatment ± antihuman ActRII antibody (20 µg/ml) for 3 days. Original magnification, x 100. Scale, 400 µm. (F) Immunoblot analysis of PC-3 cells described in Figure 3 for caspase 3 (32-kDa inactive and 17- to 22-kDa active forms) using a mouse monoclonal antibody. (G) Immunoblot analysis of PC-3 cells described in (A) for ADAM-15 (70 kDa) using a rabbit antihuman ADAM-15 antibody and normalization against β-actin (47 kDa) using a goat antihuman β-actin antibody. Experiments were repeated two times.
To confirm that the ActRII blocking antibody was inhibiting activin A signaling, we probed immunoblots of the cells treated for 3 days in Figure 2A with antibodies to ActRII and P-Smad2. Normalization of ActRII signals against GAPDH indicated that the ActRII blocking antibody markedly decreased the expression of the 70-kDa form of ActRII (90%) compared with day 3 control (Figure 2B). The decline in the expression of the 70-kDa form of ActRII was marked by an increase in the expression of a 45-kDa variant of ActRII, indicating that suppression of ActRII signaling promotes the proteolytic processing of the 70-kDa variant (the 45-kDa band is unlikely to represent residual added ActRII antibody or cross-reactivity because it was absent in the control). This suggests a negative feedback mechanism of Smad signaling on ActRII expression and that any decline in signaling through the ActRII/Smad2/Smad4 pathway might induce ActRII proteolysis (and internalization?) and decrease cell viability. The ActRII blocking antibody also decreased P-Smad2 signal (normalized to GAPDH) by 67% compared with the day 3 control (Figure 2C). These changes in the expression of ActRII and P-Smad2 reaffirm the specificity of the blocking antibody for ActRII signaling. Together, these results indicate that activin A signaling through ActRII is a basal requirement for normal PCC viability and proliferation.
Activin has previously been shown to suppress androgen-sensitive PCC proliferation [23]. Under low serum conditions (1%), activin A did not markedly alter the proliferation of androgen-sensitive PCCs cells (LNCaP; data not shown). We next blocked ActRII signaling in LNCaP cells expecting that this would either not affect cell viability or increase cell proliferation. Surprisingly, blocking ActRII signaling with the ActRII blocking antibody blocked LNCaP cell growth as well as decreased cell viability, as was observed for the PC-3 cells (Figure 2A). After 3 days of treatment with blocking antibody, LNCaP cell number had decreased by 92%.
Blocking ActRII Signaling Induces ADAM-15 Expression and Morphologic Changes in PCC
Suppression of ActRII signaling with the blocking antibody induced significant morphological changes in PC-3 cells; cells became rounded with fewer processes, had a smaller appearance (Figure 2D), and readily detached from the plate. Decreasing ActRII signaling with double-stranded (ds) antisense oligonucleotides to ActRIIA, ActRIIB, and Smad2 induced similar morphological changes in PC-3 cells (Figure W1 and Table 1). Blocking ActRII signaling with the blocking antibody also induced similar morphological changes in LNCaP cells (Figure 2E, right panels). To examine whether suppression of activin signaling induces cell detachment through an apoptotic mechanism, we measured the expression of the inactive (32 kDa) and active (17 to 22 kDa) forms of caspase 3 in cells treated with the ActRII blocking antibody or antisense-P to ActRIIA or ActRIIB (Figure 2F). Suppression of ActRII signaling with antisense-P did not alter the ratio of active/inactive caspase 3, whereas the ActRII antibody induced a 40% decrease in active/inactive caspase 3 expression. These results indicate that suppression of ActRII signaling does not induce apoptosis.
Table 1.
Oligonucleotide Sequences Used for Gene Silencing.
| Gene | Sequence No. | Oligonucleotides | G/C Content |
| ActRIIA | |||
| Antisense | 1 | 5′-GCCAACTTTGCAGCAGCGTCCA-3′ | 22nt, 59% |
| 2 | 5′-AGCTCCCATTTTCCCGAGGCG-3′ | 21nt, 62% | |
| 3 | 5′-GGCAAACGCCAACTTTGCAGCAG-3′ | 23nt, 56% | |
| Sense | 1 | 5′-TGGACGCTGCTGCAAAGTTGGC-3′ | |
| 2 | 5′-CGCCTCGGGAAAATGGGAGCT-3′ | ||
| 3 | 5′-CTGCTGCAAAGTTGGCGTTTGCC-3′ | ||
| ActRIIB | |||
| Antisense | 1 | 5′-GCCACCCAGGCGCCGTCATG-3′ | 21nt, 76% |
| 2 | 5′-AGAGGAGGGCGAGGGCCA-3′ | 18nt, 72% | |
| 3 | 5′-AGGGCCACCCAGGGCGCCGTCA-3′ | 22nt, 77% | |
| Sense | 1 | 5′-CATGACGGCGCCTGGGTGGC-3′ | |
| 2 | 5′-TGGCCCTCGCCCTCCTCT-3′ | ||
| 3 | 5′-TGACGGCGCCCTGGGTGGCCCT-3′ |
Together with our previous findings that blocking ActRII signaling decreases PC-3 and LNCaP viability (Figure 2A) and increases cell detachment, our results suggested that ActRII signaling may modulate the attachment of cells through extracellular matrix (ECM) proteins to surrounding cells and to the surface of the plate. This increased cell detachment may be attributed to increased ECM protein degradation as a result of the increase in the expression/activity of ECM-degrading enzymes such as matrix metalloproteinases. In this context, it has recently been demonstrated that the expression of ADAM-15 disintegrin, which cleaves integrin molecules (see [45] for a recent review), is strongly correlated with the metastatic potential of prostate, breast [46], and pancreatic cancers [47]. Furthermore, ADAM-15 has been shown to be involved in cell migration and invasion [48,49]. To test the hypothesis that activin A signaling mediates the adhesion of cells through the regulation of ECM proteins, PC-3 cells were incubated with the ActRII blocking antibody and the expression of ADAM-15 was measured. Suppression of ActRII signaling with the blocking antibody for 3 days increased the expression of ADAM-15 3.4-fold (Figure 2G). ADAM-15 expression after treatment with ActRII blocking antibody was time-dependent, increasing 14-fold after 24 hours and declining to ∼2- to 3-fold after 3 days (data not shown). These results suggest that blocking ActRII signaling decreases the proliferative potential of PC-3 cells by decreasing cell adhesion.
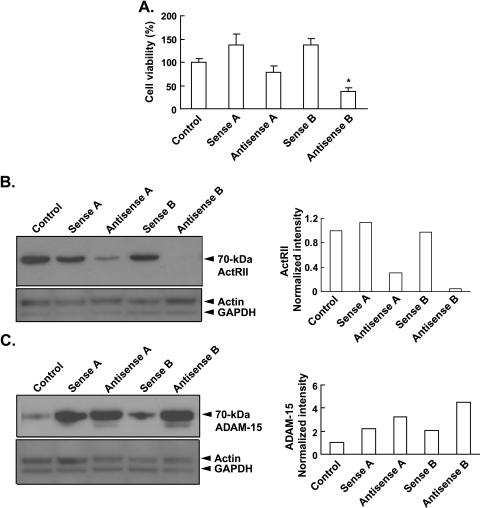
To further confirm these results, we used antisense-P to suppress ActRIIA and ActRIIB signaling. Like the blocking antibody (Figure 2A), antisense-P to ActRIIA and ActRIIB decreased cell viability by 43% and 73% compared with sense controls, respectively (Figure 3A). Antisense-P to both ActRIIA and ActRIIB potently suppressed the expression of ActRII in PC-3 cells by 73% and 96%, respectively (Figure 3B). This suppression of ActRIIA and ActRIIB expression correlated with 1.5- and 2.1-fold increases in ADAM-15 expression, respectively, above the respective sense controls (Figure 3C). These results are consistent with that observed using the blocking antibody (Figure 2) and together indicate that blocking ActRII signaling increases ADAM-15 expression and cell detachment and therefore decreases cell viability.
Figure 3.
Antisense oligonucleotide suppression of ActRII signaling inhibits PCC adhesion and viability. PC-3 cells were plated in 10% serum at 40% confluence for 24 hours, after which cells were either collected (day 0 control) or treated every day for 3 days with 1) medium containing 10% serum plus lipofectamine (control), plus 2) sense-P oligonucleotide to ActRIIA (sense A), 3) antisense-P oligonucleotide to ActRIIA (antisense A), 4) sense-P oligonucleotide to ActRIIB (sense B), or 5) antisense-P oligonucleotides to ActRIIB (antisense B; final concentration of 0.4 µM each). (A) PC-3 cell viability was measured using the trypan blue staining assay. Results are presented as number of cells (mean ± SEM; n = 3–6; (*significantly different from day 3 control, P < .005). (B) Immunoblot analysis of ActRII (70 kDa) was performed using a mouse monoclonal antibody against human ActRII and normalization against β-actin (47 kDa) using a goat antihuman β-actin antibody. Quantitation of blot is shown on the right. (C) Immunoblot analysis of PC-3 cells for ADAM-15 (70 kDa) using a rabbit antihuman ADAM-15 antibody and normalization against β-actin (47 kDa) using a goat antihuman β-actin antibody. Quantitation of blot is shown on the right.
ActRII Signaling Regulates Neuroblastoma Cell Viability and Proliferation
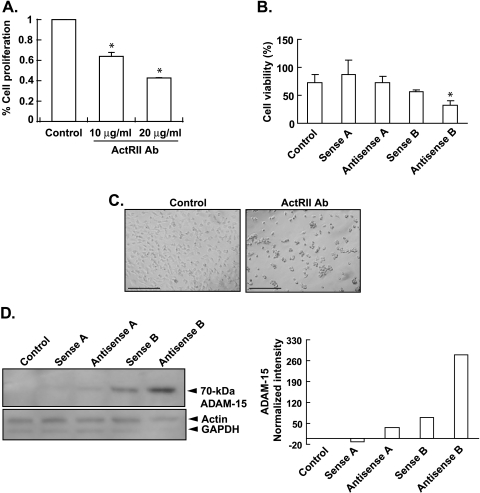
To determine whether activin signaling was a general requirement for the viability and proliferation of other cancer cell lines, we treated a neuroblastoma cell line (M17) with increasing concentrations of the ActRII blocking antibody. The ActRII blocking antibody induced a dose-dependent decrease in cell viability (Figure 4A). Similarly, antisense-P to ActRIIB (but not ActRIIA) induced a significant 43% decrease in cell viability compared with sense control (Figure 4B), indicating that ActRIIB signaling in neuroblastoma cells, like prostate cancer cells, is necessary for cell viability. Like PC-3 and LNCaP cells, treatment of M17 neuroblastoma cells with anti-ActRII blocking antibody induced rounded cells with fewer processes, a generally smaller appearance, and increased detachment from the plate (Figure 4C). Suppression of ActRIIB expression with antisense-P increased ADAM-15 expression four-fold (Figure 4D), suggesting that ActRIIB signaling also regulates cell attachment in neuroblastoma cells. These results indicate that ActRIIB signaling may be universally important for regulating cell viability and proliferation.
Figure 4.
ActRII signaling regulates cell proliferation, viability, morphology, and attachment of M17 neuroblastoma cells. (A) Human M17 neuroblastoma cells cultured in OPTI-MEM medium were plated in 0.5% FBS at 40% confluence. After 24 hours, wells were treated with the antihuman ActRII mouse monoclonal antibody (0, 10, or 20 µg/ml). Cell proliferation was assessed using the MTS assay after 3 days. Results are presented as percentage change from the control (mean ± SEM; n = 3; *significantly different from control, P < .001). Ab = antibody. (B) M17 cells were plated in 10% serum at 40% confluence for 24 hours, after which cells were either collected (day 0 control) or treated every day for 3 days with 1) medium containing 10% serum plus lipofectamine (control), plus 2) sense-P oligonucleotide to ActRIIA (sense A), 3) antisense-P oligonucleotide to ActRIIA (antisense A), 4) sense-P oligonucleotide to ActRIIB (sense B), or 5) antisense-P oligonucleotides to ActRIIB (antisense B; final concentration of 0.4 µM each). M17 cell viability was measured using the trypan blue staining assay. Results are presented as number of cells (mean ± SEM; n = 4; *significantly different from day 3 control, P < .005). (C) M17 neuroblastoma cells cultured in OPTI-MEM medium were plated in 0.5% FBS for 1 day before treatment ± antihuman ActRII antibody (20 µg/ml) for 3 days. Original magnification, x 100. Scale, 400 µm. (D) M17 cells were plated exactly as in Figure 4B, and immunoblot analysis was performed for ADAM-15 as in Figure 3C. Quantitation of blot is shown on the right.
Synergistic Effect of Hormones on PCC Proliferation
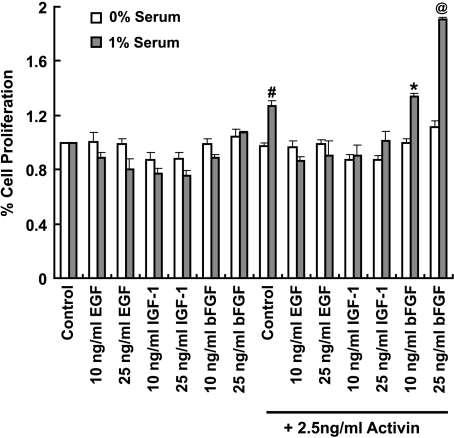
Our results indicate that ActRII signaling is an absolute requirement for cell proliferation but that it is not sufficient because activin A, in the absence of serum, does not significantly increase PCC proliferation (Figures 1 and 5). Because fibroblast growth factors (FGFs) are expressed at increased levels in prostate cancer [50–52] and given that bFGF (FGF2) is required along with transforming growth factor β (TGF-β)/activin signaling for the growth of human embryonic stem cells [53], we determined the effect of bFGF with and without activin A in the presence and absence of serum. Interestingly, like activin A, bFGF, either in the presence or in the absence of serum, did not increase PCC proliferation (Figure 5). However, bFGF cultured in the presence of 1% serum plus activin A increased proliferation 30%, compared with control (1% serum plus activin A).
Figure 5.
Basic fibroblast growth factor synergizes with activin A to induce serum-dependent PCC proliferation. PC-3 cells were plated at 40% confluence in medium ± 1% serum for 24 hours before being treated with EGF, IGF-1, and bFGF (10 or 25 ng/ml) ± activin (2.5 ng/ml). Cell proliferation was assessed using the MTS assay after 3 days. Results are presented as a mean percentage of untreated control ± SEM; n = 4(#significantly different from 1% serum control without activin A, P < .01; *significantly different from 1% serum control without activin A, P < .01; @significantly different from 1% serum control with activin A, P < .001).
Because there also is an increased localization of IGF [54–56] and EGF [57–60] in the prostate tumor epithelium, we next tested these growth factors for PCC proliferation with and without activin A in the absence and presence of serum. Epidermal growth factor and IGF-1 did not induce PC-3 cell proliferation in the presence or absence of 1% serum and/or activin A (Figure 5). There was a trend for a decrease in cell proliferation with IGF-1, which returned to control levels with addition of activin A (Figure 5). These results suggest that bFGF can synergize with activin A to induce cell proliferation, but only in the presence of serum, indicating that other serum factors contribute to PCC proliferation. Together, these results indicate that multiple (at least two) mitogenic/differentiation signals are required for PCC proliferation.
Discussion
Multiple Cell Signaling Factors Are Required for PCC Proliferation
That cancer cells require FBS for growth in vitro and that circulating serum factors are required for cancer cell growth in vivo are well recognized. From our experiments using antisense and antibody-blocking strategies, we have identified activin signaling through ActRII as an absolute requirement for serum induced PCC and neuroblastoma cell proliferation (Figures 1–5). These data support previous findings indicating that whereas mutational changes to apoptotic genes are required for the growth of tumors, this alone is insufficient to promote cancer cell proliferation [1–7]. Although activin A was found to be necessary, it was not sufficient, to stimulate prostatic and neuroblastoma cell proliferation in vitro (Figures 1–5), suggesting that at least two serum factors are required for in vitro cancer cell proliferation. Indeed, the only other system examined to date assessing the hormonal requirements for cell proliferation in vitro has found that, under defined culture conditions, both a bFGF and a TGF-β family member are necessary for the proliferation of pluripotent human embryonic stem cells [53]. However, whereas bFGF synergized with activin A to enhance PCC proliferation, neither hormone alone nor in combination induced PCC proliferation in the absence of serum (Figure 5). Importantly, these results indicate that PCC, like human embryonic stem cells, also requires more than one mitogenic factor to proliferate. Other than activin A and bFGF, the specific serum components required to promote PCC proliferation in defined conditions remain to be identified. Given our findings, it is important that any in vitro cell culture experiment performed using serum take into account the synergistic effect of the added hormonal factor(s) with serum factors.
ActRII Signaling Is Obligatory for Serum-Induced PCC and Neuroblastoma Cell Proliferation
The absolute requirement for ActRII signaling for normal PCC viability and proliferation was indicated by 1) activin A-induced Smad2 phosphorylation and proliferation of PCC; 2) inhibition of FBS-induced PCC proliferation by both a specific activin A antibody and inhibin A; 3) suppression of FBS-mediated PCC viability, concurrent with the dramatic decrease in both Smad2 phosphorylation and ActRII expression, by an ActRII blocking antibody; 4) inhibition of activin A-induced PC-3 cell growth in the presence of ActRII blocking antibody; 5) inhibition of PCC proliferation in the presence of ActRII antisense-P oligonucleotides; and 6) the increase in ADAM-15 expression, alteration in cell morphology, and increase in cell detachment with suppressed ActRII signaling (Figures 1–5). Suppression of ActRII signaling induced similar morphological changes for both LNCaP and PC-3 cells, suggesting a critical role for ActRII signaling independent of androgen receptor signaling. Similar biochemical, morphological, and viability data indicated that M17 neuroblastoma cell adhesion, viability, and proliferation also were modulated by ActRII signaling (Figure 4).
A growing body of data suggests potential roles of activin and inhibin, members of the TGF-β superfamily of growth hormones, as local regulators of normal and abnormal prostate gland growth [61,62]. Evidence supporting the role of these proteins in the regulation of prostatic epithelial cell (PEC) proliferation includes the different expression and localization of inhibin α and β subunits by normal, malignant, and benign prostatic cells. Importantly, the βA subunit and dimeric activin (βA, βA) have been shown to be expressed by normal [63,64], malignant [28], benign [25], and prostate cancer cell lines [27], whereas inhibin a has been shown to be expressed by normal differentiated epithelial cells only [63,65] in benign prostate hyperplasia and in nonmalignant regions of prostate carcinoma [66] but is not detected in malignant prostatic cells or prostate cancer cell lines [66,67]. Whether activin A-induced PCC proliferation is normal or abnormal may depend on the expression of inhibin because inhibin serves to antagonize the action of activin [22] by competing for binding to ActRII, consequently, blocking activin action [68,69]. Indeed, addition of inhibin A to FBS reduced PC-3 cell number. A role for inhibin in tumor suppression is indicated from studies of inhibin-deficient mice where both male and female animals develop gonadal tumors [70]. The loss of inhibin expression has been correlated with numerous cancers (see [61] for review). For example, a loss of expression of the inhibin α subunit is associated with high-grade prostate [66] and breast cancer [71], and as mentioned above, inhibin is not detected in malignant prostatic cells or prostate cancer cell lines [66]. Thus, the age-related increase in activin A signaling with the loss of inhibin expression with the dysregulation of the HPG axis [36] may well explain the prostatic neoplasia observed with aging. In support of this, activin A serum concentrations are significantly increased in both prostate and breast cancer patients compared with sex-matched controls; within prostate patients, those with bone metastasis had higher serum activin A compared with those without [29].
Given this in vivo data, it is therefore puzzling why activin has been shown to actually inhibit the proliferation of primary cultured prostate cells [72] and androgen-sensitive prostate cancer cell lines [22,73,74] in a dose- and time-dependent manner. One explanation is that these studies used relatively high FBS concentrations (∼10%). We have demonstrated that under such conditions, the expression of ActRII and the downstream Smad2 signaling cascade is significantly downregulated in PC-3 cells (Vadakkadath Meethal et al., unpublished data). Thus, activin A signaling may be suppressed, or alternatively, addition of exogenous activin A may further downregulate ActRII expression and signaling such as is seen with GnRHR agonists [75], thereby limiting cell proliferation. This is supported by the inhibition of PCC proliferation observed with the ActRII blocking antibody and antisense treatments (Figures 2 and 3).
About androgen-insensitive PCCs, previous studies using exogenous activin A failed to demonstrate any change in PC-3 cell proliferation [23,73], whereas in our experiments, activin A induced proliferation of PC-3 cells when used at physiological concentrations (∼1–2 µg/L; Figure 1B) [37]. The lack of activin-induced PC-3 cell proliferation in previous studies may be due to the higher FBS (and therefore activin A) concentrations used, thereby masking the effect of exogenous activin A [23]. Along these lines, high concentrations of serum may promote considerable expression of the activin ligand follistatin [27], thereby blocking activin signaling. Alternatively, as discussed above, the increased concentration of inhibin α subunit in high concentrations of FBS may antagonize activin A [22,68,69], thereby inhibiting activin-induced PC-3 cell proliferation.
ActRII signaling was found to be essential for the proliferation of both androgen-sensitive LNCaP cells and androgen-insensitive PC-3 cells (Figure 2); receptors for ActRIIB are expressed in both cell types [23,74]. Previous studies have shown that activin A and follistatin proteins are expressed and secreted by PC-3 cells, but although LNCaP cells express activin A, they do not seem to secrete it [27,73]. These findings indicate a gain of function by androgen-insensitive and metastatic cells in the production of a growth/detachment factor crucial for cell proliferation/mobilization. Similarly, because activin A is necessary for LNCaP cell proliferation, the results suggest paracrine production of activin A within the prostate [63,64,73] for normal PEC growth. That only activin A, but not other HPG hormones, induce a proliferative response in the presence of serum is consistent with the fact that the suppression of serum LH, FSH, and testosterone concentrations with GnRH superagonists eventually becomes ineffective at suppressing tumor growth [8,76].
Considerable evidence exists that activin signaling plays a role in cancer. Activin A has been found to be overexpressed in stage IV colorectal cancer [77], whereas activin A mRNA levels correlate with poor prognosis for esophageal carcinoma [78]. Activins are known to stimulate cell growth in ovarian cells [22] and are elevated in the serum of women with ovarian tumors [79]. High levels of activin postoperatively strongly correlate with recurrence of ovarian cancer [79]. Elevated serum activin also has been reported in endometrial tumors, and levels decline after surgical removal of the tumor [80].
Basic Fibroblast Growth Factor Synergizes with Activin A to Enhance PCC Proliferation
Growth of PCC is dependent on interactions with the prostate stroma and ECM through integrin and growth factor receptor-mediated systems [81–86]. Specifically, growth factors including, but not limited to, members of the FGF family such as bFGF, FGF7, and FGF10 [87–90] as well as IGF [91] and EGF [92,93], are expressed and secreted primarily by the stromal compartment of the prostate and act on their cognate receptors expressed in the luminal or basal epithelial cells. We did not observe any affect of bFGF, IGF, or EGF on the proliferation of androgen-insensitive PCC in the presence or absence of serum in vitro. However, we found that bFGF, but not other growth factors, enhanced activin A-induced serum-dependent cell proliferation in androgen-insensitive PCCs (Figure 5). Basic fibroblast growth factor as well as FGF1, FGF6, FGF8, and FGF9 are all expressed at increased levels in the prostate tumor epithelium [50–52,94–96], and bFGF has been associated with metastatic progression of prostate cancer [96]. Although we observe no effect of bFGF alone on PC-3 cell proliferation in 1% serum, the synergy observed between activin A and bFGF on cell proliferation is consistent with previous in vivo findings that bFGF promotes metastasis in mouse models [96]. The synergistic effect of bFGF and activin A on PCC proliferation is important in the context that both bFGF and activin A are actively synthesized by PEC and that activin A secretion occurs in more aggressive cell phenotypes [27,28], supporting an autocrine function in the growth of advanced prostate cancers. Thus, whether activin A-induced PCC proliferation is normal or abnormal may depend on the microenvironmental expression levels of bFGF and vice versa.
ActRII Signaling Modulates ADAM-15 Expression in PCCs and Neuroblastoma Cells
Suppression of ActRII signaling did not induce PCC apoptosis (Figure 2F), indicating that ActRII-mediated attachment mediates cell fate. In this respect, we have shown for the first time that ActRII signaling regulates ADAM-15 expression, a disintegrin whose expression has been highly correlated with the metastatic potential of PECs [46]. Suppression of ActRII signaling increased ADAM-15 expression and promoted cell detachment in PCCs and neuroblastoma cells (Figures 2 and 3), two factors associated with metastasis of primary tumors [46–49]. Thus ActRII seems to mediate cell attachment via ADAM-15, which has been shown by Najy et al. [97] to support prostate cancer metastasis by modulating tumor cell-endothelial cell interaction. Interestingly, substantial decreases in P-Smad2 and Smad4 levels are found in tumor specimens with primary Gleason grades 3 and 4, whereas in grade 5, levels are markedly higher [98]. Because the cellular phenotype of Gleason grades 3 and 4 is indicative of cellular invasion into the surrounding stroma, these results strongly support our findings that activin A regulates ECM adhesion and indicate that PCC may be migrating toward tissue environments of higher activin A secretion. Thus, the metastatic potential of PCCs may depend on ActRII signaling; a decrease in activin A (or high inhibin expression) would promote cell detachment from its ECM, with relocalization to a new tissue; reattachment would be dependent on a high microenvironmental level of activin A (or low inhibin) expression. Put another way, the microenvironmental expression of activins may dictate where PCCs take residence and explain why certain tumors preferentially metastasize to particular tissues.
Conclusions
Given the finding that activin A is pro-proliferative and, based on our novel finding, that ActRII signaling regulates the expression of the disintegrin ADAM-15, we propose a model for aggressive cancer progression, i.e., when there is sufficient ActRII signaling, ADAM-15 expression decreases and PCCs will be adhered to the ECM and can proliferate. When ActRII signaling is insufficient, ADAM-15 expression dramatically increases, leading to detachment of cells from the ECM. Cell detachment can lead to either apoptotic cell death or metastasis should the cell find an environment containing adequate activin A and bFGF. Thus, our findings have important implications for the determination of cell fate.
That neither activin A nor bFGF alone was sufficient for PCC proliferation indicates that additional serum factors are required to induce cell proliferation. Alterations in serum hormone composition with the dysregulation of the HPG axis during aging and in disease states will have a marked effect on both the proliferative and metastatic potential of PCC. For example, inhibin levels decline markedly with reproductive senescence during aging leading to unopposed activin signaling due to a high serum ratio of activin to inhibin. That the activin/inhibin ratio is important is indicated by the fact that inhibin α is not detectable in malignant prostatic cells or prostate cancer cell lines [66,67]. Further identification of the exact endocrine and autocrine/paracrine factors that synergize with activin A is therefore important.
There are currently few options for the treatment of androgen-insensitive metastatic prostate cancers. Aside from androgen signaling, our results indicate that blocking ActRII signaling may be a therapeutic strategy for age-related cancers. However, given the metastatic potential associated with decreased ActRII signaling, therapeutic strategies will need to consider suppressing ActRII signaling along with antimetastatic or tumor-specific proapoptotic agents. Conversely, therapeutic strategies that upregulate ActRII signaling to suppress metastasis may inadvertently promote tumor growth. Because ActRII signaling also was necessary for neuroblastoma cell proliferation and cell adhesion, these therapeutic strategies also may apply to other cancer types.
Supplementary Material
Abbreviations
- ActRII
activin receptor type II
- bFGF
basic fibroblast growth factor
- EGF
epidermal growth factor
- ECM
extracellular matrix protein
- FBS
fetal bovine serum
- FSH
follicle-stimulating hormone
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GnRH
gonadotropin-releasing hormone
- HPG
hypothalamic-pituitary-gonadal
- IGF-1
insulin-like growth factor 1
- LH
luteinizing hormone
- PEC
prostatic epithelial cell
- PCC
prostatic cancer cell
Footnotes
This article refers to supplementary material, which is designated by Figure W1 and is available online at www.neoplasia.com.
References
- 1.McCullough KD, Coleman WB, Smith GJ, Grisham JW. Age-dependent induction of hepatic tumor regression by the tissue microenvironment after transplantation of neoplastically transformed rat liver epithelial cells into the liver. Cancer Res. 1997;57:1807–1813. [PubMed] [Google Scholar]
- 2.McCullough KD, Coleman WB, Ricketts SL, Wilson JW, Smith GJ, Grisham JW. Plasticity of the neoplastic phenotype in vivo is regulated by epigenetic factors. Proc Natl Acad Sci USA. 1998;95:15333–15338. doi: 10.1073/pnas.95.26.15333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coleman WB, McCullough KD, Esch GL, Faris RA, Hixson DC, Smith GJ, Grisham JW. Evaluation of the differentiation potential of WB-F344 rat liver epithelial stem-like cells in vivo. Differentiation to hepatocytes after transplantation into dipeptidylpeptidase-IV-deficient rat liver. Am J Pathol. 1997;151:353–359. [PMC free article] [PubMed] [Google Scholar]
- 4.Lee LM, Seftor EA, Bonde G, Cornell RA, Hendrix MJ. The fate of human malignant melanoma cells transplanted into zebrafish embryos: assessment of migration and cell division in the absence of tumor formation. Dev Dyn. 2005;233:1560–1570. doi: 10.1002/dvdy.20471. [DOI] [PubMed] [Google Scholar]
- 5.Topczewska JM, Postovit LM, Margaryan NV, Sam A, Hess AR, Wheaton WW, Nickoloff BJ, Topczewski J, Hendrix MJ. Embryonic and tumorigenic pathways converge via Nodal signaling: role in melanoma aggressiveness. Nat Med. 2006;12:925–932. doi: 10.1038/nm1448. [DOI] [PubMed] [Google Scholar]
- 6.Hendrix MJ, Seftor EA, Seftor RE, Kasemeier-Kulesa J, Kulesa PM, Postovit LM. Reprogramming metastatic tumour cells with embryonic microenvironments. Nat Rev Cancer. 2007;7:246–255. doi: 10.1038/nrc2108. [DOI] [PubMed] [Google Scholar]
- 7.Maffini MV, Calabro JM, Soto AM, Sonnenschein C. Stromal regulation of neoplastic development: age-dependent normalization of neoplastic mammary cells by mammary stroma. Am J Pathol. 2005;167:1405–1410. doi: 10.1016/S0002-9440(10)61227-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schally AV, Comaru-Schally AM, Plonowski A, Nagy A, Halmos G, Rekasi Z. Peptide analogs in the therapy of prostate cancer. Prostate. 2000;45:158–166. doi: 10.1002/1097-0045(20001001)45:2<158::aid-pros10>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 9.Miyamoto H, Messing EM, Chang C. Androgen deprivation therapy for prostate cancer: current status and future prospects. Prostate. 2004;61:332–353. doi: 10.1002/pros.20115. [DOI] [PubMed] [Google Scholar]
- 10.Sharifi N, Gulley JL, Dahut WL. Androgen deprivation therapy for prostate cancer. JAMA. 2005;294:238–244. doi: 10.1001/jama.294.2.238. [DOI] [PubMed] [Google Scholar]
- 11.Navarro D, Luzardo OP, Fernandez L, Chesa N, Diaz-Chico BN. Transition to androgen-independence in prostate cancer. J Steroid Biochem Mol Biol. 2002;81:191–201. doi: 10.1016/s0960-0760(02)00064-x. [DOI] [PubMed] [Google Scholar]
- 12.Lee EC, Tenniswood MP. Emergence of metastatic hormone-refractory disease in prostate cancer after anti-androgen therapy. J Cell Biochem. 2004;91:662–670. doi: 10.1002/jcb.20040. [DOI] [PubMed] [Google Scholar]
- 13.Barki-Harrington L, Daaka Y. Bradykinin induced mitogenesis of androgen independent prostate cancer cells. J Urol. 2001;165:2121–2125. doi: 10.1097/00005392-200106000-00081. [DOI] [PubMed] [Google Scholar]
- 14.Berteaux N, Lottin S, Adriaenssens E, Van Coppenolle F, Leroy X, Coll J, Dugimont T, Curgy JJ. Hormonal regulation of H19 gene expression in prostate epithelial cells. J Endocrinol. 2004;183:69–78. doi: 10.1677/joe.1.05696. [DOI] [PubMed] [Google Scholar]
- 15.Miyazaki H, Watabe T, Kitamura T, Miyazono K. BMP signals inhibit proliferation and in vivo tumor growth of androgen-insensitive prostate carcinoma cells. Oncogene. 2004;23:9326–9335. doi: 10.1038/sj.onc.1208127. [DOI] [PubMed] [Google Scholar]
- 16.Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, Rosenfeld MG, Sawyers CL. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–39. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki H, Ueda T, Ichikawa T, Ito H. Androgen receptor involvement in the progression of prostate cancer. Endocr Relat Cancer. 2003;10:209–216. doi: 10.1677/erc.0.0100209. [DOI] [PubMed] [Google Scholar]
- 18.Bahk JY, Hyun JS, Lee H, Kim MO, Cho GJ, Lee BH, Choi WS. Expression of gonadotropin-releasing hormone (GnRH) and GnRH receptor mRNA in prostate cancer cells and effect of GnRH on the proliferation of prostate cancer cells. Urol Res. 1998;26:259–264. doi: 10.1007/s002400050054. [DOI] [PubMed] [Google Scholar]
- 19.Halmos G, Arencibia JM, Schally AV, Davis R, Bostwick DG. High incidence of receptors for luteinizing hormone-releasing hormone (LHRH) and LHRH receptor gene expression in human prostate cancers. J Urol. 2000;163:623–629. [PubMed] [Google Scholar]
- 20.Gnanapragasam VJ, Darby S, Khan MM, Lock WG, Robson CN, Leung HY. Evidence that prostate gonadotropin-releasing hormone receptors mediate an anti-tumourigenic response to analogue therapy in hormone refractory prostate cancer. J Pathol. 2005;206:205–213. doi: 10.1002/path.1767. [DOI] [PubMed] [Google Scholar]
- 21.Ben-Josef E, Yang SY, Ji TH, Bidart JM, Garde SV, Chopra DP, Porter AT, Tang DG. Hormone-refractory prostate cancer cells express functional follicle-stimulating hormone receptor (FSHR) J Urol. 1999;161:970–976. [PubMed] [Google Scholar]
- 22.Wang QF, Tilly KI, Tilly JL, Preffer F, Schneyer AL, Crowley WF, Jr, Sluss PM. Activin inhibits basal and androgen-stimulated proliferation and induces apoptosis in the human prostatic cancer cell line, LNCaP. Endocrinology. 1996;137:5476–5483. doi: 10.1210/endo.137.12.8940374. [DOI] [PubMed] [Google Scholar]
- 23.Dalkin AC, Gilrain JT, Bradshaw D, Myers CE. Activin inhibition of prostate cancer cell growth: selective actions on androgen-responsive LNCaP cells. Endocrinology. 1996;137:5230–5235. doi: 10.1210/endo.137.12.8940339. [DOI] [PubMed] [Google Scholar]
- 24.van Schaik RH, Wierikx CD, Timmerman MA, Oomen MH, van Weerden WM, van der Kwast TH, van Steenbrugge GJ, de Jong FH. Variations in activin receptor, inhibin/activin subunit and follistatin mRNAs in human prostate tumour tissues. Br J Cancer. 2000;82:112–117. doi: 10.1054/bjoc.1999.0886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas TZ, Chapman SM, Hong W, Gurusingfhe C, Mellor SL, Fletcher R, Pedersen J, Risbridger GP. Inhibins, activins, and follistatins: expression of mRNAs and cellular localization in tissues from men with benign prostatic hyperplasia. Prostate. 1998;34:34–43. doi: 10.1002/(sici)1097-0045(19980101)34:1<34::aid-pros5>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 26.Dirnhofer S, Berger C, Hermann M, Steiner G, Madersbacher S, Berger P. Coexpression of gonadotropic hormones and their corresponding FSH- and LH/CG-receptors in the human prostate. Prostate. 1998;35:212–220. doi: 10.1002/(sici)1097-0045(19980515)35:3<212::aid-pros7>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 27.McPherson SJ, Mellor SL, Wang H, Evans LW, Groome NP, Risbridger GP. Expression of activin A and follistatin core proteins by human prostate tumor cell lines. Endocrinology. 1999;140:5303–5309. doi: 10.1210/endo.140.11.7141. [DOI] [PubMed] [Google Scholar]
- 28.Thomas TZ, Wang H, Niclasen P, O'Bryan MK, Evans LW, Groome NP, Pedersen J, Risbridger GP. Expression and localization of activin subunits and follistatins in tissues from men with high grade prostate cancer. J Clin Endocrinol Metab. 1997;82:3851–3858. doi: 10.1210/jcem.82.11.4374. [DOI] [PubMed] [Google Scholar]
- 29.Leto G, Incorvaia L, Badalamenti G, Tumminello FM, Gebbia N, Flandina C, Crescimanno M, Rini G. Activin A circulating levels in patients with bone metastasis from breast or prostate cancer. Clin Exp Metastasis. 2006;23:117–122. doi: 10.1007/s10585-006-9010-5. [DOI] [PubMed] [Google Scholar]
- 30.Kaighn ME, Lechner JF, Narayan KS, Jones LW. Prostate carcinoma: tissue culture cell lines. Natl Cancer Inst Monogr. 1978;49:17–21. [PubMed] [Google Scholar]
- 31.Kaighn ME, Narayan KS, Ohnuki Y, Lechner JF, Jones LW. Establishment and characterization of a human prostatic carcinoma cell line (PC-3) Invest Urol. 1979;17:16–23. [PubMed] [Google Scholar]
- 32.Horoszewicz JS, Leong SS, Chu TM, Wajsman ZL, Friedman M, Papsidero L, Kim U, Chai LS, Kakati S, Arya SK, et al. The LNCaP cell line—a new model for studies on human prostatic carcinoma. Prog Clin Biol Res. 1980;37:115–132. [PubMed] [Google Scholar]
- 33.Horoszewicz JS, Leong SS, Kawinski E, Karr JP, Rosenthal H, Chu TM, Mirand EA, Murphy GP. LNCaP model of human prostatic carcinoma. Cancer Res. 1983;43:1809–1818. [PubMed] [Google Scholar]
- 34.Bowen RL, Verdile G, Liu T, Parlow AF, Perry G, Smith MA, Martins RN, Atwood CS. Luteinizing hormone, a reproductive regulator that modulates the processing of amyloid-beta precursor protein and amyloid-beta deposition. J Biol Chem. 2004;279:20539–20545. doi: 10.1074/jbc.M311993200. [DOI] [PubMed] [Google Scholar]
- 35.Chahal HS, Drake WM. The endocrine system and ageing. J Pathol. 2007;211:173–180. doi: 10.1002/path.2110. [DOI] [PubMed] [Google Scholar]
- 36.Bowen RL, Atwood CS. Living and dying for sex. A theory of aging based on the modulation of cell cycle signaling by reproductive hormones. Gerontology. 2004;50:265–290. doi: 10.1159/000079125. [DOI] [PubMed] [Google Scholar]
- 37.Harada K, Shintani Y, Sakamoto Y, Wakatsuki M, Shitsukawa K, Saito S. Serum immunoreactive activin A levels in normal subjects and patients with various diseases. J Clin Endocrinol Metab. 1996;81:2125–2130. doi: 10.1210/jcem.81.6.8964839. [DOI] [PubMed] [Google Scholar]
- 38.Welt C, Sidis Y, Keutmann H, Schneyer A. Activins, inhibins, and follistatins: from endocrinology to signaling. A paradigm for the new millennium. Exp Biol Med. 2002;227:724–752. doi: 10.1177/153537020222700905. [DOI] [PubMed] [Google Scholar]
- 39.Pangas SA, Woodruff TK. Activin signal transduction pathways. Trends Endocrinol Metab. 2000;11:309–314. doi: 10.1016/s1043-2760(00)00294-0. [DOI] [PubMed] [Google Scholar]
- 40.Abe Y, Minegishi T, Leung PC. Activin receptor signaling. Growth Factors. 2004;22:105–110. doi: 10.1080/08977190410001704688. [DOI] [PubMed] [Google Scholar]
- 41.Xu G, Zhou H, Wang Q, Auersperg N, Peng C. Activin receptor-like kinase 7 induces apoptosis through up-regulation of Bax and down-regulation of Xiap in normal and malignant ovarian epithelial cell lines. Mol Cancer Res. 2006;4:235–246. doi: 10.1158/1541-7786.MCR-05-0174. [DOI] [PubMed] [Google Scholar]
- 42.Lebrun JJ, Takabe K, Chen Y, Vale W. Roles of pathway-specific and inhibitory Smads in activin receptor signaling. Mol Endocrinol. 1999;13:15–23. doi: 10.1210/mend.13.1.0218. [DOI] [PubMed] [Google Scholar]
- 43.Wu JW, Hu M, Chai J, Seoane J, Huse M, Li C, Rigotti DJ, Kyin S, Muir TW, Fairman R, et al. Crystal structure of a phosphorylated Smad2. Recognition of phosphoserine by the MH2 domain and insights on Smad function in TGF-beta signaling. Mol Cell. 2001;8:1277–1289. doi: 10.1016/s1097-2765(01)00421-x. [DOI] [PubMed] [Google Scholar]
- 44.Wu JW, Fairman R, Penry J, Shi Y. Formation of a stable heterodimer between Smad2 and Smad4. J Biol Chem. 2001;276:20688–20694. doi: 10.1074/jbc.M100174200. [DOI] [PubMed] [Google Scholar]
- 45.Rocks N, Paulissen G, El Hour M, Quesada F, Crahay C, Gueders M, Foidart JM, Noel A, Cataldo D. Emerging roles of ADAM and ADAMTS metalloproteinases in cancer. Biochimie. 2008;90:367–379. doi: 10.1016/j.biochi.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 46.Kuefer R, Day KC, Kleer CG, Sabel MS, Hofer MD, Varambally S, Zorn CS, Chinnaiyan AM, Rubin MA, Day ML. ADAM15 disintegrin is associated with aggressive prostate and breast cancer disease. Neoplasia. 2006;8:319–329. doi: 10.1593/neo.05682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamada D, Ohuchida K, Mizumoto K, Ohhashi S, Yu J, Egami T, Fujita H, Nagai E, Tanaka M. Increased expression of ADAM 9 and ADAM 15 mRNA in pancreatic cancer. Anticancer Res. 2007;27:793–799. [PubMed] [Google Scholar]
- 48.Martin J, Eynstone LV, Davies M, Williams JD, Steadman R. The role of ADAM 15 in glomerular mesangial cell migration. J Biol Chem. 2002;277:33683–33689. doi: 10.1074/jbc.M200988200. [DOI] [PubMed] [Google Scholar]
- 49.Charrier-Hisamuddin L, Laboisse CL, Merlin D. ADAM-15: a metalloprotease that mediates inflammation. FASEB J. 2008;22:641–653. doi: 10.1096/fj.07-8876rev. [DOI] [PubMed] [Google Scholar]
- 50.Nakamoto T, Chang CS, Li AK, Chodak GW. Basic fibroblast growth factor in human prostate cancer cells. Cancer Res. 1992;52:571–577. [PubMed] [Google Scholar]
- 51.Cronauer MV, Hittmair A, Eder IE, Hobisch A, Culig Z, Ramoner R, Zhang J, Bartsch G, Reissigl A, Radmayr C, et al. Basic fibroblast growth factor levels in cancer cells and in sera of patients suffering from proliferative disorders of the prostate. Prostate. 1997;31:223–233. doi: 10.1002/(sici)1097-0045(19970601)31:4<223::aid-pros3>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 52.Dorkin TJ, Robinson MC, Marsh C, Neal DE, Leung HY. aFGF immunoreactivity in prostate cancer and its co-localization with bFGF and FGF8. J Pathol. 1999;189:564–569. doi: 10.1002/(SICI)1096-9896(199912)189:4<564::AID-PATH480>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 53.Ludwig TE, Bergendahl V, Levenstein ME, Yu J, Probasco MD, Thomson JA. Feeder-independent culture of human embryonic stem cells. Nat Methods. 2006;3:637–646. doi: 10.1038/nmeth902. [DOI] [PubMed] [Google Scholar]
- 54.Chan JM, Stampfer MJ, Giovannucci E, Gann PH, Ma J, Wilkinson P, Hennekens CH, Pollak M. Plasma insulin-like growth factor-I and prostate cancer risk: a prospective study. Science. 1998;279:563–566. doi: 10.1126/science.279.5350.563. [DOI] [PubMed] [Google Scholar]
- 55.Mantzoros CS, Tzonou A, Signorello LB, Stampfer M, Trichopoulos D, Adami HO. Insulin-like growth factor 1 in relation to prostate cancer and benign prostatic hyperplasia. Br J Cancer. 1997;76:1115–1118. doi: 10.1038/bjc.1997.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wolk A, Mantzoros CS, Andersson SO, Bergstrom R, Signorello LB, Lagiou P, Adami HO, Trichopoulos D. Insulin-like growth factor 1 and prostate cancer risk: a population-based, case-control study. J Natl Cancer Inst. 1998;90:911–915. doi: 10.1093/jnci/90.12.911. [DOI] [PubMed] [Google Scholar]
- 57.Connolly JM, Rose DP. Production of epidermal growth factor and transforming growth factor-alpha by the androgen-responsive LNCaP human prostate cancer cell line. Prostate. 1990;16:209–218. doi: 10.1002/pros.2990160304. [DOI] [PubMed] [Google Scholar]
- 58.Hofer DR, Sherwood ER, Bromberg WD, Mendelsohn J, Lee C, Kozlowski JM. Autonomous growth of androgen-independent human prostatic carcinoma cells: role of transforming growth factor alpha. Cancer Res. 1991;51:2780–2785. [PubMed] [Google Scholar]
- 59.Carruba G, Leake RE, Rinaldi F, Chalmers D, Comito L, Sorci C, Pavone-Macaluso M, Castagnetta LA. Steroid-growth factor interaction in human prostate cancer. 1: Short-term effects of transforming growth factors on growth of human prostate cancer cells. Steroids. 1994;59:412–420. doi: 10.1016/0039-128x(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 60.McEleny KR, Watson RW, Coffey RN, O'Neill AJ, Fitzpatrick JM. Inhibitors of apoptosis proteins in prostate cancer cell lines. Prostate. 2002;51:133–140. doi: 10.1002/pros.10061. [DOI] [PubMed] [Google Scholar]
- 61.Risbridger GP, Schmitt JF, Robertson DM. Activins and inhibins in endocrine and other tumors. Endocr Rev. 2001;22:836–858. doi: 10.1210/edrv.22.6.0450. [DOI] [PubMed] [Google Scholar]
- 62.Chen YG, Lui HM, Lin SL, Lee JM, Ying SY. Regulation of cell proliferation, apoptosis, and carcinogenesis by activin. Exp Biol Med (Maywood) 2002;227:75–87. doi: 10.1177/153537020222700201. [DOI] [PubMed] [Google Scholar]
- 63.Risbridger GP, Thomas T, Gurusinghe CJ, McFarlane JR. Inhibinrelated proteins in rat prostate. J Endocrinol. 1996;149:93–99. doi: 10.1677/joe.0.1490093. [DOI] [PubMed] [Google Scholar]
- 64.Al-Omari R, Shidaifat F, Dardaka M. Castration induced changes in dog prostate gland associated with diminished activin and activin receptor expression. Life Sci. 2005;77:2752–2759. doi: 10.1016/j.lfs.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 65.Cancilla B, Jarred RA, Wang H, Mellor SL, Cunha GR, Risbridger GP. Regulation of prostate branching morphogenesis by activin A and follistatin. Dev Biol. 2001;237:145–158. doi: 10.1006/dbio.2001.0364. [DOI] [PubMed] [Google Scholar]
- 66.Mellor SL, Richards MG, Pedersen JS, Robertson DM, Risbridger GP. Loss of the expression and localization of inhibin alpha-subunit in high grade prostate cancer. J Clin Endocrinol Metab. 1998;83:969–975. doi: 10.1210/jcem.83.3.4640. [DOI] [PubMed] [Google Scholar]
- 67.Ying C, Zhang Z, Ying SY. Expression and localization of activin beta A-subunit and activin receptors in TM3, a mouse Leydig cell line. Endocr Res. 1995;21:815–824. doi: 10.1080/07435809509030494. [DOI] [PubMed] [Google Scholar]
- 68.Gray PC, Greenwald J, Blount AL, Kunitake KS, Donaldson CJ, Choe S, Vale W. Identification of a binding site on the type II activin receptor for activin and inhibin. J Biol Chem. 2000;275:3206–3212. doi: 10.1074/jbc.275.5.3206. [DOI] [PubMed] [Google Scholar]
- 69.Xu J, McKeehan K, Matsuzaki K, McKeehan WL. Inhibin antagonizes inhibition of liver cell growth by activin by a dominant-negative mechanism. J Biol Chem. 1995;270:6308–6313. doi: 10.1074/jbc.270.11.6308. [DOI] [PubMed] [Google Scholar]
- 70.Matzuk MM, Finegold MJ, Su JG, Hsueh AJ, Bradley A. alpha-Inhibin is a tumour-suppressor gene with gonadal specificity in mice. Nature. 1992;360:313–319. doi: 10.1038/360313a0. [DOI] [PubMed] [Google Scholar]
- 71.Mylonas I, Jeschke U, Shabani N, Kuhn C, Friese K, Gerber B. Inhibin/activin subunits (inhibin-alpha, -betaA and -betaB) are differentially expressed in human breast cancer and their metastasis. Oncol Rep. 2005;13:81–88. [PubMed] [Google Scholar]
- 72.Wang M, Liu A, Garcia FU, Rhim JS, Stearns ME. Growth of HPV-18 immortalized human prostatic intraepithelial neoplasia cell lines. Influence of IL-10, follistatin, activin-A, and DHT. Int J Oncol. 1999;14:1185–1195. doi: 10.3892/ijo.14.6.1185. [DOI] [PubMed] [Google Scholar]
- 73.McPherson SJ, Thomas TZ, Wang H, Gurusinghe CJ, Risbridger GP. Growth inhibitory response to activin A and B by human prostate tumour cell lines, LNCaP and DU145. J Endocrinol. 1997;154:535–545. doi: 10.1677/joe.0.1540535. [DOI] [PubMed] [Google Scholar]
- 74.Zhang Z, Zhao Y, Batres Y, Lin MF, Ying SY. Regulation of growth and prostatic marker expression by activin A in an androgen-sensitive prostate cancer cell line LNCAP. Biochem Biophys Res Commun. 1997;234:362–365. doi: 10.1006/bbrc.1997.6649. [DOI] [PubMed] [Google Scholar]
- 75.Schally AV. [The discovery of hypothalamic hormones and the development of antitumor analogs] Ann Urol (Paris) 2005;39(Suppl 3):S46–S50. doi: 10.1016/s0003-4401(05)80007-5. [DOI] [PubMed] [Google Scholar]
- 76.Labrie F, Belanger A, Luu-The V, Labrie C, Simard J, Cusan L, Gomez J, Candas B. Gonadotropin-releasing hormone agonists in the treatment of prostate cancer. Endocr Rev. 2005;26:361–379. doi: 10.1210/er.2004-0017. [DOI] [PubMed] [Google Scholar]
- 77.Wildi S, Kleeff J, Maruyama H, Maurer CA, Buchler MW, Korc M. Overexpression of activin A in stage IV colorectal cancer. Gut. 2001;49:409–417. doi: 10.1136/gut.49.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yoshinaga K, Mimori K, Yamashita K, Utsunomiya T, Inoue H, Mori M. Clinical significance of the expression of activin A in esophageal carcinoma. Int J Oncol. 2003;22:75–80. [PubMed] [Google Scholar]
- 79.Lambert-Messerlian GM, DePasquale SE, Maybruck WM, Steinhoff MM, Gajewski WH. Secretion of activin A in recurrent epithelial ovarian carcinoma. Gynecol Oncol. 1999;74:93–97. doi: 10.1006/gyno.1999.5417. [DOI] [PubMed] [Google Scholar]
- 80.Petraglia F, Florio P, Luisi S, Gallo R, Gadducci A, Vigano P, Di Blasio AM, Genazzani AR, Vale W. Expression and secretion of inhibin and activin in normal and neoplastic uterine tissues. High levels of serum activin A in women with endometrial and cervical carcinoma. J Clin Endocrinol Metab. 1998;83:1194–1200. doi: 10.1210/jcem.83.4.4689. [DOI] [PubMed] [Google Scholar]
- 81.Mori H, Maki M, Oishi K, Jaye M, Igarashi K, Yoshida O, Hatanaka M. Increased expression of genes for basic fibroblast growth factor and transforming growth factor type beta 2 in human benign prostatic hyperplasia. Prostate. 1990;16:71–80. doi: 10.1002/pros.2990160108. [DOI] [PubMed] [Google Scholar]
- 82.Cohen RJ, Glezerson G, Haffejee Z. Neuro-endocrine cells—a new prognostic parameter in prostate cancer. Br J Urol. 1991;68:258–262. doi: 10.1111/j.1464-410x.1991.tb15318.x. [DOI] [PubMed] [Google Scholar]
- 83.Chung LW, Li W, Gleave ME, Hsieh JT, Wu HC, Sikes RA, Zhau HE, Bandyk MG, Logothetis CJ, Rubin JS. Human prostate cancer model: roles of growth factors and extracellular matrices. J Cell Biochem Suppl. 1992;16H:99–105. doi: 10.1002/jcb.240501222. [DOI] [PubMed] [Google Scholar]
- 84.Comoglio PM, Trusolino L. Invasive growth: from development to metastasis. J Clin Invest. 2002;109:857–862. doi: 10.1172/JCI15392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cunha GR, Hayward SW, Wang YZ. Role of stroma in carcinogenesis of the prostate. Differentiation. 2002;70:473–485. doi: 10.1046/j.1432-0436.2002.700902.x. [DOI] [PubMed] [Google Scholar]
- 86.Sung SY, Chung LW. Prostate tumor-stroma interaction: molecular mechanisms and opportunities for therapeutic targeting. Differentiation. 2002;70:506–521. doi: 10.1046/j.1432-0436.2002.700905.x. [DOI] [PubMed] [Google Scholar]
- 87.Story MT. Regulation of prostate growth by fibroblast growth factors. World J Urol. 1995;13:297–305. doi: 10.1007/BF00185973. [DOI] [PubMed] [Google Scholar]
- 88.Story MT, Livingston B, Baeten L, Swartz SJ, Jacobs SC, Begun FP, Lawson RK. Cultured human prostate-derived fibroblasts produce a factor that stimulates their growth with properties indistinguishable from basic fibroblast growth factor. Prostate. 1989;15:355–365. doi: 10.1002/pros.2990150408. [DOI] [PubMed] [Google Scholar]
- 89.Sherwood ER, Fong CJ, Lee C, Kozlowski JM. Basic fibroblast growth factor: a potential mediator of stromal growth in the human prostate. Endocrinology. 1992;130:2955–2963. doi: 10.1210/endo.130.5.1374018. [DOI] [PubMed] [Google Scholar]
- 90.Alarid ET, Rubin JS, Young P, Chedid M, Ron D, Aaronson SA, Cunha GR. Keratinocyte growth factor functions in epithelial induction during seminal vesicle development. Proc Natl Acad Sci USA. 1994;91:1074–1078. doi: 10.1073/pnas.91.3.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cohen P, Peehl DM, Lamson G, Rosenfeld RG. Insulin-like growth factors (IGFs), IGF receptors, and IGF-binding proteins in primary cultures of prostate epithelial cells. J Clin Endocrinol Metab. 1991;73:401–407. doi: 10.1210/jcem-73-2-401. [DOI] [PubMed] [Google Scholar]
- 92.Cohen DW, Simak R, Fair WR, Melamed J, Scher HI, Cordon-Cardo C. Expression of transforming growth factor-alpha and the epidermal growth factor receptor in human prostate tissues. J Urol. 1994;152:2120–2124. doi: 10.1016/s0022-5347(17)32335-2. [DOI] [PubMed] [Google Scholar]
- 93.Freeman MR, Paul S, Kaefer M, Ishikawa M, Adam RM, Renshaw AA, Elenius K, Klagsbrun M. Heparin-binding EGF-like growth factor in the human prostate: synthesis predominantly by interstitial and vascular smooth muscle cells and action as a carcinoma cell mitogen. J Cell Biochem. 1998;68:328–338. doi: 10.1002/(sici)1097-4644(19980301)68:3<328::aid-jcb4>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 94.Sciavolino PJ, Abate-Shen C. Molecular biology of prostate development and prostate cancer. Ann Med. 1998;30:357–368. doi: 10.3109/07853899809029935. [DOI] [PubMed] [Google Scholar]
- 95.Giri D, Ropiquet F, Ittmann M. FGF9 is an autocrine and paracrine prostatic growth factor expressed by prostatic stromal cells. J Cell Physiol. 1999;180:53–60. doi: 10.1002/(SICI)1097-4652(199907)180:1<53::AID-JCP6>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 96.Polnaszek N, Kwabi-Addo B, Peterson LE, Ozen M, Greenberg NM, Ortega S, Basilico C, Ittmann M. Fibroblast growth factor 2 promotes tumor progression in an autochthonous mouse model of prostate cancer. Cancer Res. 2003;63:5754–5760. [PubMed] [Google Scholar]
- 97.Najy AJ, Day KC, Day ML. ADAM15 supports prostate cancer metastasis by modulating tumor cell-endothelial cell interaction. Cancer Res. 2008;68:1092–1099. doi: 10.1158/0008-5472.CAN-07-2432. [DOI] [PubMed] [Google Scholar]
- 98.Perttu MC, Martikainen PM, Huhtala HS, Blauer M, Tammela TL, Tuohimaa PJ, Syvala H. Altered levels of Smad2 and Smad4 are associated with human prostate carcinogenesis. Prostate Cancer Prostatic Dis. 2006;9:185–189. doi: 10.1038/sj.pcan.4500871. [DOI] [PubMed] [Google Scholar]
- 99.Vadakkadath Meethal S, Atwood CS. The role of hypothalamic-pituitary-gonadal hormones in the normal structure and functioning of the brain. Cell Mol Life Sci. 2005;62:257–270. doi: 10.1007/s00018-004-4381-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.