Abstract
There is growing evidence about the role of mesenchymal stem cells (MSCs) as cancer stem cells in many sarcomas. Nevertheless, little is still known about the cellular and molecular mechanisms underlying MSCs transformation. We aimed at investigating the role of p53 and p21, two important regulators of the cell cycle progression and apoptosis normally involved in protection against tumorigenesis. Mesenchymal stem cells from wild-type, p21-/-p53+/+, and p21-/-p53+/- mice were cultured in vitro and analyzed for the appearance of tumoral transformation properties after low, medium, and high number of passages both in vitro and in vivo. Wild-type or p21-/-p53+/+ MSCs did not show any sign of tumoral transformation. Indeed, after short-term in vitro culture, wild-type MSCs became senescent, and p21-/-p53+/+ MSCs showed an elevated spontaneous apoptosis rate. Conversely, MSCs carrying a mutation in one allele of the p53 gene (p21-/-p53+/- MSCs) completely lost p53 expression after in vitro long-term culture. Loss of p53 was accompanied by a significant increase in the growth rate, gain of karyotypic instability, loss of p16 expression, and lack of senescence response. Finally, these cells were able to form fibrosarcomas partially differentiated into different mesenchymal lineages when injected in immunodeficient mice both after subcutaneous and intrafemoral injection. These findings show that MSCs are very sensitive to mutations in genes involved in cell cycle control and that these deficiencies can be at the origin of some mesodermic tumors.
Introduction
The cyclin-dependent kinase (CDK) inhibitor p21Cip1/Waf1 (hereafter referred to as p21) is regulated by both p53-dependent and -independent mechanisms on cellular stress, and the induction of p21 may cause cell cycle arrest [1,2]. In addition to the loss of cell cycle checkpoints, p21 deficiency commonly results in the lack of both replicative and DNA damage-induced senescence [3,4] and the increase of apoptosis after several stresses [5,6].
Unlike p53-deficient (p53-/-) mice, p21-deficient (p21-/-) mice are resistant to early onset of tumorigenesis [7], although they develop spontaneous tumors at an average age of 16 months. Remarkably, half of these tumors long-term developed by p21-/- mice display a mesoderm origin (i.e., histiocytic sarcomas) [8]. Moreover, p21 deficiency was shown to facilitate the susceptibility to sarcomas in p53+/- and p53-/- mice [9]. Mouse embryonic fibroblasts (MEFs) derived from p21-/- mice show a greater capacity to grow at late passages compared with wild-type MEFs [7]. Similarly, p21 knockdown in human fibroblasts allows cells to bypass senescence, although eventually, they enter a nonproliferative crisis state [3,10].
The consequences of p21 deficiency in adult stem cells have been studied in hematopoietic stem cells (HSCs), neural stem cells, and endothelial progenitor cells. In these cell types, p21 seems to control their development and proliferation. p21-/- mice have more HSCs than normal mice and fewer stem cells are in a quiescent state [11]. Similar to HSCs, p21 contributes to the relative quiescence of adult neural stem cells and the loss of p21 lead to the exhaustion of their self-renewal capacity [12]. Endothelial progenitor cells number and their clonal expansion capacity are also elevated in p21-/- mice compared with wild-type counterparts [13].
Studies in mesenchymal stem cells (MSCs) show that the overexpression of p21 protect them from programmed cell death induced by low-density culture [14] and that there was an age-dependent decrease in the proliferation of MSCs associated with an up-regulation of p21 [15]. In addition, p53, the better known activator of p21, has also been reported to be mutated in a model of spontaneously transformed MSCs, which recapitulated the naturally occurring fibrosarcomas observed in aged mice [16]. However, there is no information about the effects of p21 deficiency in MSCs.
Previous reports suggest that MSCs could behave as cancer stem cells of certain sarcomas [17–21] and that their in vitro transformation would also generate both sarcomas [16,22–24] and carcinomas [25,26]. Little is still known, however, about the cellular and molecular mechanisms underlying MSCs transformation. The process of MSCs transformation has been associated to the accumulation of chromosome instability (CIN) [22–24] and high resistance to apoptosis [27], suggesting the relevance of an accurate cell cycle control in MSCs. In fact, alterations in cell cycle regulators such as p16INK4A, p53, and CDK-1, CDK-2, and CDK-6 [21,23,28] have been detected in transformed MSCs.
On the basis of the relation between p21 deficiency and sarcomagenesis and in the ability of transformed MSCs to generate sarcomas, we aimed to study the role of p21 and p53 during in vitro culture of murine MSCs (mMSCs). We have derived and grown MSCs from p21-/- and p21-/-p53+/- mice. We found that p21-/- MSCs grow with similar characteristics to that of wild-type MSCs, except for a greater apoptotic rate, and that these cells were not tumorigenic. Strikingly, on the introduction of a mutation in one allele of p53 gene generating p21-/-p53+/- MSCs, we observed a complete loss of p53 function after continuous in vitro culture associated with a malignant phenotype as shown by the ability of these cells to form sarcomas in immunodeficient mice.
Materials and Methods
Obtaining and In Vitro Culture of Mouse MSCs
p21-/- and p21-/- p53+/- mice on a C57BL/6J background have been previously described and characterized [9,29]. Mesenchymal stem cells were derived from adipose tissue. The tissue was cut into small pieces and disaggregated for 2 hours, with the digestion medium consisting of Dulbecco's modified Eagle's medium (Gibco, Carlsbad, CA) with 1 mg/ml collagenase A (Roche, Basel, Switzerland). The sample was centrifuged, filtered through 40-µm nylon filter (Becton Dickinson, Franklin Lakes, NJ), seeded at a density of 1.6 x 105 cells/cm2 in flasks with MesenCult for mouse cells medium and MSC supplements (Stem Cell Technologies, Vancouver, Canada), and incubated at 37°C in a 5% humidified CO2 atmosphere. After 24 hours, nonadherent cells were discarded, and fresh medium was added. When cell culture achieved more than 90% of density, adherent cells were trypsinized (0.25% trypsin; Sigma, St. Louis, MO), washed, and replated at a concentration of 4 x 103 cells/cm2.
Flow Cytometry Analysis of MSCs
The immunophenotype of cultured MSCs was analyzed by flow cytometry. Cells were trypsinized, washed, and suspended in PBS with 1% bovine serum albumin (Sigma). A total of 2 x 105 cells were incubated in the dark for 30 minutes with fluorochrome-conjugated monoclonal antibodies (Sca-1, CD11b, CD34, CD45, CD44, and CD29; BD Biosciences, San Jose, CA), washed, and analyzed in a FACSCanto II cytometer (BD Biosciences).
Differentiation Studies of MSCs
Mesenchymal stem cells were plated at 5 x 103 cells/cm2 in MesenCult medium and were allowed to adhere for 24 hours. Culture medium was then replaced with specific differentiation inductive media. For adipogenic differentiation, cells were cultured in Adipogenic MSCs Differentiation BulletKit (Lonza, Basel, Switzerland) for 2 weeks. Differentiated cell cultures were stained with Oil Red O (Amresco, Solon, OH). For osteogenic differentiation, cells were cultured in Osteogenic MSCs Differentiation BulletKit (Lonza) for 2 weeks. Differentiated cell cultures were stained with Alizarin Red S (Sigma).
In Vitro Cell Growth Analysis
Cell cultures were checked daily for changes in growth rates and morphology. Growth curves were performed by assessing the cell number in triplicate MSC cultures for 8 days. For colony-forming assays, cells were plated in triplicate at low density (2000 cells/100-mm dish). Cells were allowed to grow for 10 to 15 days before staining them with 0.5% crystal violet (Sigma)/25% methanol. Only colonies of >50 cells were scored.
Karyotypic Analysis
Cells were cultured in medium supplemented with 0.1 mg/ml colcemid (Sigma) for 3 to 4 hours, washed with PBS, and trypsinized. The subsequent pellet was carefully resuspended in hypotonic solution of KCl (0.075 M), incubated for 20 minutes at 37°C, spun down, and fixed in Carnoy's solution (methanol/acetic acid ratio, 3:1). The fixing procedure was repeated three times, and the pellet was finally resuspended in 1 ml of fixative. Cells were then dropped on glass slides, and the chromosomes were visualized by using modified Wright's staining. Metaphases were analyzed using conventional microscope (Leica DM 5500B, Solms, Germany), and the karyotypes were prepared using IKAROS software (Metasystems, North Royalton, OH). Fifty metaphases were analyzed for each culture.
Cell Cycle Analysis
Cell cycle analysis was carried out by flow cytometry after propidium iodide staining of 70% ethanol-fixed cells as previously described [6].
Detection of Apoptosis
Apoptotic cells were assessed by flow cytometry using PE-Annexin V according to the manufacturer's instructions (BD Biosciences).
Senescence-Associated β-Galactosidase Staining
After the indicated treatments, cells were fixed and incubated overnight with X-gal solution (pH 6.0) as previously described [10].
In Vivo Tumorigenesis Assay
Nonobese diabetic/severe combined immunodeficient NOD.Cg-Prkdcscid IL2rgtm1Wjl/SzJ (NOD/SCID-IL2R-/-) mice were obtained from The Jackson Laboratory (Bar Harbor, ME). All mice were housed under specific pathogen-free conditions, fed ad libitum according to Animal Facilities guidelines, and used at 8 to 12 weeks of age. All protocols involving mice were approved by the Institutional Animal Care and Use Committee. Female NOD/SCID-IL2R-/- mice were infused subcutaneously with 3 x 106 cells. According to the UKCCCR guidelines for the welfare of animals in experimental neoplasia, animals were killed when their tumors reached approximately a diameter of 10 mm or 5 months after inoculation. Tumors and organs extracted for further analyses. In some experiments, a fraction of tumors was incubated in culture medium containing 1 mg/ml collagenase A for 3 hours. Then, disaggregated samples were centrifuged, filtered through 40-µm nylon filter, and seeded in culture as described above to reestablish ex vivo culture of tumor cells.
Histologic Analysis
Tumor samples were fixed in 4% buffered formalin, embedded in paraffin wax, cut into 4 µm sections, and stained with hematoxylin and eosin. The histologic diagnosis was performed following the criteria of the World Health Organization described by Ernst et al. [30] taking in account the infiltrative growth, cellular pleomorphism, and cellular malignancy. Immunohistochemical staining was carried out using the ABC Vectasain Elite Kit (Vector Laboratories, Burlingame, CA) according to manufacturer's instructions and counterstained with hematoxylin. After a previous unmasking antigen with heat and blocking unspecific protein with MOM kit (Vector Laboratories), the primary antibody antihuman β-tubulin III (Chemicon, Billerica, MA) was incubated at a dilution 1:100 for 1 hour at room temperature. Immunoreactivity was observed with 3-3′ diaminobenzidine tretrachloride (Vector Laboratories).
Western Blot Analysis
Whole-cell extracts were prepared after treatment with or without 0.5 µM camptothecin (CPT; Sigma) for 24 hours as described previously [31], resolved on 10% SDS-PAGE gels, and blotted onto nitrocellulose (BioRad, Hercules, CA). Proteins were detected with the enhanced chemiluminescence detection system (Amersham, Buckinghamshire, England) using anti-p21 (sc-6246), anti-p53 (sc-6243), anti-p16 (sc-74401), and anti-c-myc (sc-41) from Santa Cruz Biotechnology, Santa Cruz, CA; anti-phospho-p53 (Ser15) (PC386) and anti-p19 (PC435) from Calbiochem, Dormstadt, Germany; or anti-β-actin (A1978) from Sigma.
Results
Characterization of p21-/- p53+/+ and p21-/- p53+/- Mouse MSCs
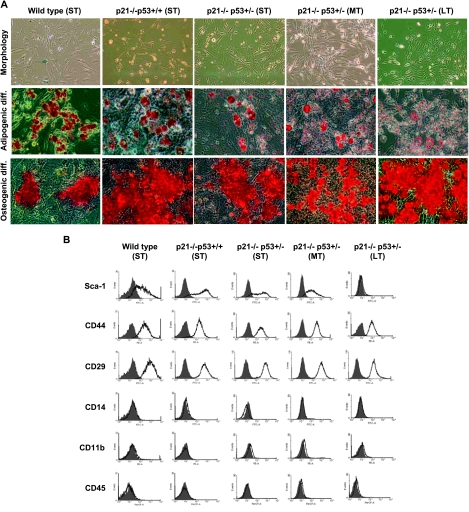
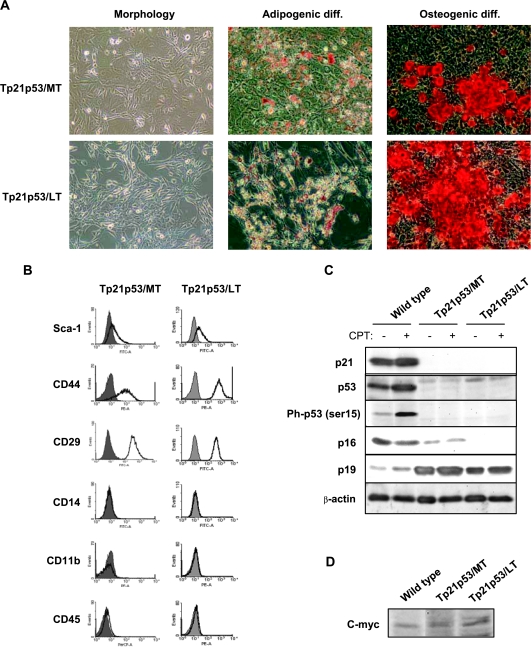
Mesenchymal stem cells from wild-type, p21-/-p53+/+, and p21-/-p53+/- mice were maintained in culture for short-term (ST; passages 3–15), medium term (MT; passages 16–35) or long-term (LT; passages 36–60) periods. During ST in vitro culture, cells from all genotypes showed a typical fibroblast-like morphology (Figure 1A). However, after continuous culture, p21-/-p53+/- (MT and LT) MSCs changed from an elongated spindle shape to a small, compact morphology (Figures 1A and 2A). All these cell types showed their capacity to differentiate to adipocytes and osteocytes after specific stimulation as usual in MSCs (Figure 1A). Nevertheless, the p21-/-p53+/- (LT) MSCs showed poorer differentiation to the adipogenic lineage. After ST culture, all MSCs expressed the cell surface antigens Sca-1, CD44, and CD29 and lacked expression of the hematopoietic markers CD45, CD14, and CD11b, therefore displaying a common mouse-MSC phenotype [32,33] (Figure 1B). Intriguingly, after LT culture, p21-/-p53+/- MSCs had almost completely lost the expression of Sca-1, although the rest of the markers remain in a similar level of expression. Taken together, the changes observed in p21-/-p53+/- MSCs after long-term culture suggest a different in vitro homeostasis behavior.
Figure 1.
MSCs characterization. (A) Morphology, Oil Red O staining (adipogenic differentiation), and Alizarin Red staining (osteogenic differentiation) of the indicated MSCs. Original magnification, x20. (B) Fluorescence-activated cell sorting analysis of MSC surface markers. Filled line, control isotype; empty line, cells incubated with the indicated antibody.
Figure 2.
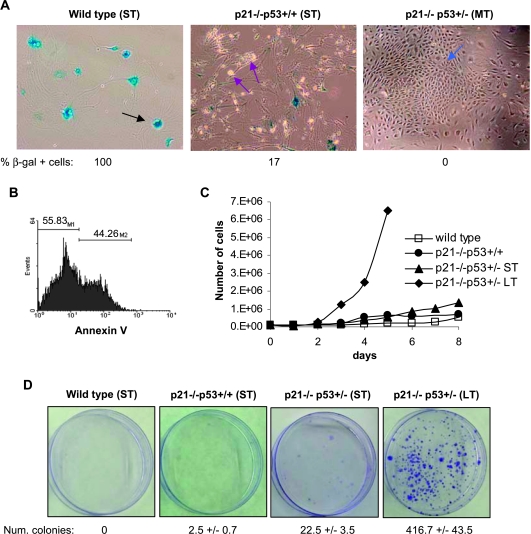
p21-/-p53+/- MSCs were transformed after extensive in vitro culture. (A) Representative images of senescence-associated β-galactosidase (SA-β-gal) assays of wild-type (passage 12), p21-/-p53+/+ (passage 11), and p21-/-p53+/+ (passage 20) MSCs. SA-β-gal senescent-positive cells (black arrows), apoptotic cells (red arrows), and the outgrowth of a transformed population (blue arrow) are indicated. Original magnification, x20. (B) Annexin V binding assay of a culture of p21-/-p53+/+ (ST) cells. (C) Growth curves of the indicated MSCs. (D) Representative plates of colony formation assays showing the gain of clonogenic capacity of p21-/-p53+/- (LT) MSCs.
In Vitro Immortalization of p21-/-p53+/- MSCs After Extensive Cell Culture
Ongoing experimental evidence suggests that normal MSCs, like most of primary cell cultures, enter senescence on several passages in vitro [34]. Here, after 10 to 15 passages, wild-type MSCs showed a pronounced increase in size with prominent and enlarged nuclei characteristic of senescent cells. In fact, the entire cell population stained for senescence-associated β-galactosidase activity (SA-β-gal; Figure 2A). After identical number of passages, p21-/-p53+/+ MSCs showed a significantly lower level of senescence cells, although in these cultures, a high proportion of smaller, rounded, and partially detached apoptotic cells were detected (Figure 2A). Almost half of the p21-/-p53+/+ MSCs undergoes spontaneous apoptotic response as quantified by Annexin V binding (Figure 2B). As a consequence of these antiproliferative effects (senescence and/or apoptosis), cultures of wild-type and p21-/-p53+/+ MSCs did not progress any further. Conversely, p21-/-p53+/- MSCs did not undergo either senescence or apoptosis. Opposite to these effects, after 17 to 22 passages, a colony showing a small, rounded morphology arose in these cultures (Figure 2A). This cell population showed a growth rate much higher than the normal spindle-shaped cells and completely took over the entire culture after a few passages. As a consequence, p21-/-p53+/- (MT) MSCs displayed an immortalized/transformed phenotype that includes the loss of contact inhibition, a growth rate much higher than wild-type, p21-/-p53+/+ or p21-/-p53+/- (ST) MSCs (Figure 2C) as a result of a higher mitotic index (data not shown), and the gain of clonogenic capacity (Figure 2D).
Karyotypic analysis showed moderate levels of chromosomal instability in wild-type and p21-/-p53+/+ MSCs as expected for primary cultures of mouse cells. Nevertheless, relevant differences in the level of instability have been found. Thus, approximately 50% to 60% of metaphases from wild-type (ST) and p21-/-p53+/+ (ST) MSCs cultures showed normal karyotypes. The rest of the metaphases gained chromosomes, showing most of them a karyotype with 41 to 70 chromosomes (Figure W1, A and B). Conversely, cultures from p21-/-p53+/- (ST) showed a higher level of chromosomal instability, because virtually, the whole population had gained extra chromosomes, with 80% of them displaying karyotypes of more than 70 chromosomes. After LT culture, these cells showed a kind of adaptation to in vitro culture conditions and lost some of the previously gained chromosomes (Figure W1, A and B). Cell cycle analysis of these cells confirm that p21-/-p53+/- (ST) had almost double DNA content than wild-type or p21-/-p53+/+ cells and that p21-/-p53+/- (LT) presented an intermediate amount of DNA content (Figure W1C). Cell cycle profiles also revealed a higher proportion of cells in S-phase in p21-/-p53+/- (LT; 23.20%) compared with p21-/-p53+/- (ST; 17.89%) cells and especially to wild-type (9.09%) and p21-/-p53+/+ (9.05%) cells according to their growth rates.
Loss of p53 and p16 Expression in p21-/-p53+/- MSCs After Long-term Cell Culture
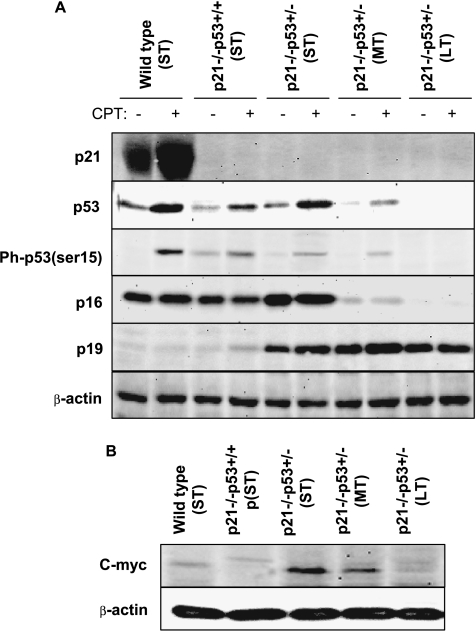
To better understand the observed transformation of p21-/-p53+/- after LT in vitro culture, we analyzed the expression of relevant cell cycle regulators in control conditions or after a 24-hour treatment with the topoisomerase I inhibitor CPT, which induces DNA damage in the form of replication fork stress, as a control for the activation of some of these proteins (Figure 3A). As expected, expression of p21 was completely absent in all of the p21-/- MSCs, whereas it was highly expressed and further upregulated after CPT treatment in wild-type cells. Conversely, expression of p53 was similar in wild-type and p21-/-p53+/- (ST) cells and was slightly lower in p21-/-p53+/+ cells. A strong activation (up-regulation and phosphorylation in serine 15) of p53 was also observed after CPT treatment of these cells. More importantly, the expression and activation of this protein were highly abrogated in p21-/-p53+/- (MT) cells and completely disappeared in p21-/-p53+/- (LT) MSCs.
Figure 3.
p53 and p16 are gradually lost after in vitro culture of p21-/-p53+/- MSCs. Levels of p21, total p53, phospho-p53 (ser 15), p16, p19 (A) and c-myc (B) in the indicated MSCs treated or not with 0.5 µM CPT for 24 hours. β-Actin levels are presented as a loading control.
We next analyzed the expression of the proteins codified by the INK4A/ARF locus p16 and p19 whose altered expressions have been reported in other models of transformed MSCs [23,35]. The expression pattern of p16 was similar to that observed for p53, showing a high expression level in wild type, p21-/-p53+/+, and p21-/-p53+/- (ST) cells, a great reduction of their levels in p21-/-p53+/- (MT), and complete absence of expression in p21-/-p53+/- (LT) MSCs. Opposite to p16, p19 was poorly expressed in wild-type and p21-/-p53+/+ cells but highly expressed in p21-/-p53+/- independently of their time in culture (Figure 3A).
Overexpression of c-myc is a common event associated with tumoral transformation of MSCs [22,23]. Compared with wild-type cells, the expression of this oncogenic protein was virtually undetectable in p21-/-p53+/+ (ST) cells but upregulated in p21-/-p53+/- (ST) and, in a lower degree, in p21-/-p53+/- (MT) cells (Figure 3B). Intriguingly, c-myc levels decrease after LT culture of p21-/-p53+/- MSCs to the levels of wild-type cells. This pattern of expression agrees with previously published data, which suggest a role for c-myc in senescence bypass during spontaneous tumoral transformation of human MSCs [23,28].
Lack of Serum Deprivation Induced Senescence in p21-/-p53+/- (LT) Cells
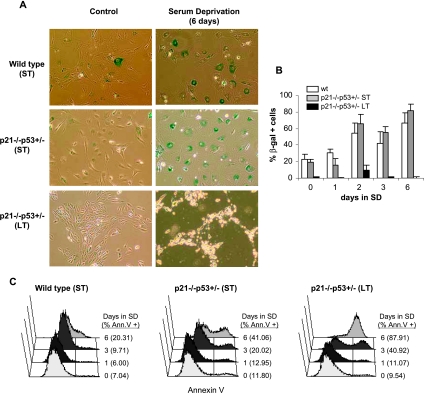
The induction of replicative or DNA damage-induced senescence constitutes one of the most important antitumoral barriers. In mammalian cells, senescence is dependent on functional p53/p21 and/or p16 pathways [36], which are both compromised in p21-/-p53+/- (LT) MSCs. To test the ability of wild-type, p21-/-p53+/-, (ST) and p21-/-p53+/- (LT) MSCs to senesce, the cells were deprived of serum for up to 6 days before being stained for SA-β-gal (Figure 4, A and B). Control cultures of wild-type and p21-/-p53+/- (ST) showed a basal level of senescence (approximately 20%). Serum deprivation of these cells showed a similar time-dependent increase in the percentage of senescent cells, reaching a maximum of 80% after 6 days. Conversely, p21-/-p53+/- (LT) cells did not stain at all for SA-β-gal even after 6 days of being deprived of serum. However, during the experiment, these cells became smaller, rounded, and highly refringent, resembling apoptotic cells (Figure 4A). Annexin V binding experiments were done to confirm this apoptotic effect (Figure 4C). Wild-type cells showed only a modest apoptotic induction of 20% after 6 days of serum deprivation. Meanwhile, this apoptotic effect increased up to 40% in p21-/-p53+/- (ST) MSCs and virtually affected the whole population in p21-/-p53+/- (LT) MSCs after the same period. These results stress the relevance of p53, p21, and p16 in the induction of senescence and suggest an important role of this mechanism in the protection against tumoral transformation of MSCs. Nevertheless, these results also suggest that agents that induce senescence could be efficient apoptotic inducers in transformed MSCs knocked down for p53/p21 and p16 pathways.
Figure 4.
Senescence induction after serum deprivation of p21-/-p53+/- MSCs. The indicated MSCs were deprived of serum for the indicated days and analyzed for senescence-associated β-galactosidase (SA-β-gal) activity (A, representative images; original magnification, x20; B, mean and SD of three independent experiments) or for the Annexin V binding (C, percentage of Annexin V-positive apoptotic cells is indicated).
In Vivo Tumor Formation Potential of p21-/-p53+/- MSCs
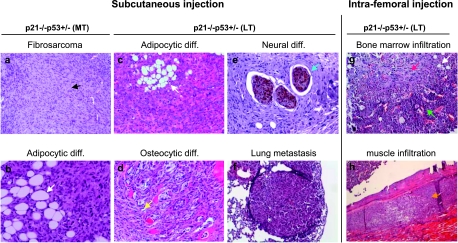
To test the ability of p21-/-p53+/- MSCs to form tumors in vivo, we introduced cells of the different studied genotypes into immunodeficient mice through subcutaneous injection. One hundred fifty days after the inoculation of wild-type, p21-/-p53+/+, or p21-/-p53+/- (ST) MSCs, no signs of illness or tumor formation were observed in any mice (Table 1). In contrast, inoculation of p21-/-p53+/- (MT) or p21-/-p53+/- (LT) cells originated tumors in all injected mice (Figure 5). Eventually, tumors developed ulcers in the skin when the diameter reached approximately 7 to 10 mm, and the mice in these groups were killed. p21-/-p53+/- (MT) and p21-/-p53+/- (LT) MSCs reached this point 105 and 74 days after inoculation, respectively, evidencing the more aggressive phenotype of p21-/-p53+/- (LT), which completely lacks p53 and p16. Anatomopathological analysis classified tumors as fibrosarcomas, showing most cells an elongated shape, anisocytosis, anisokaryosis, and an elevated mitotic index (Figure 5). Tumors obtained from p21-/-p53+/- (MT) showed areas of adipose tissue differentiation, whereas tumors from p21-/-p53+/- (LT) MSCs displayed not only adipose but also osteocytic differentiation as well as signs of neural differentiation, as indicated by β-tubulin III staining of cells residing inside nerve-like structures (Figure 5). Moreover, small nodules of metastasis were found in lungs from some mice (Figure 5).
Table 1.
In Vivo Tumor Formation Ability of p21-/- MSCs.
| Subcutaneous Injection | Intrafemoral Injection | ||||
| Tumors/Mice | Days to Tumor Development* | Histologic Analysis | Tumors/Mice | Histologic Analysis | |
| wild-type | 0/5 | NT† | Normal | — | — |
| p21-/-p53+/+ | 0/5 | NT† | Normal | 0/4 | Normal |
| p21-/-p53+/- ST | 0/5 | NT† | Normal | — | — |
| p21-/-p53+/- MT | 5/5 | 105 | Fibrosarcoma + fat | — | — |
| p21-/-p53+/- LT | 5/5 | 74 | Fibrosarcoma + fat + bone + nerve | 4/4 | Fibrosarcoma + fat |
Average number of days needed to observe tumor ulceration and an approximate tumor diameter of 8 mm. Mice carrying tumors were killed at this point.
NT, no tumors were detected. Mice were killed 150 days after injection.
Figure 5.
In vivo tumor formation ability of transformed p21-/-p53+/- MSCs. Hematoxylin and eosin staining (and β-tubulin III immunohistochemistry for e) of tumors obtained after subcutaneous or intrafemoral injection of p21-/-p53+/- (MT) and p21-/-p53+/- (LT) as indicated. Tumors generated after subcutaneous injection were mostly composed of elongated cells typical of fibrosarcomas (a, black arrow) showing anisocytosis, anisokaryosis and an elevated mitotic index. These tumors also showed areas of adipose tissue differentiation (b, c, white arrows), osteogenic differentiation (d, yellow arrow) and nerve-like structures showing positive staining for β-tubulin III (e, blue arrow). Lungs infiltrated with p21-/-p53+/- transformed MSCs were observed in some mice (f). Tumors generated after intrafemoral injection of p21-/-p53+/- (LT) MSCs showed the same fibrosarcoma morphology and were present both inside the bone marrow (g, see red arrow for tumor cells, white arrow for adipose differentiation, and green arrow for bone marrow) and infiltrated between the muscle surrounding the bone (h, orange arrow). Original magnifications: all (except in b and f, x10), x4.
In addition, we have also tested the ability of p21-/-p53+/+ and p21-/-p53+/- (LT) MSCs to form tumors in vivo in an environment characteristic of MSCs such as bone marrow. Thus, MSCs were injected into the femurs of immunodeficient mice (Table 1). Again, no tumors were observed from p21-/-p53+/+ MSCs. Conversely, all the mice injected with p21-/-p53+/- (LT) MSCs developed fibrosarcomas with some level of fat differentiation, present both inside the bone marrow and infiltrated between the muscles surrounding the bone (Figure 5). Altogether, these results confirm that p21 knockdown per se is not enough to transform MSCs, but simultaneous and gradual loss of p53 and p16 expression cooperate to induce an increasingly aggressive tumorigenic phenotype.
Characterization of Cells Derived from p21-/-p53+/- MSC-Induced Tumors
We next extracted and cultured cells from tumors derived from p21-/-p53+/- (MT) (Tp21p53/MT) and from p21-/-p53+/- (LT) cells (Tp21p53/LT). Cells from both tumors showed similar growth properties compared with their parental MSCs, including morphology (Figure 6A), absence of contact inhibition, elevated mitotic index, and clonogenic capacity (data not shown). Tp21p53/MT and LT cells were negative for CD45, CD14 and CD11b, positive for CD44 and CD29 and weakly positive for Sca-1 (Figure 6B), similar to the parental MSCs. These cells showed a strong ability for osteogenic differentiation together with a weak differentiation to adipocytes (Figure 6A), similar to that reported for p53-/- MSCs [37]. Indeed, both Tp21p53/MT and Tp21p53/LT cells did not express p21 or p53 proteins (Figure 6C). Like the parental MSCs, Tp21p53/MT showed a reduced expression of p16, which was completely absent in Tp21p53/LT cells, whereas the p19 levels were highly upregulated in both type of cells compared with wild-type MSCs. Likewise, c-myc levels were similar in wild-type and both tumor-derived cells, following the pattern of expression of their parental MSCs (Figure 6D).
Figure 6.
Characterization of cell lines derived from p21-/-p53+/- MSCs-induced tumors. (A) Morphology, Oil Red O staining (adipogenic differentiation), and Alizarin Red staining (osteogenic differentiation) of the indicated tumor-derived cells. Original magnification, x20. (B) Fluorescence-activated cell sorting analysis of MSCs surface markers. (C) Levels of p21, total p53, phospho-p53 (ser 15), p16, and p19 in wild-type MSCs or tumor-derived cell lines treated or not with 0.5 µM CPT for 24 hours. β-Actin levels are presented as a loading control. (D) c-myc levels in the indicated cells. Loading controls are the same as in (C).
Tumor-derived cells also showed a great increase in the number of chromosomes, presenting a medium of 63 chromosomes in Tp21p53/MT and 75 in Tp21p53/LT cells (Figure W2A). The differences in DNA content were also confirmed by cell cycle analysis (Figure W2B). Percentage of cells in the S phase in tumor-derived cells (22.07% for Tp21p53/MT cells and 23.81% for Tp21p53/LT cells) was again similar to that observed in their parental MSCs. In summary, the cells recovered from tumors showed a phenotype and biological behavior similar to that observed in the parental transformed MSCs, p21-/-p53+/- (MT) and p21-/-p53+/- (LT).
Discussion
p21 plays important roles in regulating key antitumoral barriers such as cell cycle control, senescence, and apoptosis, and its deficiency predisposes mice to spontaneous and induced tumorigenesis. Most of these spontaneous tumors observed in p21 knockout mice were sarcomas [8], and p21 deficiency also increased the percentage of sarcomas in p53-deficient mice [9]. These data suggest a potential link between p21 deficiency and sarcomagenesis. In addition, human and mouse MSCs transformed after in vitro culture generated sarcomas in vivo [21–24], and human MSCs have been proposed as cancer stem cells for certain sarcomas [16–20]. Thus, we wanted to test if p21-deficient MSCs could be transformed in vitro and form sarcomas in vivo.
Our study suggests that p21 deficiency of is not enough for MSC immortalization, even losing one allele of p53. However, serial cultivation of p21-/-p53+/- mMSCs was accompanied of a total loss of heterozygosity in p53 function. As a result, long-term cultured p21-/-p53+/- mMSCs became immortalized and were able to induce tumor formation into immunodeficient mice, according to the two-hit model of tumorigenesis (see Figure W3 for a summary of the transforming events observed in the MSCs cultures). Altogether, we conclude that the deficiency of p21 and p53 would generate sarcomas from MSCs.
According to our data, it has been reported that the loss of p21 accelerated tumor onset in mice deficient for p53-induced apoptotic function, and these tumors showed an increased CIN [38]. We found a similar increase in CIN in p21-/-p53+/- MSCs even after a short number of passages when p53 protein is still expressed and can be phosphorylated after DNA damage, suggesting that p53 is haploinsufficient to maintain chromosomal stability in a p21-/- background.
Loss of heterozygosity has previously been reported after in vitro culture of p21, p53, Rb, or p16 heterozygous human fibroblasts [39]. In this case, cells that lost heterozygosity for p21, p53, and Rb but not for p16 were able to bypass senescence, although the extended life span of these cells eventually terminated in a crisis state. Likewise, fibroblast from patients with Li-Fraumeni syndrome (p53+/-) spontaneously lost the wild-type p53 allele and gained extended life span [40]. Moreover, spontaneous immortalization of murine fibroblasts is frequently associated with a mutation of p53 [41], and wild-type and p21-/- MEF cultures acquire alterations in p53 [42]. In MSCs, genetic changes, in particular, p53 mutations, occur after long-term in vitro proliferation [21]. In our model, p21-/-p53+/- mMSCs, which completely lost p53 expression, became not only immortalized but also transformed, suggesting an important role for p21 in the prevention of tumorigenesis in MSCs.
Loss of p21 also permits carcinogenesis in chronically damaged epithelial cells despite having lost the p21 antiapoptotic functions [43]. We have also noticed a higher apoptosis rate in p21-/- MSC cultures, and similar observations have been reported in other cell types. Thus, p21-/- mice show a defect in the induction of p53-mediated G1/S arrest in response to γ-radiation [7] but maintain an intact apoptotic response [29]. In addition, irradiation-induced thymic lymphomas in p21-null mice show a significantly higher level of apoptosis compared with that in wild-type mice [8]. Moreover, lymphocytes in the spleen of p21-deficient mice display a higher level of constitutive apoptosis compared with wild-type mice [44].
Other alterations detected during the in vitro culture of p21-/-p53+/- MSCs included the loss of p16 expression accompanied by a high up-regulation of the related tumor-suppressor protein p19 and a transient up-regulation of c-myc. p16 and p19 are encoded by overlapping reading frames in the same gene (INK4/ARF), so p16 silencing is not due to the loss of this locus. In this regard, it has been reported that p16 expression is closely associated with senescence of human MSCs and could be silenced by DNA methylation during in vitro expansion of these cells [45]. Repression of p16 expression has also been reported in a case of spontaneous transformation of human MSCs [23,28]. Another study shows that the loss of p16 with retention of p19 is enough to predispose mice to tumorigenesis [46]. p19 regulates a route for p53 stabilization in response to aberrant growth or oncogenic stresses [47]. We hypothesized that the high levels of p19 observed could be due to an insufficient activation of p53 in p53+/- and p53-/- MSCs in response to the high level of CIN and/or the c-myc up-regulation observed after in vitro culture of these cells.
Activation of c-myc has been demonstrated as a very relevant event in the transformation process of human MSCs [48], being commonly upregulated in cases of transformation of human and mMSCs [22,23]. The transient up-regulation of c-myc in MSCs has been associated with the bypass of cellular senescence [28].
In mammalian cells, senescence is initiated by one of these two signaling pathways: p53/p21 and p16/Rb [36]. In human fibroblasts, it has been shown that the up-regulation of p21 is the primary response-inducing cellular senescence, whereas the up-regulation of p16 occurs weeks after the induction of cell cycle arrest [49]. In this regard, the inactivation of p21 and INK4 pathways in a mouse model strongly cooperates in suppressing cellular senescence [50]. Interestingly, this double mutant displays an increased incidence of sarcomas compared with the single mutants. In our model, the loss of p21, p53, and p16 suppresses not only replicative but also serum deprivation-induced senescence. Intriguingly, p21-/-p53+/- (LT) cells that do not senesce after 6 days of serum deprivation undergo a p53-independent apoptotic cell death, suggesting that agents that induce senescence could be efficient apoptotic inducers in transformed MSCs deficient for p53/p21 and p16 pathways.
In summary, transformation of MSCs seems to be highly dependent on alterations in the p21/p53 pathway. Our data indicate that p21-deficiency itself is not sufficient to allow MSCs immortalization. However, when the p21 deficiency is coupled to p53 deficiency, the MSCs bypass senescence in vitro and generate tumors that resemble typical mesenchymal sarcomas in vivo. Our study suggests the hypothesis that MSCs might require few genetic alterations to undergo transformation and may act as the cellular origin for sarcomas.
Supplementary Material
Abbreviations
- CDK
cyclin-dependent kinase
- CIN
chromosome instability
- CPT
camptothecin
- HSC
hematopoietic stem cell
- MEF
mouse embryonic fibroblast
- MSC
mesenchymal stem cell
Footnotes
This work was funded by the Consejería de Salud de la Junta de Andalucía (grants 0108/2007 to R.R., 0028/2006 and 0029/2006 to P.M., and 0027/2006 to J.G.-C.), Consejería de Educación de la Comunidad Autónoma de Madrid (S-BIO-0204-2006; MESENCAM), The International Jose Carreras Foundation against the Leukemia to PM (EDThomas-05), and The Spanish Ministry of Health to P.M. (FIS PI070026) and to J.G.-C. (FIS PI052217).
This article refers to supplementary materials, which are designated by Figures W1 to W3 and are available online at www.neoplasia.com.
References
- 1.el-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 2.Xiong Y, Hannon GJ, Zhang H, Casso D, Kobayashi R, Beach D. p21 is a universal inhibitor of cyclin kinases. Nature. 1993;366:701–704. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]
- 3.Brown JP, Wei W, Sedivy JM. Bypass of senescence after disruption of p21CIP1/WAF1 gene in normal diploid human fibroblasts. Science. 1997;277:831–834. doi: 10.1126/science.277.5327.831. [DOI] [PubMed] [Google Scholar]
- 4.Han Z, Wei W, Dunaway S, Darnowski JW, Calabresi P, Sedivy J, Hendrickson EA, Balan KV, Pantazis P, Wyche JH. Role of p21 in apoptosis and senescence of human colon cancer cells treated with camptothecin. J Biol Chem. 2002;277:17154–17160. doi: 10.1074/jbc.M112401200. [DOI] [PubMed] [Google Scholar]
- 5.Janicke RU, Sohn D, Essmann F, Schulze-Osthoff K. The multiple battles fought by anti-apoptotic p21. Cell Cycle. 2007;6:407–413. doi: 10.4161/cc.6.4.3855. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez R, Meuth M. Chk1 and p21 cooperate to prevent apoptosis during DNA replication fork stress. Mol Biol Cell. 2006;17:402–412. doi: 10.1091/mbc.E05-07-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deng C, Zhang P, Harper JW, Elledge SJ, Leder P. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell. 1995;82:675–684. doi: 10.1016/0092-8674(95)90039-x. [DOI] [PubMed] [Google Scholar]
- 8.Martin-Caballero J, Flores JM, Garcia-Palencia P, Serrano M. Tumor susceptibility of p21(Waf1/Cip1)-deficient mice. Cancer Res. 2001;61:6234–6238. [PubMed] [Google Scholar]
- 9.De la Cueva E, Garcia-Cao I, Herranz M, Lopez P, Garcia-Palencia P, Flores JM, Serrano M, Fernandez-Piqueras J, Martin-Caballero J. Tumorigenic activity of p21Waf1/Cip1 in thymic lymphoma. Oncogene. 2006;25:4128–4132. doi: 10.1038/sj.onc.1209432. [DOI] [PubMed] [Google Scholar]
- 10.Wei W, Sedivy JM. Differentiation between senescence (M1) and crisis (M2) in human fibroblast cultures. Exp Cell Res. 1999;253:519–522. doi: 10.1006/excr.1999.4665. [DOI] [PubMed] [Google Scholar]
- 11.Cheng T, Rodrigues N, Shen H, Yang Y, Dombkowski D, Sykes M, Scadden DT. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science. 2000;287:1804–1808. doi: 10.1126/science.287.5459.1804. [DOI] [PubMed] [Google Scholar]
- 12.Kippin TE, Martens DJ, van der Kooy D. p21 loss compromises the relative quiescence of forebrain stem cell proliferation leading to exhaustion of their proliferation capacity. Genes Dev. 2005;19:756–767. doi: 10.1101/gad.1272305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruhl T, Heeschen C, Aicher A, Jadidi AS, Haendeler J, Hoffmann J, Schneider MD, Zeiher AM, Dimmeler S, Rossig L. p21Cip1 levels differentially regulate turnover of mature endothelial cells, endothelial progenitor cells, and in vivo neovascularization. Circ Res. 2004;94:686–692. doi: 10.1161/01.RES.0000119922.71855.56. [DOI] [PubMed] [Google Scholar]
- 14.van den Bos C, Silverstetter S, Murphy M, Connolly T. p21(cip1) rescues human mesenchymal stem cells from apoptosis induced by low-density culture. Cell Tissue Res. 1998;293:463–470. doi: 10.1007/s004410051138. [DOI] [PubMed] [Google Scholar]
- 15.Zhou S, Greenberger JS, Epperly MW, Goff JP, Adler C, Leboff MS, Glowacki J. Age-related intrinsic changes in human bone-marrow-derived mesenchymal stem cells and their differentiation to osteoblasts. Aging Cell. 2008;7:335–343. doi: 10.1111/j.1474-9726.2008.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li H, Fan X, Kovi RC, Jo Y, Moquin B, Konz R, Stoicov C, Kurt-Jones E, Grossman SR, Lyle S, et al. Spontaneous expression of embryonic factors and p53 point mutations in aged mesenchymal stem cells: a model of agerelated tumorigenesis in mice. Cancer Res. 2007;67:10889–10898. doi: 10.1158/0008-5472.CAN-07-2665. [DOI] [PubMed] [Google Scholar]
- 17.Matushansky I, Hernando E, Socci ND, Mills JE, Matos TA, Edgar MA, Singer S, Maki RG, Cordon-Cardo C. Derivation of sarcomas from mesenchymal stem cells via inactivation of the Wnt pathway. J Clin Invest. 2007;117:3248–3257. doi: 10.1172/JCI31377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riggi N, Cironi L, Provero P, Suva ML, Kaloulis K, Garcia-Echeverria C, Hoffmann F, Trumpp A, Stamenkovic I. Development of Ewing's sarcoma from primary bone marrow-derived mesenchymal progenitor cells. Cancer Res. 2005;65:11459–11468. doi: 10.1158/0008-5472.CAN-05-1696. [DOI] [PubMed] [Google Scholar]
- 19.Riggi N, Cironi L, Provero P, Suva ML, Stehle JC, Baumer K, Guillou L, Stamenkovic I. Expression of the FUS-CHOP fusion protein in primary mesenchymal progenitor cells gives rise to a model of myxoid liposarcoma. Cancer Res. 2006;66:7016–7023. doi: 10.1158/0008-5472.CAN-05-3979. [DOI] [PubMed] [Google Scholar]
- 20.Riggi N, Suva ML, Suva D, Cironi L, Provero P, Tercier S, Joseph JM, Stehle JC, Baumer K, Kindler V, et al. EWS-FLI-1 expression triggers a Ewing's sarcoma initiation program in primary human mesenchymal stem cells. Cancer Res. 2008;68:2176–2185. doi: 10.1158/0008-5472.CAN-07-1761. [DOI] [PubMed] [Google Scholar]
- 21.Tirode F, Laud-Duval K, Prieur A, Delorme B, Charbord P, Delattre O. Mesenchymal stem cell features of Ewing tumors. Cancer Cell. 2007;11:421–429. doi: 10.1016/j.ccr.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 22.Miura M, Miura Y, Padilla-Nash HM, Molinolo AA, Fu B, Patel V, Seo BM, Sonoyama W, Zheng JJ, Baker CC, et al. Accumulated chromosomal instability in murine bone marrow mesenchymal stem cells leads to malignant transformation. Stem Cells. 2006;24:1095–1103. doi: 10.1634/stemcells.2005-0403. [DOI] [PubMed] [Google Scholar]
- 23.Rubio D, Garcia-Castro J, Martin MC, de la Fuente R, Cigudosa JC, Lloyd AC, Bernad A. Spontaneous human adult stem cell transformation. Cancer Res. 2005;65:3035–3039. doi: 10.1158/0008-5472.CAN-04-4194. [DOI] [PubMed] [Google Scholar]
- 24.Tolar J, Nauta AJ, Osborn MJ, Panoskaltsis Mortari A, McElmurry RT, Bell S, Xia L, Zhou N, Riddle M, Schroeder TM, et al. Sarcoma derived from cultured mesenchymal stem cells. Stem Cells. 2007;25:371–379. doi: 10.1634/stemcells.2005-0620. [DOI] [PubMed] [Google Scholar]
- 25.Liu C, Chen Z, Chen Z, Zhang T, Lu Y. Multiple tumor types may originate from bone marrow-derived cells. Neoplasia. 2006;8:716–724. doi: 10.1593/neo.06253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rubio D, Garcia S, De la Cueva T, Paz MF, Lloyd AC, Bernad A, Garcia-Castro J. Human mesenchymal stem cell transformation is associated with a mesenchymal-epithelial transition. Exp Cell Res. 2008;314:691–698. doi: 10.1016/j.yexcr.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 27.Mueller LP, Luetzkendorf J, Mueller T, Reichelt K, Simon H, Schmoll HJ. Presence of mesenchymal stem cells in human bone marrow after exposure to chemotherapy: evidence of resistance to apoptosis induction. Stem Cells. 2006;24:2753–2765. doi: 10.1634/stemcells.2006-0108. [DOI] [PubMed] [Google Scholar]
- 28.Rubio D, Garcia S, Paz MF, De la Cueva T, Lopez-Fernandez LA, Lloyd AC, Garcia-Castro J, Bernad A. Molecular characterization of spontaneous mesenchymal stem cell transformation. PLoS ONE. 2008;3:e1398. doi: 10.1371/journal.pone.0001398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brugarolas J, Chandrasekaran C, Gordon JI, Beach D, Jacks T, Hannon GJ. Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature. 1995;377:552–557. doi: 10.1038/377552a0. [DOI] [PubMed] [Google Scholar]
- 30.Ernst H, Carlton WW, Courtney C, Rinke M, Greaves P, Isaacs KR, Krinke G, Konishi Y, Mesfin GM, Sandusky G. Soft tissue and skeletal muscle. In: Mohr U, editor. International Classification of Rodent Tumors. The Mouse. Berlin, Germany: Springer-Velag; 2001. pp. 361–388. [Google Scholar]
- 31.Rodriguez R, Gagou ME, Meuth M. Apoptosis induced by replication inhibitors in Chk1-depleted cells is dependent upon the helicase cofactor Cdc45. Cell Death Differ. 2008;15:889–898. doi: 10.1038/cdd.2008.4. [DOI] [PubMed] [Google Scholar]
- 32.Nadri S, Soleimani M, Hosseni RH, Massumi M, Atashi A, Izadpanah R. An efficient method for isolation of murine bone marrow mesenchymal stem cells. Int J Dev Biol. 2007;51:723–729. doi: 10.1387/ijdb.072352ns. [DOI] [PubMed] [Google Scholar]
- 33.Sun S, Guo Z, Xiao X, Liu B, Liu X, Tang PH, Mao N. Isolation of mouse marrow mesenchymal progenitors by a novel and reliable method. Stem Cells. 2003;21:527–535. doi: 10.1634/stemcells.21-5-527. [DOI] [PubMed] [Google Scholar]
- 34.Bruder SP, Jaiswal N, Haynesworth SE. Growth kinetics, self-renewal, and the osteogenic potential of purified human mesenchymal stem cells during extensive subcultivation and following cryopreservation. J Cell Biochem. 1997;64:278–294. doi: 10.1002/(sici)1097-4644(199702)64:2<278::aid-jcb11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 35.Shima Y, Okamoto T, Aoyama T, Yasura K, Ishibe T, Nishijo K, Shibata KR, Kohno Y, Fukiage K, Otsuka S, et al. In vitro transformation of mesenchymal stem cells by oncogenic H-rasVal12. Biochem Biophys Res Commun. 2007;353:60–66. doi: 10.1016/j.bbrc.2006.11.137. [DOI] [PubMed] [Google Scholar]
- 36.Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120:513–522. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 37.Tataria M, Quarto N, Longaker MT, Sylvester KG. Absence of the p53 tumor suppressor gene promotes osteogenesis in mesenchymal stem cells. J Pediatr Surg. 2006;41:624–632. doi: 10.1016/j.jpedsurg.2005.12.001. discussion 624–632. [DOI] [PubMed] [Google Scholar]
- 38.Barboza JA, Liu G, Ju Z, El-Naggar AK, Lozano G. p21 delays tumor onset by preservation of chromosomal stability. Proc Natl Acad Sci USA. 2006;103:19842–19847. doi: 10.1073/pnas.0606343104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wei W, Herbig U, Wei S, Dutriaux A, Sedivy JM. Loss of retinoblastoma but not p16 function allows bypass of replicative senescence in human fibroblasts. EMBO Rep. 2003;4:1061–1066. doi: 10.1038/sj.embor.7400001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rogan EM, Bryan TM, Hukku B, Maclean K, Chang AC, Moy EL, Englezou A, Warneford SG, Dalla-Pozza L, Reddel RR. Alterations in p53 and p16INK4 expression and telomere length during spontaneous immortalization of Li-Fraumeni syndrome fibroblasts. Mol Cell Biol. 1995;15:4745–4753. doi: 10.1128/mcb.15.9.4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harvey DM, Levine AJ. p53 alteration is a common event in the spontaneous immortalization of primary BALB/c murine embryo fibroblasts. Genes Dev. 1991;5:2375–2385. doi: 10.1101/gad.5.12b.2375. [DOI] [PubMed] [Google Scholar]
- 42.Pantoja C, Serrano M. Murine fibroblasts lacking p21 undergo senescence and are resistant to transformation by oncogenic Ras. Oncogene. 1999;18:4974–4982. doi: 10.1038/sj.onc.1202880. [DOI] [PubMed] [Google Scholar]
- 43.Willenbring H, Sharma AD, Vogel A, Lee AY, Rothfuss A, Wang Z, Finegold M, Grompe M. Loss of p21 permits carcinogenesis from chronically damaged liver and kidney epithelial cells despite unchecked apoptosis. Cancer Cell. 2008;14:59–67. doi: 10.1016/j.ccr.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Komarova EA, Christov K, Faerman AI, Gudkov AV. Different impact of p53 and p21 on the radiation response of mouse tissues. Oncogene. 2000;19:3791–3798. doi: 10.1038/sj.onc.1203717. [DOI] [PubMed] [Google Scholar]
- 45.Shibata KR, Aoyama T, Shima Y, Fukiage K, Otsuka S, Furu M, Kohno Y, Ito K, Fujibayashi S, Neo M. Expression of the p16INK4A gene is associated closely with senescence of human mesenchymal stem cells and is potentially silenced by DNA methylation during in vitro expansion. Stem Cells. 2007;25:2371–2382. doi: 10.1634/stemcells.2007-0225. [DOI] [PubMed] [Google Scholar]
- 46.Sharpless NE, Bardeesy N, Lee KH, Carrasco D, Castrillon DH, Aguirre AJ, Wu EA, Horner JW, DePinho RA. Loss of p16Ink4a with retention of p19Arf predisposes mice to tumorigenesis. Nature. 2001;413:86–91. doi: 10.1038/35092592. [DOI] [PubMed] [Google Scholar]
- 47.Kim WY, Sharpless NE. The regulation of INK4/ARF in cancer and aging. Cell. 2006;127:265–275. doi: 10.1016/j.cell.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 48.Funes JM, Quintero M, Henderson S, Martinez D, Qureshi U, Westwood C, Clements MO, Bourboulia D, Pedley RB, Moncada S, et al. Transformation of human mesenchymal stem cells increases their dependency on oxidative phosphorylation for energy production. Proc Natl Acad Sci USA. 2007;104:6223–6228. doi: 10.1073/pnas.0700690104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stein GH, Drullinger LF, Soulard A, Dulic V. Differential roles for cyclin-dependent kinase inhibitors p21 and p16 in the mechanisms of senescence and differentiation in human fibroblasts. Mol Cell Biol. 1999;19:2109–2117. doi: 10.1128/mcb.19.3.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Quereda V, Martinalbo J, Dubus P, Carnero A, Malumbres M. Genetic cooperation between p21Cip1 and INK4 inhibitors in cellular senescence and tumor suppression. Oncogene. 2007;26:7665–7674. doi: 10.1038/sj.onc.1210578. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.