Abstract
The effects of serotonin (5-HT) on tumor growth are inconsistent. We investigated whether a decreased level of 5-HT affected tumor growth using 5-HT transporter knockout (5-HTT-/-) mice, which showed 5-HT depletion. When cancer cells were injected subcutaneously into both 5-HTT-/- and 5-HTT+/+ mice, the tumor growth was markedly attenuated in 5-HTT-/- mice. Serotonin levels in the blood, forebrain, and tumors of 5-HTT-/- mice bearing tumors were significantly smaller than those of their 5-HTT+/+ littermates. However, 5-HT did not increase cancer cells' proliferation in vitro. When we applied 5-HTT inhibitors to the wild mice bearing tumors, they did not inhibit tumor growth. The endothelial nitric oxide synthase (eNOS) expressions in tumors were reduced in 5-HTT-/- mice compared with 5-HTT+/+ mice. Stimulations with 5-HT (1–50 µM) induced eNOS expressions in human umbilical vein endothelial cell (HUVEC) in a concentration-dependent manner. When we measured activations of multiple signaling pathways by using a high-throughput phosphospecific antibodies platform, 5-HT stimulated the extracellular signal-regulated kinase 1/2 (ERK1/2) in HUVEC. Moreover, we found that the physiological level of 5-HT induced phosphorylation of both ERK1/2 and eNOS in HUVEC. Human umbilical vein endothelial cell expressed both 5-HT2B and 5-HT2C receptors. SB204741, a specific 5-HT2B receptor inhibitor, blocked 5-HT-induced ERK1/2 and eNOS phosphorylations, whereas RS102221, a specific 5-HT2C receptor inhibitor, did not in HUVEC. SB204741 reduced microvessel density in tumors and inhibited the proliferation of HUVEC in vitro. These results suggest that regulation of 5-HT and 5-HT receptors, especially the 5-HT2B receptor, may serve as a therapeutic strategy in cancer therapy.
Introduction
The results of clinical studies have thus far failed to show whether psychological factors affect cancer development [1]. We previously reported that emotions induced by hyperdopaminergic transmission, such as haloperidol-sensitive delirium, attenuate tumor growth [2,3]. The neurotransmitter serotonin (5-hydoxytryptamine [5-HT]) is causally involved in multiple central nervous facets of mood in regulating sleep, anxiety, alcoholism, drug abuse, food intake, and sexual behavior. The effect of 5-HT on tumor growth is still unclear [4–7]. Moreover, it has been reported that exogeneously applied high doses of 5-HT exert a direct mitogenic effect on tumor cells, whereas low doses of 5-HT reduce tumor growth by decreasing the blood supply to the tumors [7], suggesting that the role of 5-HT on tumor growth is concentration-dependent on 5-HT. Among the subtypes of 5-HT receptors, which have been identified (5-HT1–7) based on their structural, functional, and pharmacological characteristics [8], several subtypes have been found to exist in cancers [9–11]. The 5-HT2 receptor subfamily consists of three members, i.e., 5-HT2A, 5-HT2B, and 5-HT2C receptors. The 5-HT2B receptor has been detected in many peripheral organs in several mammalian species including humans, primarily in the cardiovascular system, the gut, and the stomach fundus [12–18]. Especially, the blockade of 5-HT2B is known to inhibit colon cancer and a prostate cancer cell line [11,19].
Nitric oxide (NO) is a major endothelium-derived relaxing factor [20]. Vasoconstriction is caused by activation of 5-HT1D and 5-HT2A receptors on vascular smooth muscle cells [21], whereas vasodilatation has been attributed to activation of 5-HT2B receptors on endothelial cells, mediating the release of relaxing factors [22,23]. Endothelial nitric oxide synthase (eNOS), which produces NO in endothelial cells, plays a major role in inhibiting vasoconstriction to 5-HT [24,25]. Endothelial nitric oxide synthase is known to be stimulated by 5-HT [26]. Moreover, eNOS plays a role in the angiogenesis of tumor growth [27]. The levels of eNOS were found to increase in the carcinoma and to promote cancer progression by providing a selective growth advantage to tumor cells [28].
In peripheral tissues, 5-HT regulates vascular tone, gut mortality, primary homeostasis, and cell-mediated immune response [29,30]. Enterochromaffin cells produce and secrete far more 5-HT than either the central or the peripheral serotonergic neurons. The secreted 5-HT overflows to reach the peripheral blood by platelets [31–34]. Platelets, which do not synthesize 5-HT, are totally dependent on uptake to obtain 5-HT [30]. In 5-HT transporter (5-HTT) knockout mice, the level of 5-HT in the peripheral blood is virtually nil [34].
Although the concentration of 5-HT in the plasma has been shown to vary depending on psychological state, the level of hypertension, polymorphism in the 5-HTT-linked promoter region, and use of 5-HT reuptake inhibitors (selective serotonin reuptake inhibitors, SSRIs), the effect of a decreased level of 5-HTT on tumor growth has not been heretofore studied. Use of SSRI has been reported to reduce the risk of colorectal cancer in a large case-control study [35]. These studies suggest the existence of a strong relationship between 5-HTand cancer growth. Therefore, using 5-HTT-/- mice, we investigated whether a decreased level of 5-HT affects tumor growth. In the present study, we found that specific inhibition of the 5-HT2B receptor inhibited implanted lung cancer tumors in mice. On the basis of the analysis of a decreased level of 5-HT on tumor growth, we clarified the molecular mechanisms of the cancer inhibition by 5-HT2B blockade.
Materials and Methods
Animals
Six- to 9-week-old male mutant mice lacking 5-HTT and littermate wild-type mice were obtained from heterozygous crosses with a 129Sv/C57BL6 mixed genetic background. Details of the generation of 5-HTT-/- mice have been described previously [36]. We generated homozygous, heterozygous, and wild mice by crossing adult heterozygotes. DNA extract for tail biopsies were genotyped using polymerase chain reaction (PCR). Mice were group housed (two to four per cage) with food and water ad libitum in a room maintained at 22 ± 2°C and 65 ± 5% humidity under a 12-hour light-dark cycle. The animals were killed with an overdose of urethane (20 g/kg). All animal experiments were performed according to the Animals (Scientific Procedures) Act 1986 and approved by the local ethics panel at the Tohoku University School of Medicine.
Cell Culture
Lewis lung carcinoma (LLC), B16F0, and KLN205 cells were purchased from American Type Culture Collection (Manassas, VA). Lewis lung carcinoma and B16F0 cells were cultured in high-glucose Dulbecco's modified Eagle's medium containing 10% fetal calf serum, 100 U/ml penicillin, and 0.1 mg/ml streptomycin. KLN205 cells were cultured in minimum essential medium containing 10% fetal calf serum, 1% nonessential amino acids, and 100 µg/ml kanamycin. Human umbilical vein endothelial cells (HUVECs) were purchased from Kurabo (Osaka, Japan) and cultured in EC growth medium (Kurabo).
In Vivo Tumor Models
LLCs or B16F0 cells were injected (1 x 106 cells per animal) subcutaneously (s.c.) into the flank of male 6- to 9-week-old wild-type and 5-HTT-/- mice on day 0. KLN205 cells were injected (5 x 105 cells per animal) s.c. into the flank of male 6- to 9-week-old BDF1 mice on day 0. In solid-tumor growth experiments, paroxetine (20 mg/kg), fluvoxamine (20 mg/kg), SB204741 (N-(1-methyl-5-indolyl)-N-(3-methyl-5-isothiazolyl) urea), or RS102221 (8-[5-(5-amino 2,4-dimethoxyphenyl) 5-oxopentyl]-1,3,8-triazaspiro[4,5] decane-2,4-dione) (Tocris Cookson, Inc, Ellisville, MO) was injected intraperitoneally (i.p.) every 2 days from day 6. Tumor size was quantified daily as width2 x length x 0.52. Mice inoculated with LLCs, those inoculated with B16F0 cells, and those inoculated with KLN205 cells were killed on days 23, 19, and 33, respectively. Control mice were treated with DMSO or saline.
Cell Proliferation Assays
The cell proliferation assay was performed as previously described [37]. Briefly, LLCs, KLN205, or HUVEC (5 x 103 cells) were plated onto 96-well plates and incubated with 0, 1, 10, and 100 µM SB204741 or RS102221. Each cell was cultured for 48 hours, and then the number of cells was determined by water-soluble tetrazolium assay using a cell counting kit (Dojindo, Kumamoto, Japan).
Detection of 5-HT Receptor mRNA by Reverse Transcription-Polymerase Chain Reaction Analysis
Total RNA was extracted from the cells using RNAzol B reagent (Tel-Test, Friendswood, TX) following the manufacturer's instructions. Reverse transcription-polymerase chain reaction analyses for 5-HT2A, 5-HT2B, and 5-HT2c receptors were performed as previously described [21,38]. cDNA obtained by oligo-dT primed reverse transcription of total RNA isolated from HUVEC was subjected to PCR amplification with receptor-specific primers: 5-HT2A receptor 5′-TCTTTAAGGCGGGGAGTTGCT-3′ and 5′-TTTTGCTCATTGCTGATGGACTG-3′; 5-HT2B receptor 5′-TGCCATTCCAGTCCCTATT-3′ and 5′-GTGGATGT- TCTTCGCATAAGT-3′; 5-HT2C receptor 5′-TGCATTCATTCCT- TGTGCAC-3′ and 5′-ATATCTAGGTAGTGGCCAGA-3′; and the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) 5′-GTCTTC- ACCACCATGGAGAA-3′ and 5′-ATCCACAGTCTTCTGGGT- GG-3′. Polymerase chain reaction products were separated by 1.5% agarose gel electrophoresis and visualized by ethidium bromide staining.
Protein Analysis
When the diameter of the tumor became 1 cm, tumor tissues were suspended in a lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.25% deoxycholic acid, 1% NP-40, 1 mM EDTA) containing protease inhibitors (20 mg/ml leupeptin, 1 mg/ml pepstatin A, and 1 mM PMSF) and then sonicated on ice. After centrifugation at 16,000g for 15 minutes, the supernatant was eluted in an SDS sample buffer (60 mM Tris-HCl, pH 6.7, 3% SDS, 2% 2-mercaptoethanol, and 5% glycerol) for 5 minutes. Next, 2 x 105 HUVECs were seeded in 10-cm dishes, cultured for 2 days, serum-starved (0.1% serum) for 24 hours, and then treated with various concentrations of 5-HT (0–50 µM). Cells treated with 5-HT or saline were suspended in a lysis buffer containing protease inhibitors and then sonicated on ice. Cell extracts were centrifuged, and the supernatant was boiled and subjected to 10% SDS-PAGE for transfer onto polyvinylidene difluoride membranes (Millipore, Billerica, MA). Each membrane was blotted with Abs to eNOS, phospho-eNOS (Ser 1177), extracellular signal-regulated kinase 1/2 (ERK1/2), and phospho-ERK1/2 (Cell Signaling Technology, Beverly, MA). Anti-5-HT2B receptor, -5-HT2C receptor, and -5-HTT were purchased from Santa Cruz Biotechnology, Santa Cruz, CA. The membranes were developed with an ECL Western Blotting Detection System Advance (Amersham Biosciences, Bucks, United Kingdom) according to the manufacturer's instructions. Phosphoprotein detection was performed by using the human phospho-MAPK assay Array kit (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions. Image analysis was performed with ImageJ 1.37 software (National Institutes of Health, Bethesda, MD).
Immunohistochemistry
When the tumor diameter became 1 cm, under deep pentobarbital anesthesia (50 mg/kg body weight, i.p.), mice were perfused transcardially with 4% formaldehyde in a 0.1-M phosphate buffer. Tumor tissues were fixed in 10% formalin, embedded in paraffin, and sectioned. They were blocked with 10% normal goat serum and incubated with polyclonal antihuman factor VIII-related Ag Ab (Dako Japan, Kyoto, Japan). Subsequently, the sections were incubated with biotinylated goat antirabbit IgG (Vector Laboratories, Burlingame, CA), and then treated with the ABC kit (Vector Laboratories), for the detection of factor VIII-related Ag by 3-amino-9-ethylcarbazole (Vector Laboratories), and counterstained with hematoxylin.
Fluorescent Immunostaining
Sample preparation was the same as above. Sections (12 µm in thickness) were cut from the frozen tumor with a cryostat (CM1900; Leica, Heidelberg, Germany) and mounted onto glass slides (Dako, Carpinteria, CA). After incubation with 10% goat serum (Nichirei, Tokyo, Japan) for 1 hour at room temperature, the sections were incubated with mice monoclonal anti.factor VIII antibody (1:100) and rabbit polyclonal anti-phsopho-eNOS antibody (1:500; Cell Signaling Technology) for 36 hours at 4°C. Sections were subsequently incubated with fluorescein isothiocyanate-conjugated antimouse and Cy3-conjugated antirabbit IgG antibodies (1:100; Chemicon, Temecula, CA) for 1 hour at room temperature. Sections were then mounted on coverslips using antifade mounting medium (Vectashield; Vector Laboratories) and viewed using a confocal laser microscope (LSM 510; Carl Zeiss Meditec, Oberkochen, Germany).
Determination of Microvessel Density
Intratumoral microvessel density (MVD) was determined as previously described [37]. In brief, intratumoral vessels were stained immunohistochemically with antihuman factor VIII-related Ag Ab. The image that contained the highest number of microvessels was chosen for each section by initial scan at a magnification of x100, and then the vessels were counted in the selected image at a magnification of x200. At least four fields were counted for each section, and the highest count was then used. Two independent investigators evaluated the number of vessels.
Measurement of 5-HT
Serotonin was measured in the forebrain, tumors, and blood of wild type and 5-HTT-/- mice. These samples were prepared as previously described [39]. The prepared samples from the forebrain, tumors, and blood were used for the assay of 5-HT by HPLC with electrochemical detection.
Cytokine Enzyme-Linked Immunosorbent Assays
The concentrations of vascular endothelial growth factor (VEGF) in the plasma, and cell culture supernatants were determined using a murine VEGF ELISA kit (R&D Systems) according to the manufacturer's recommendation. Lewis lung carcinomas or B16F0 cells were injected (1 x 106 cells per animal) subcutaneously (s.c.) into the flank of male 6- to 9-week-old wild-type and 5-HTT-/- mice on day 0. Tumor size was quantified daily as width2 x length x 0.52. Mice inoculated with LLCs were killed on day 23. The inferior vena cava of the mouse was then punctured, peripheral blood was collected, and plasma was isolated.
Data Analysis
Statistical analysis of the results was performed using analysis of variance (ANOVA) with Fisher's least significant difference test for multiple comparisons. A value of P < .05 was considered significant.
Results
Decreased Tumor Growth in 5-HTT Knockout Mice
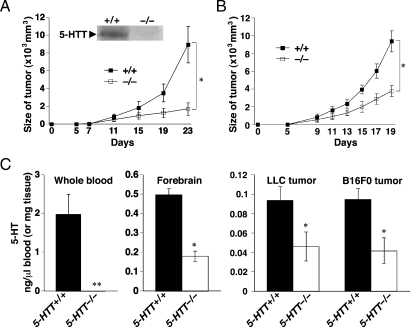
The immunoblot analysis of implanted tumor homogenate by murine 5-HTT antibody showed the existence of 5-HTT in the 5-HTT+/+ mice but not in the 5-HTT-/- mice (Figure 1A, insert). As shown in Figure 1, A and B, the tumor growth was markedly attenuated in 5-HTT-/- mice. To test whether this growth might reflect a direct effect of 5-HT on LLCs or B16F0 cells, we examined the response of LLCs or B16F0 cell to 5-HT in vitro. We found that 5-HT did not increase LLCs or B16F0 cells proliferation in vitro (data not shown). To quantify tumor angiogenesis, we stained tumors with an antibody against the factor VIII-related antigen, a blood vessel marker (Figure W1) [40]. There was no difference in tumor MVD estimated by factor VIII-related antigen staining between 5-HTT-/- and 5-HTT+/+ mice. Moreover, to examine the level of VEGF in the culture supernatant fluid, we performed enzyme-linked immunosorbent assay. There were no differences among LLCs and B16F0 cells with 5-HT or saline treatment (data not shown).
Figure 1.
Effects of 5-HT on tumor growth in mice. (A) A total of 1 x 106 LLC cells were implanted into 5-HTT-/- and 5-HTT+/+ mice. Tumor volumes were calculated from tumor measurements scored on the indicated day. Results are presented as the mean tumor volume ± SD (n = 6, 7 per group). Insert shows Western blot analysis of 5-HTT protein in tumors of 5-HTT-/- and 5-HTT+/+ mice. (B) A total of 1 x 106 B16F0 cells were implanted into 5-HTT-/- and 5-HTT+/+ mice. Tumor volumes were calculated from tumor measurements scored on the indicated day. Results are presented as the mean tumor volume ± SD (n = 6 per group). (C) Serotonin in whole blood, forebrain, and tumors of 5-HTT-/- and 5-HTT+/+ mice on day 23 (LLC) after implantation. Serotonin in tumors of 5-HTT-/- and 5-HTT+/+ mice on day 19 (B16F0) after implantation. Values represent the mean ± SD (n = 6, 7 per group). Statistically significant (*P < .05, **P < .01) compared with 5-HTT+/+ mice.
Decreased Level of 5-HT in Tumors of 5-HTT-/- Mice
We reported that 5-HTT-/- mice displayed reduced levels of 5-HT in the frontal cortex [36]. Next, we focused on 5-HT analyses of the tumor and peripheral blood. Mice lacking 5-HTT were generated, and either LLCs or B16F0 cells were injected (1 x 106 cells per animal) s.c. into the flank of both 5-HTT-/- and 5-HTT+/+ 6-week-old mice on day 0. The tumor size was quantified daily, and mice were killed on day 23. As shown in Figure 1C, in 5-HTT-/- mice bearing LLC tumors, electrochemical detection of 5-HT showed a level of 5-HT in the blood below the level of detection. Serotonin levels in the forebrain of 5-HTT-/- mice bearing LLC tumors were significantly lower than those of their 5-HTT+/+ littermates with LLC tumors. Serotonin levels of LLC or B16F0 tumors were also significantly lower in 5-HTT-/- mice than in 5-HTT+/+ mice.
5-HTT Inhibitors Did Not Decrease Tumor Growth
It has been reported that 5-HTT inhibitors inhibit cancer initiation by inhibition of 5-HT reuptake [41]. We thus investigated whether the 5-HTT inhibitors influence tumor growth. Lewis lung carcinoma cells were inoculated into the flank of C57BL/6J mice s.c. on day 0. From day 6 after tumor identification, we i.p. injected paroxetine, a 5-HTT inhibitor; fluvoxamine, a 5-HTT inhibitor; or saline every 2 days. Compared with saline treatment, paroxetine or fluvoxamine treatment did not inhibit tumor growth (Figure 2A). Moreover, we investigated the influence between 5-HTT inhibitors and another cancer cell line, KLN205. In the KLN205 tumor growth model, there were no differences between saline treatment and 5-HTT inhibitors (Figure 2B). Serotonin levels in the whole blood of mice treated with paroxetine or fluvoxamine were significantly lower than those of mice treated with saline (Figure 2C), but the 5-HT levels in tumors did not differ among saline-, paroxetine-, and fluvoxamine-treated mice (Figure 2D). These results suggest that levels of 5-HT in the tumor but not in the whole blood might influence tumor growth.
Figure 2.
Paroxetine and fluvoxamine did not decrease tumor growth. (A) Mice were injected s.c. with LLC on day 0 and were treated with saline (closed squares), paroxetine (circles), or fluvoxamine (open squares) from day 6, every 2fs days. (B) Mice were injected s.c. with KLN205 on day 0 and were treated with saline (closed squares), paroxetine (circles), or fluvoxamine (open squares) from day 6, every 2 days. Tumor volumes were calculated from tumor measurement scored on the indicated day. Results are presented as the mean tumor volume ± SD. (C, D) On day 23 (LLC) or 33 (KLN205) after implantation 5-HT in whole blood and tumors of mice with paroxetine, fluvoxamine, or saline. *Statistically significant (P < .05) compared with saline-treated mice. The values represent the mean ± SD (n = 8 per group).
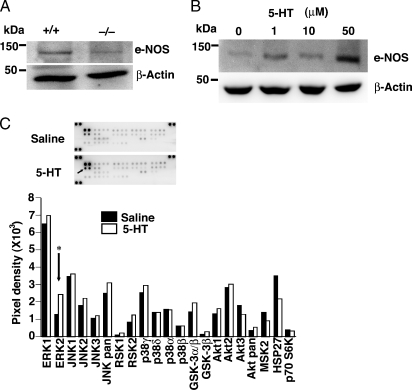
Decreased eNOS Expression in 5-HTT-/- Mice
Because eNOS plays a major role in inhibiting contraction of arteries to 5-HT, we next investigated the expression of eNOS in LLC tumors from 5-HTT-/- mice, which had 5-HT levels in the whole blood. We found that eNOS protein expression in tumors was lower in 5-HTT-/- mice than in 5-HTT+/+ mice (Figure 3A). As shown in Figure 3B, stimulation with 5-HT (1–50 µM) for 24 hours induced eNOS expression in HUVEC in a concentration-dependent manner. Next, we investigated whether 5-HT induced signaling pathways in HUVEC. According to previous reports, 5-HT induced phosphorylation of MAPK and Akt in various cell types [42]. However, HUVEC has not been studied yet. We measured the phosphorylation of multiple signaling pathways by using a high-throughput phosphospecific antibodies platform. Human umbilical vein endothelial cells were treated with or without 1-µM 5-HT, and cell signaling was assessed. As shown in Figure 3C, 5-HT stimulation modulated the ERK1/2 in HUVEC. These results suggest that 5-HT stimulation may induce eNOS expression through the ERK1/2 phosphorylation in HUVEC.
Figure 3.
Association between 5-HT and eNOS. (A) LLC tumors from 5-HTT-/- mice decreased eNOS. (B) Effects of 5-HT on 5-HT-induced eNOS in cultured HUVEC. Serotonin or saline was added to cultured HUVEC for 24 hours. Cells were collected for extraction and immunoblot analysis with antibodies to eNOS and β-actin. (C) Effects of serotonin on 5-HT-induced signaling pathways in cultured HUVEC. Saline or 5-HT, 1 µM, was added to cultured HUVEC for 10 minutes. Phosphorylation of intracellular signaling molecules was assessed by using the human Phospho-MAPK Array kit. The columns present the results of the densitometric analysis of the dot images corresponding to the phosphorylation status of individual protein. *Statistical significance was determined by 2-tailed-Student's t test.
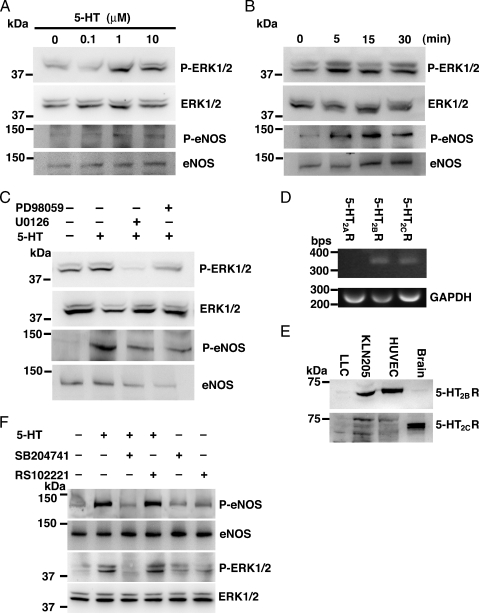
Stimulation of 5-HT2B Receptor Induced the Phosphorylation of eNOS through ERK1/2 in HUVEC
Phosphorylation of eNOS produced more bioavailability of the signaling molecule nitric oxide (NO) than eNOS. Endothelial nitric oxide synthase is phosphorylated in response to various forms of cellular stimulation in endothelial cells [43]. In the present study, we investigated whether eNOS phosphorylation was induced by 5-HT in HUVEC in detail. We stimulated HUVEC with various concentrations of 5-HT for 10 minutes. phosphorylations of ERK1/2 and eNOS were concentration-dependent of 5-HT (Figure 4A). As shown in Figure 4B, 1-µM 5-HT caused transient phosphorylation of both ERK1/2 and eNOS. Next, we examined the effect of U0126 or PD 98059, which are ERK1/2 inhibitors, on 5-HT-induced ERK1/2 phosphorylation. Human umbilical vein endothelial cells treated with U0126 or PD 98059 blocked not only ERK1/2 phosphorylation but also eNOS phosphorylation (Figure 4C). Moreover, we investigated what types of 5-HT receptors induced ERK1/2 phosphorylation in HUVEC. Reverse transcription-polymerase chain reaction and Western blot analysis revealed that 5-HT2B and 5-HT2C receptors were expressed in HUVEC (Figure 4, D and E). SB204741, which is a specific 5-HT2B receptor inhibitor, blocked 5-HT-induced ERK1/2 and eNOS phosphorylation, whereas RS102221, which is a specific 5-HT2C receptor inhibitor, did not (Figure 4F). These results suggest that the 5-HT2B receptor plays a pivotal role in HUVEC and that inhibition of the 5-HT2B receptor may reduce tumor growth.
Figure 4.
Stimulation of 5-HT2B receptor induced the phosphorylation of eNOS through ERK1/2 in HUVEC. (A) Effect of doses of 5-HT incubated for 10 minutes on phosphorylation of ERK1/2 and eNOS in HUVEC. (B) Serotonin induced activation of ERK1/2 and eNOS. Human umbilical vein endothelial cells were treated with 1-µM 5-HT for the indicated time. (C) Inhibition of ERK1/2 activity by U0126 and PD98059. Human umbilical vein endothelial cells were preincubated with 10-µM U0126 or 10-µM PD98059 for 30 minutes, then treated with 1-µM 5-HT for 5 minutes. (D) Reverse transcription-polymerase chain reaction analysis of selected 5-HT receptors in HUVEC. The bands representing the 5-HT2A receptor (301 bp), the 5-HT2B receptor (363 bp), the 5-HT2C receptor (354 bp), and GAPDH (268 bp) are indicated. (E) Western blot analysis to demonstrate the expression of 5-HT2B and 5-HT2C receptors in LLC, KLN205, and HUVEC. The proteins were detected with polyclonal 5-HT2B receptor and polyclonal 5-HT2C receptor antibodies. Mice forebrain expressed 5-HT2B and 5-HT2C receptors as a positive control. (F) Inhibition of ERK1/2 activity by SB204741. Human umbilical vein endothelial cells were preincubated with 10-µM SB204741 or 10-µM RS102221 for 30 minutes, then treated with 1-µM 5-HT for 5 minutes. Phosphorylation of ERK1/2 and eNOS was determined by Western blot analysis using phosphospecific antibodies.
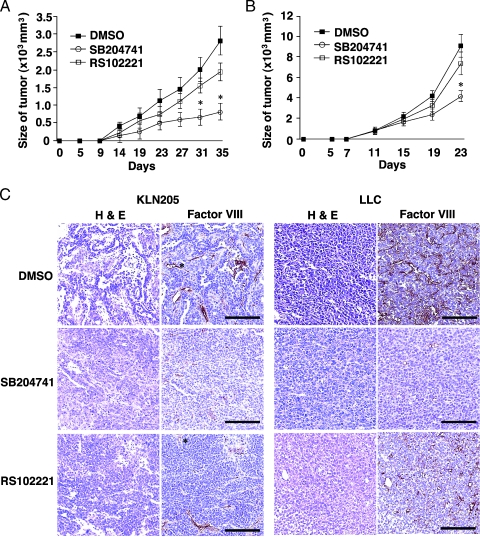
SB204741 Reduced Tumor Growth
We investigated whether inhibition of the 5-HT2B receptor influenced tumor growth in vivo because the 5-HT2B receptor antagonist reduced 5-HT-induced ERK1/2 and eNOS phosphorylation. KLN205 cells were inoculated into the flank of BDF1 mice s.c. on day 0. From day 6 after tumor identification, we injected SB204741 (20 mg/kg), RS102221 (20 mg/kg), or DMSO, as a control, i.p. every 2 days. Compared with DMSO treatment, SB204741 treatment inhibited tumor growth (Figure 5A). Similar results were obtained from the inoculation of LLCs (Figure 5B). We next examined the direct effect of SB204741 or RS102221 on the proliferation of HUVEC, LLCs, and KLN205 cells with various concentrations of these inhibitors. SB204741 reduced the proliferation of HUVEC and KLN205 cells in vitro (Figure W2). These results suggest that SB204741 reduced tumor growth because SB204741 inhibited the proliferation of the endothelial cells and KLN205 cells.
Figure 5.
SB204741 reduced tumor growth. (A) Mice were injected s.c. with KLN205 on day 0 and were treated with saline (closed squares), SB204741 (circles), or RS102221 (open squares) from day 6, every 2 days. (B) Mice were injected s.c. with LLC on day 0 and were treated with saline (closed squares), SB204741 (circles), or RS102221 (open squares) from day 6, every 2 days. Tumor volumes were calculated from tumor measurement scores on the indicated day. Results are presented as the mean tumor volume ± SD. (n = 8 per group). *Statistically significant (P < .05) compared with saline-treated mice. (C) When the diameter of the tumors reached 1 cm, mice were killed. Bars, 100 µm. Hematoxylin and eosin-stained sections of KLN205 or LLC tumors (left). Representative sections of tumors stained for factor VIII as a vascular endothelial marker (right; original magnification, x200).
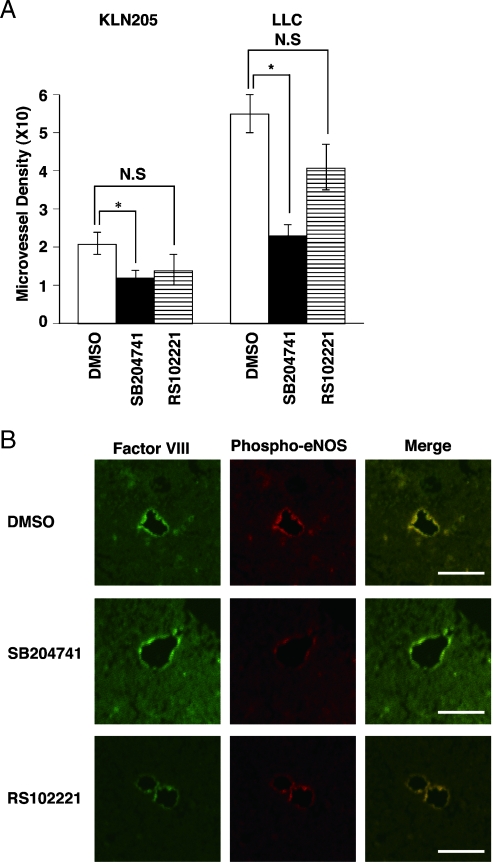
SB204741 Treatment Decreased Blood Vessel Density in Tumor Tissues
Hematoxylin and eosin staining of the tumor tissues revealed a decrease in vessels in KLN205 and LLC tumor tissues taken from SB204741-treated mice (Figure 5, C and D). To confirm the vessels, we stained paraffin sections immunohistochemically using an antibody against factor VIII-related Ag. Compared with DMSO-treated mice, we found fewer tumor vessels in SB204741-treated mice. The localization of phospho-eNOS in the LLC tumor tissues was determined by fluorescent immunostaining (Figure 6B). Immunostainning of phospho-eNOS was observed in the endothelial cells and completely overlapped that of factor VIII-related Ag. The differences in MVD between control and SB204741 mice were statistically significant (Figure 6A).
Figure 6.
SB204741 treatment induced a decrease in vessel density in tumor tissues. (A) Microvessel densities were calculated. Results are indicated as the mean ± SD of eight mice in each group. The difference in MVD between control and SB204741 treatment mice was statistically significant (*P < .05). N.S.: not significant by one-way ANOVA with Fisher's least significant difference test. (B) Location of phospho-eNOS in the endothelial cells. The LLC tumor tissues were stained with anti-factor VIII-related Ag (green) and anti-phospho-eNOS (red). The images were merged. Bars, 50 µm.
Discussion
This is the first study to extensively examine the effect of depletion of 5-HT on tumor growth in vivo. Conflicting results have been reported on the effect of exogeneously applied 5-HT on tumor growth [6]. In this study, we therefore investigated the effect of a low level of 5-HT, i.e., which is lower than the physiological level of 5-HT, on tumor growth. Using 5-HTT-deficient mice, we found that 5-HT levels in the tumor played a crucial role in tumor growth, suggesting that physiological levels of 5-HT in the tumor are needed for tumor growth. In addition, we found that SB204741, which is a 5-HT2B receptor inhibitor, suppressed the phosphorylation of ERK1/2 and eNOS in HUVEC. Moreover, we found that SB204741 reduced tumor growth by suppressing angiogenesis.
Although the inoculated cancer cells were not knocked out of 5-HTT, there was no 5-HTT protein in tumors implanted in 5-HTT mice, suggesting that the 5-HTT protein is completely absent in our 5-HT depletion models. Other methods to deplete 5-HT, such as deleting enzymes involved in 5-HT synthesis (tryptophan hydroxylases), may not work as long as LLC and B16F0 have the enzyme. If we knock out the enzyme in LLC and B16F0, we cannot exclude the possibility that the absence of the enzyme affects the proliferation and synthesis of LLCs and B16F0 cells.
Selective serotonin reuptake inhibitors are the most commonly prescribed drugs for treating depression. Therefore, the effects of SSRIs on cancer have been studied. Although it has been reported that SSRIs have cytotoxic activity against colorectal cancer [7,41,44], our results showed that paroxetine and fluvoxamine did not reduce tumor growth in vivo. Selective serotonin reuptake inhibitors are known to decrease whole-blood 5-HT concentrations in patients [45]. In this study, although paroxetine and fluvoxamine decreased the whole blood 5-HT concentration, it did not influence the 5-HT level in the tumor. These results suggest that the 5-HT levels in the tumor could be important, whereas SSRIs might have a different influence in each cancer type [44].
The effect of 5-HT depletion may be attributed to the specific vasodilating effect of 5-HT on the vessels supplying oxygen and nutrients to tumors. This hypothesis is supported by intravital microscopic evidence [6]. Moreover, by investigating eNOS expression in 5-HTT-/- tumors, we examined the underlying mechanism of why the physiological level of 5-HT is important for the maintenance of blood vessels supplying the blood flow to tumors.We found that eNOS protein was reduced in the tumors of 5-HTT-/- mice. Furthermore, we confirmed the importance of 5-HT in eNOS protein expression and phosphorylation in endothelial cells. Under the physiological level of 5-HT, 5-HT induced eNOS protein and phosphorylation of eNOS in a concentration-dependent manner, suggesting that constitutive eNOS is regulated in the presence of 5-HT in tumors.
It has been reported that 5-HT stimulates phosphorylation of ERK1/2 in bovine endothelial cells [26,46], and the 5-HT2B receptor was reported to play a role in the activation of eNOS in human endothelial cells [21]. Therefore, we focused on the 5-HT receptor 2 family, especially the 5-HT2B receptor. Our results indicate that the 5-HT2B receptor plays a pivotal role in the phosphorylation of both ERK1/2 and eNOS in HUVEC. The 5-HT2B receptor antagonists have been found to inhibit colon cancer [19], and our results suggest that 5-HT2B receptor antagonists reduce tumor growth by inhibiting angiogenesis through phosphorylation of ERK1/2 and eNOS.
There are no differences in MVD between tumor-bearing 5-HTT+/+ and 5-HTT-/- mice, whereas SB204741 treatment decrease MVD. Because the level of 5-HT in the tumor of 5-HTT-/- mice was reduced to half of that in 5-HTT+/+ mice (Figure 1C), 5-HT2B receptor in the tumor of 5-HTT-/- mice was stimulated to a certain extent. However, because SB204741 is specific and strong antagonist of 5-HT2B receptor, 5-HT2B receptor in the tumor of SB204741-treated mice was strongly blocked. Our datamight suggest that less 5-HT2B receptor stimulation could not decrease MVD, but completely blocking the 5-HT2B receptor could decrease MVD.
From the standpoint of therapeutic applications, there is an increasing evidence that the 5-HT2B receptor located on endothelial cells of meningeal blood may be implicated in the pathophysiology of migraine through the NO pathway [47]. In addition, recent studies have shown that the 5-HT2B receptor mediates the excitatory effects of 5-HT in the human colon [48], indicating that antagonists of this receptor might be valuable for treatment of irritable bowel syndrome [49]. We found that selective blockade of the 5-HT2B receptor resulted in the inhibition of tumor angiogenesis and growth through the inhibition effect of ERK1/2 and eNOS.
Recently, 5-HT-related medicines have been extensively used in many clinical settings such as psychiatric and coagulation disorders. In addition to the possibility of the 5-HT2B receptor as a molecular target, our study suggests that we should be cautious in the use of 5-HT-related medicines in patients with solid tumors or with a risk of cancer.
Supplementary Material
Abbreviations
- 5-HT
serotonin
- 5-HTT
serotonin transporter
- eNOS
endothelial nitric oxide synthase
- LLC
Lewis lung carcinoma
Footnotes
This study was supported by Grants-In-Aid for Scientific Research from the Ministry of Education, Science, and Culture of the Japanese government to M. A. (no. 19790553) and to S. E. (nos. 18014004 and 19590688).
This article refers to supplementary materials, which are designated by Figures W1 and W2 and are available online at www.neoplasia.com.
References
- 1.Nakaya N, Tsubono Y, Hosokawa T, Nishino Y, Ohkubo T, Hozawa A, Shibuya D, Fukudo S, Fukao A, Tsuji I, et al. Personality and the risk of cancer. J Natl Cancer Inst. 2003;95:799–805. doi: 10.1093/jnci/95.11.799. [DOI] [PubMed] [Google Scholar]
- 2.Asada M, Ebihara S, Okazaki T, Takahashi H, Yasuda H, Sasaki H. Tiapride may accelerate lung cancer in older people: a case report. J Am Geriatr Soc. 2005;53:731–732. doi: 10.1111/j.1532-5415.2005.53228_4.x. [DOI] [PubMed] [Google Scholar]
- 3.Asada M, Ebihara S, Numachi Y, Okazaki T, Yamanda S, Ikeda K, Yasuda H, Sora I, Arai H. Reduced tumor growth in a mouse model of schizophrenia, lacking the dopamine transporter. Int J Cancer. 2008;123:511–518. doi: 10.1002/ijc.23562. [DOI] [PubMed] [Google Scholar]
- 4.Cattaneo MG, Codignola A, Vicentini LM, Clementi F, Sher E. Nicotine stimulates a serotonergic autocrine loop in human small-cell lung carcinoma. Cancer Res. 1993;53:5566–5568. [PubMed] [Google Scholar]
- 5.Cattaneo MG, Fesce R, Vicentini LM. Mitogenic effect of serotonin in human small cell lung carcinoma cells via both 5-HT1A and 5-HT1D receptors. Eur J Pharmacol. 1995;291:209–211. doi: 10.1016/0922-4106(95)90145-0. [DOI] [PubMed] [Google Scholar]
- 6.Vicaut E, Laemmel E, Stucker O. Impact of serotonin on tumour growth. Ann Med. 2000;32:187–194. doi: 10.3109/07853890008998826. [DOI] [PubMed] [Google Scholar]
- 7.Siddiqui EJ, Thompson CS, Mikhailidis DP, Mumtaz FH. The role of serotonin in tumour growth [Review] Oncol Rep. 2005;14:1593–1597. [PubMed] [Google Scholar]
- 8.Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav. 2002;71:533–554. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- 9.Sheehan PF, Baker T, Tutton PJ, Barkla DH. Effects of histamine and 5-hydroxytryptamine on the growth rate of xenografted human bronchogenic carcinomas. Clin Exp Pharmacol Physiol. 1996;23:465–471. doi: 10.1111/j.1440-1681.1996.tb02762.x. [DOI] [PubMed] [Google Scholar]
- 10.Takai D, Yagi Y, Wakazono K, Ohishi N, Morita Y, Sugimura T, Ushijima T. Silencing of HTR1B and reduced expression of EDN1 in human lung cancers, revealed by methylation-sensitive representational difference analysis. Oncogene. 2001;20:7505–7513. doi: 10.1038/sj.onc.1204940. [DOI] [PubMed] [Google Scholar]
- 11.Dizeyi N, Bjartell A, Hedlund P, Tasken KA, Gadaleanu V, Abrahamsson PA. Expression of serotonin receptors 2B and 4 in human prostate cancer tissue and effects of their antagonists on prostate cancer cell lines. Eur Urol. 2005;47:895–900. doi: 10.1016/j.eururo.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 12.Ellis ES, Byrne C, Murphy OE, Tilford NS, Baxter GS. Mediation by 5-hydroxytryptamine2B receptors of endothelium-dependent relaxation in rat jugular vein. Br J Pharmacol. 1995;114:400–404. doi: 10.1111/j.1476-5381.1995.tb13240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonhaus DW, Bach C, DeSouza A, Salazar FH, Matsuoka BD, Zuppan P, Chan HW, Eglen RM. The pharmacology and distribution of human 5-hydroxytryptamine2B (5-HT2B) receptor gene products: comparison with 5-HT2A and 5-HT2C receptors. Br J Pharmacol. 1995;115:622–628. doi: 10.1111/j.1476-5381.1995.tb14977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi DS, Maroteaux L. Immunohistochemical localisation of the serotonin 5-HT2B receptor in mouse gut, cardiovascular system, and brain. FEBS Lett. 1996;391:45–51. doi: 10.1016/0014-5793(96)00695-3. [DOI] [PubMed] [Google Scholar]
- 15.Duxon MS, Kennett GA, Lightowler S, Blackburn TP, Fone KC. Activation of 5-HT2B receptors in the medial amygdala causes anxiolysis in the social interaction test in the rat. Neuropharmacology. 1997;36:601–608. doi: 10.1016/s0028-3908(97)00042-7. [DOI] [PubMed] [Google Scholar]
- 16.Duxon MS, Flanigan TP, Reavley AC, Baxter GS, Blackburn TP, Fone KC. Evidence for expression of the 5-hydroxytryptamine-2B receptor protein in the rat central nervous system. Neuroscience. 1997;76:323–329. doi: 10.1016/s0306-4522(96)00480-0. [DOI] [PubMed] [Google Scholar]
- 17.Borman RA, Burleigh DE. 5-HT1D and 5-HT2B receptors mediate contraction of smooth muscle in human small intestine. Ann N Y Acad Sci. 1997;812:222–223. doi: 10.1111/j.1749-6632.1997.tb48182.x. [DOI] [PubMed] [Google Scholar]
- 18.Lauder JM, Wilkie MB, Wu C, Singh S. Expression of 5-HT(2A), 5-HT(2B) and 5-HT(2C) receptors in the mouse embryo. Int J Dev Neurosci. 2000;18:653–662. doi: 10.1016/s0736-5748(00)00032-0. [DOI] [PubMed] [Google Scholar]
- 19.Tutton PJ, Steel GG. Influence of biogenic amines on the growth of xenografted human colorectal carcinomas. Br J Cancer. 1979;40:743–749. doi: 10.1038/bjc.1979.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palmer RMJ, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 21.Ullmer C, Schmuck K, Kalkman HO, Lubbert H. Expression of serotonin receptor mRNAs in blood vessels. FEBS Lett. 1995;370:215–221. doi: 10.1016/0014-5793(95)00828-w. [DOI] [PubMed] [Google Scholar]
- 22.Glusa E, Roos A. Endothelial 5-HT receptors mediate relaxation of porcine pulmonary arteries in response to ergotamine and dihydroergotamine. Br J Pharmacol. 1996;119:330–334. doi: 10.1111/j.1476-5381.1996.tb15990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson KW, Phebus LA, Cohen ML. Serotonin in migraine: theories, animal models and emerging therapies. Prog Drug Res. 1998;51:219–244. doi: 10.1007/978-3-0348-8845-5_6. [DOI] [PubMed] [Google Scholar]
- 24.Brian JE, Jr, Kennedy RH. Modulation of cerebral arterial tone by endothelium-derived relaxing factor. Am J Physiol. 1993;264:H1245–H1250. doi: 10.1152/ajpheart.1993.264.4.H1245. [DOI] [PubMed] [Google Scholar]
- 25.Cappelli-Bigazzi M, Nuno DW, Lamping KG. Evidence of a role for compounds derived from arginine in coronary response to serotonin in vivo. Am J Physiol. 1991;261:H404–H409. doi: 10.1152/ajpheart.1991.261.2.H404. [DOI] [PubMed] [Google Scholar]
- 26.McDuffie JE, Coaxum SD, Maleque MA. 5-Hydroxytryptamine evokes endothelial nitric oxide synthase activation in bovine aortic endothelial cell cultures. Proc Soc Exp Biol Med. 1999;221:386–390. doi: 10.1046/j.1525-1373.1999.d01-97.x. [DOI] [PubMed] [Google Scholar]
- 27.Lala PK, Orucevic A. Role of nitric oxide in tumor progression: lessons from experimental tumors. Cancer Metastasis Rev. 1998;17:91–106. doi: 10.1023/a:1005960822365. [DOI] [PubMed] [Google Scholar]
- 28.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 29.Veenstra-VanderWeele J, Anderson GM, Cook EH., Jr Pharmacogenetics and the serotonin system: initial studies and future directions. Eur J Pharmacol. 2000;410:165–181. doi: 10.1016/s0014-2999(00)00814-1. [DOI] [PubMed] [Google Scholar]
- 30.Erspamer V. Occurrence of indolealkylamines in nature. In: Erspamer V, editor. Handbook of Experimental Pharmacology: 5-Hydroxytryptamine and Related Indolealkylamines. Vol. 19. New York, NY: Springer-Verlag; 1966. pp. 132–181. [Google Scholar]
- 31.Toh CC. Release of 5-hydroxytryptamine (serotonin) from the dog's gastrointestinal tract. J Physiol (Lond) 1954;126:248–254. doi: 10.1113/jphysiol.1954.sp005206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morrissey JJ, Walker MN, Lovenberg W. The absence of tryptophan hydroxylase activity in blood platelets. Proc Soc Exp Biol Med. 1977;154:496–499. doi: 10.3181/00379727-154-39702. [DOI] [PubMed] [Google Scholar]
- 33.Tamir H, Payette RF, Huang YL, Liu KP, Gershon MD. Human serotonectin: a blood glycoprotein that binds serotonin and is associated with platelets and white blood cells. J Cell Sci. 1985;73:187–206. doi: 10.1242/jcs.73.1.187. [DOI] [PubMed] [Google Scholar]
- 34.Chen JJ, Li Z, Pan H, Murphy DL, Tamir H, Koepsell H, Gershon MD. Maintenance of serotonin in the intestinal mucosa and ganglia of mice that lack the high-affinity serotonin transporter: abnormal intestinal motility and the expression of cation transporters. J Neurosci. 2001;21:6348–6361. doi: 10.1523/JNEUROSCI.21-16-06348.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu W, Tamim H, Shapiro S, Stang MR, Collet JP. Use of antidepressants and risk of colorectal cancer: a nested case-control study. Lancet Oncol. 2006;7:301–308. doi: 10.1016/S1470-2045(06)70622-2. [DOI] [PubMed] [Google Scholar]
- 36.Sora I, Hall FS, Andrews AM, Itokawa M, Li XF, Wei HB, Wichems C, Lesch KP, Murphy DL, Uhl GR. Molecular mechanisms of cocaine reward: combined dopamine and serotonin transporter knockouts eliminate cocaine place preference. Proc Natl Acad Sci USA. 2001;98:5300–5305. doi: 10.1073/pnas.091039298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okazaki T, Ebihara S, Takahashi H, Asada M, Kanda A, Sasaki H. Macrophage colony-stimulating factor induces vascular endothelial growth factor production in skeletal muscle and promotes tumor angiogenesis. J Immunol. 2005;174:7531–7538. doi: 10.4049/jimmunol.174.12.7531. [DOI] [PubMed] [Google Scholar]
- 38.Verheggen R, Meier A, Werner I, Wienekamp A, Kruschat T, Brattelid T, Levy FO, Kaumann A. Functional 5-HT receptors in human occipital artery. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:391–401. doi: 10.1007/s00210-004-0878-9. [DOI] [PubMed] [Google Scholar]
- 39.Seretis E, Gavrill A, Agnantis N, Golematis V, Voloudakis-Baltatzis IE. Comparative study of serotonin and bombesin in adenocarcinomas and neuroendocrine tumors of the colon. Ultrastruct Pathol. 2001;25:445–454. doi: 10.1080/019131201753343485. [DOI] [PubMed] [Google Scholar]
- 40.Okazaki T, Ebihara S, Asada M, Yamanda S, Niu K, Arai H. Erythropoietin promotes the growth of tumors lacking its receptor and decreases survival of tumor-bearing mice by enhancing angiogenesis. Neoplasia. 2008;10:932–939. doi: 10.1593/neo.08140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tutton PJ, Barkla DH. Influence of inhibitors of serotonin uptake on intestinal epithelium and colorectal carcinomas. Br J Cancer. 1982;46:260–263. doi: 10.1038/bjc.1982.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cowen DS. Serotonin and neuronal growth factors—a convergence of signaling pathways. J Neurochem. 2007;101:1161–1171. doi: 10.1111/j.1471-4159.2006.04420.x. [DOI] [PubMed] [Google Scholar]
- 43.Shaul PW. Regulation of endothelial nitric oxide synthase: location, location, location. Annu Rev Physiol. 2002;64:749–774. doi: 10.1146/annurev.physiol.64.081501.155952. [DOI] [PubMed] [Google Scholar]
- 44.Rosetti M, Frasnelli M, Tesei A, Zoli W, Conti M. Cytotoxicity of different selective serotonin reuptake inhibitors (SSRIs) against cancer cells. J Exp Ther Oncol. 2006;6:23–29. [PubMed] [Google Scholar]
- 45.Kremer HP, Goekoop JG, Van Kempen GM. Clinical use of the determination of serotonin in whole blood. J Clin Psychopharmacol. 1990;10:83–87. doi: 10.1097/00004714-199004000-00002. [DOI] [PubMed] [Google Scholar]
- 46.McDuffie JE, Motley ED, Limbird LE, Maleque MA. 5-Hydroxytryptamine stimulates phosphorylation of p44/p42 mitogen-activated protein kinase activation in bovine aortic endothelial cell cultures. J Cardiovasc Pharmacol. 2000;35:398–402. doi: 10.1097/00005344-200003000-00008. [DOI] [PubMed] [Google Scholar]
- 47.Schmuck K, Ullmer C, Kalkman HO, Probst A, Lubbert H. Activation of meningeal 5-HT2B receptors: an early step in the generation of migraine headache? Eur J Neurosci. 1996;8:959–967. doi: 10.1111/j.1460-9568.1996.tb01583.x. [DOI] [PubMed] [Google Scholar]
- 48.Borman RA, Tilford NS, Harmer DW, Day N, Ellis ES, Sheldrick RL, Carey J, Coleman RA, Baxter GS. 5-HT(2B) receptors play a key role in mediating the excitatory effects of 5-HT in human colon in vitro. Br J Pharmacol. 2002;135:1144–1151. doi: 10.1038/sj.bjp.0704571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McLean PG, Borman RA, Lee K. 5-HT in the enteric nervous system: gut function and neuropharmacology. Trends Neurosci. 2007;30:9–13. doi: 10.1016/j.tins.2006.11.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.