Abstract
Objective
To examine cardiovascular reactivity and recovery to laboratory stress among a naturalistic sample of individuals diagnosed with major depressive disorder (MDD) and healthy control participants. Prospective evidence suggests that MDD confers risk for cardiovascular disease equal to or greater than the risk associated with depressed mood. Enhanced cardiovascular reactivity has been proposed as a mechanism explaining increased risk, but data are inconsistent as to whether depressed individuals exhibit enhanced or attenuated reactivity. Further, few studies have examined appraisal and recovery differences.
Design
Participants diagnosed with MDD (N = 25) and healthy control participants (N = 25) engaged in a cardiovascular reactivity protocol including two tasks, each followed by a brief recovery period.
Main outcome measures
Blood pressure, heart rate, pre-ejection period, cardiac output and total peripheral resistance were assessed. Appraisals of tasks were assessed prior to each task.
Results
Depressed participants exhibited significantly less systolic blood pressure, heart rate and cardiac output reactivity during speech, less heart rate reactivity during mirror tracing and less heart rate recovery after speech and mirror tracing than controls. Depressed participants appraised the tasks as more demanding, threatening, and stressful and reported being less able to cope than controls. Appraisals were related to heart rate reactivity, but appraisals did not mediate the relationship between depression group and reactivity.
Conclusion
Impaired recovery rather than exaggerated cardiovascular reactivity may partially explain the increased prospective cardiovascular disease risk in depressed individuals.
Keywords: Depression, cardiovascular reactivity, recovery, cognitive appraisals
Prospective evidence suggests that depression confers risk for the development of CVD (Kuper, Marmot & Hemingway, 2002; Wuslin & Singal, 2003;) with an overall relative risk of 1.64 (Rugulies, 2002). Much of this research has focused on depressed mood, but a few studies have shown that diagnosed major depressive disorder (MDD) is associated with at least equal to if not higher risk than that of depressed mood (Aromaa et al., 1994; Ford et al., 1999; Pratt, et al., 1998). In a recent meta-analysis of prospective studies involving initially physically-healthy samples, MDD was associated with a relative risk of 2.69 for the development of CVD whereas depressed mood was associated with a relative risk of 1.49 (Rugulies, 2002). Similarly, depressed mood has been prospectively linked to the development of hypertension (Jonas & Lando, 2000) and stroke (Gump, Matthews, Eberly & Chang, 2005).
Several mechanisms have been proposed to explain the increased CVD risk CVD associated with depression including poorer health behaviors, obesity, hypothalamic-pituitary-adrenocortical axis hyperactivity, elevated catecholamine levels, and hypercoaguability (see Joynt, Whellan & O’Connor, 2003 for a review). One proposal receiving research attention within the framework of the reactivity hypothesis (Krantz & Manuck, 1984; K. Matthews et al., 2006) is that depression is associated with exaggerated cardiovascular responses to laboratory stressors, which serves as a marker of CVD risk. However, the idea that depression is related to exaggerated cardiovascular reactivity (i.e., larger changes from resting to task) has not received consistent support. While some investigations report a positive association between depressed mood and reactivity (e.g., S. Matthews, Nelson & Dimsdale, 2005; Thornton & Hallas, 1999), other studies report no association (Guinjoan, Bernabó,& Cardinali, 1995; Taylor et al., 2006) and still others report either a negative association overall (e.g., Carroll, Phillips, Hunt & Der, 2007; Straneva-Meuse, Light, Allen, Golding & Girdler, 2004; York, et al., 2007), or among subgroups (e.g., Knight & McCallum, 1998; Delehanty, Dimsdale & Mills, 1991). A recent meta-analysis reported small, nonsignificant effect sizes linking depression to blood pressure reactivity and a significant modest effect size for HR reactivity (Kibler & Ma, 2004). However, a recent report based on over 1600 adults showed that depressed mood was negatively related to systolic blood pressure and heart rate reactivity (Carroll et al., 2007).
Critically, inconsistency in prior findings is likely to reflect heterogeneity between studies. Prior studies vary along critical dimensions, such as whether or not the sample is free from CVD, or is selected on the basis of a dysphoric mood versus a diagnosis of MDD. The presence of CVD may alter cardiovascular reactivity through peripheral physiological mechanisms unrelated to psychosocial factors (Dimsdale, Siegler, Mills, Delahanty & Berry, 1990; Lovallo & Gerin, 2003), and depressed mood has been shown to alter a number of physiological responses (Allen, Trinder & Brennen, 1999; Dawson, Schell & Catania, 1977). To provide evidence that exaggerated reactivity contributes to the prospective risk that MDD confers, medically healthy depressed individuals should exhibit exaggerated reactivity relative to healthy controls. To our knowledge only two studies have carefully excluded participants who are positive for a history of cardiovascular disease. One showed no differences in cardiovascular reactivity between MDD participants and healthy controls during autonomic function tests (Guinjoan et al., 1995). The other showed evidence that those with MDD exhibited attenuated reactivity (Straneva-Meuse et al., 2004), although all depressed participants were taking one of two specific antidepressants, so these results may not generalize to a more naturalistic sample. Therefore, one goal of the present study was to examine cardiovascular reactivity to two laboratory stressors in a naturalistic sample of participants with diagnosed depression and healthy controls with no self-reported history of CVD.
Perceived task difficulty is an important construct in the cardiovascular reactivity literature, and one that may be useful for explaining depression-related differences in reactivity and between-study heterogeneity. Depression involves changes in cognition that are likely to alter perceptions of demanding situations. For example, dysphoria and rumination, two common features of depression, have been related to perceiving stressors as severe and unsolvable as well as reduced self-confidence, optimism and perceived control (Lyubormirsky, Tucker, Caldwell & Berg, 1999). Further, those in a negative mood, compared to those in a positive mood, exhibit greater reactivity when presented with easy tasks but less reactivity when presented with difficult tasks (Gendolla & Krusken, 2005). Thus, a second goal of the present study was to examine differences between depressed individuals and healthy controls in cognitive appraisals of laboratory stressors and if appraisal differences partially explain group differences in reactivity.
A third goal of the present study was to examine whether depressed persons exhibit impaired recovery from laboratory stressors. Impaired cardiovascular recovery has been identified as a CVD risk factor that is independent of cardiovascular reactivity (Hocking Schuler & O’Brien, 1997; Steptoe & Marmot, 2006). Incomplete recovery after stress predicts hypertension risk (e.g., Hocking Schuler & O’Brien, 1997; Steptoe & Marmot, 2005; Stewart, Janicki & Kamarck, 2006; Trieber et al., 2001) and has been related to carotid intima-media thickness (Steptoe, Donald, O’Donnell, Marmot & Deanfield, 2006). Perseverative cognitive styles associated with negative affect, such as worry and rumination, have been linked to delayed recovery from laboratory stressors (Brosschot, Gerin & Thayer, 2006; Glynn, Christenfeld & Gerin, 2002); in turn, high positive emotionality has been linked to accelerated cardiovascular recovery (Tugade & Frederickson, 2004). This is important because MDD is a disorder that is often characterized by excessive worry and rumination (Nolen-Hoeksema, 2000; Spasojevic & Alloy, 2001) and low positive emotionality (Kasch, Rottenberg, Arnow & Gotlib, 2002). Thus the hypothesis that depression impairs cardiovascular recovery from stress is compelling.
Unfortunately, few studies have examined cardiovascular recovery in depressed individuals. Two studies found that depressed mood was related to higher cardiovascular levels across phases (i.e., baseline, tasks, recovery) but did not find interactions between phase and depression group, suggesting recovery did not differ by group (Hamer, Tanaka, Okamura, Tsuda & Steptoe, 2007; S. Matthews et al., 2005). One study of an older sample at risk for CVD reported no differences in level across phases or interactions between diagnosed depressed and control groups (Taylor et al., 2006). Importantly, these prior studies did not examine recovery independent of baseline and reactivity, a limitation that the present study attempts to remedy.
Hypotheses
We predicted that significant differences in reactivity would emerge between MDD participants and controls. Because the literature suggests that both enhanced or diminished reactivity is possible in MDD, we did not make directional predictions.
Two common features of depression, dysphoria and rumination, are related to perceiving stressors as severe and reduced self-confidence. Therefore, we expect that MDD participants will exhibit higher levels of threat, demand, and stress appraisals, and report less ability to cope with the task than control participants.
For those in a negative mood, appraisals are related to greater reactivity for easy tasks and less reactivity for difficult tasks. Therefore, we expect that cognitive appraisals will mediate the relationship between group (MDD and control) and reactivity.
Method
The study was conducted from January 2005 to May 2006 and was approved by the Social & Behavioral Sciences Institutional Review Board at the University of South Florida.
Participants and Clinical Assessment
Participants were 25 unipolar depressed persons and 25 healthy nonpsychiatric controls. Groups were matched on average age, self-reported ethnicity, gender, and income (see Table 1).
Table 1.
Demographic and Clinical Characteristics of the Sample
| Group
|
||
|---|---|---|
| Variable | MDD (n = 25) | Control (n = 25) |
| Age (years), M (SD) | 30.36 (9.37) | 32.95 (11.93) |
| Age, Range | 19 – 52 | 19 – 57 |
| Caucasian Ethnicity, N (%) | 17 (68%) | 17 (68%) |
| Female gender, N (%) | 20 (80%) | 17 (68%) |
| BMI, M (SD) | 27.29 (5.39) | 23.59 (3.66) |
| Waist circumference, M (SD) | 89.60 (14.39)a | 82.83 (13.05) |
| Incomeb | 2.09 (1.12) | 2.70 (1.52) |
| BDI, M (SD)* | 29.84 (5.60) | 1.48 (1.69) |
| BDI, Range | 19 – 45 | 0 – 7 |
| BAI, M (SD)* | 19.24 (11.17) | 2.64 (2.55) |
| BAI, Range | 5 – 49 | 0 – 9 |
Note.
Significant difference between depressed and healthy control groups, p < .001.
BDI = Beck Depression Inventory, 2nd Edition, BAI = Beck Anxiety Inventory, BMI = Body Mass Index
N = 24,
Income was assessed on a 6-point scale with higher numbers representing higher income—a score of 2.09 represents an income of $10,000 to $25,000
General selection criteria
Participants responded to local ads or flyers in the immediate campus area, including the waiting areas at the university counseling services centers. Ads targeted either depressed persons suffering currently from common depressive symptoms or healthy controls who had no history of psychiatric illness. Diagnostic evaluations were based on DSM-IV (American Psychiatric Association, 1994) criteria and were determined by an initial telephone screening and the Structured Clinical Interview for DSM-IV Axis I (SCID-I; First, Gibbon, Spitzer & Williams, 1995). All participants met the following inclusion and exclusion criteria: English fluency, aged 18 - 60; no reported history of brain injury; no lifetime history of primary psychotic ideation, no lifetime diagnoses of bipolar disorder, no behavioral indications of impaired mental status, and no reported abuse of any psychoactive substances, including alcohol, within the past six months.
Exclusion of cardiovascular disease was based on self-report by participants during initial screening and during the SCID-I interview. Upon screening, participants were asked whether they had a history of CVD, such as hypertension or heart attacks. During the SCID-I interview, participants’ medical history was extensively probed for past and present medical problems, including diagnoses, prescribed medications, and hospitalizations. Participants were excluded for a self-reported history of medication-dependent diabetes, heart disease, hypertension, mood episodes secondary to general medical conditions or medical conditions specific to the central nervous system, or current use of medications known to have significant effects on cardiovascular function (e.g., tricyclic antidepressants, antipsychotic agents, antihistamines, and beta-blockers). Six participants reported use of oral contraceptives; all were in the control group. One control participant reported use of barbiturate medication for the treatment of a seizure condition. Nine depressed participants reported use of psychotropic medication.
Depressed and healthy control diagnostic criteria
The SCID-I interview (First et al., 1995) was conducted by clinical doctoral students to confirm that the depressed group met current diagnostic criteria for current MDD. Consistent with high levels of anxiety comorbidity reported elsewhere (Kessler, Chiu, Demler, & Walters, 2005), 17 MDD participants also met diagnostic criteria for at least one anxiety disorder. Fifteen participants met criteria for melancholic MDD and four met criteria for atypical MDD. The average duration of the current depressive episode was 24.3 months (SD = 36.6). Six MDD participants reported one episode of major depression, three reported two episodes, and sixteen reported three or more episodes. Five MDD participants reported history of attempted suicide and six reported history of psychiatric hospitalization. The average age of onset was 18.8 years (SD = 9.6). Healthy control participants met the medical exclusion criteria and did not have any SCID-I assessed lifetime diagnoses of Axis I disorders.
Severity of depression and anxiety
The Beck Depression Inventory-II (BDI-II) and the Beck Anxiety Inventory (BAI) were used to measure depression symptom severity and anxiety symptom severity, respectively. Both are 21 item self-report measures that have demonstrated validity and reliability (Beck, Epstein, Brown & Steer, 1988; Beck, Steer & Brown, 1996).
Assessment of Cognitive Appraisals
We assessed pre-task cognitive appraisals of demand, threat, stress, and ability to cope based on prior research suggesting that pre-task appraisals are related to cardiovascular responses during laboratory reactivity tasks (Tomaka, Blascovich, Kelsey & Leitten, 1993; Tomaka, Blascovich, Kibler & Ernst, 1997). We generated four appraisal items: How demanding do you expect the upcoming task to be, how threatening (or intimidating) do you expect the upcoming task to be, how stressful do you expect the upcoming task to be, and how able are you to cope with the upcoming task. Responses ranged from “not at all” to “very much” on a 5-point Likert-type scale. We formed a challenge-threat ratio by dividing coping appraisal score by demand appraisal score. Items and scoring were virtually identical to those used in prior research (Tomaka et al., 1993; Tomaka et al., 1997).
Cardiovascular Measures
An Accutorr Plus blood pressure monitor (Datascope Corp., Mahwah, NJ) collected systolic (SBP) and diastolic (DBP) blood pressure according to published guidelines (Shapiro et al., 1990). Heart rate was measured via electrocardiogram (ECG), using Cleartrace LT disposable Ag/AgCl electrodes (Conmed Andover Medical, Haverhill, MA), placed in a modified Lead II configuration on the chest. ECG was amplified using a Biopac MP150 system with an ECG100 amplifier (Biopac Instruments Inc., Goleta, CA). Impedance cardiography was collected according to the Sherwood et al (1990) using four mylar-band electrodes fully encircling the neck and torso with the Biopac EBI100C monitor. ECG and impedance (Z0) signals were digitized, acquired and stored using a PC and Biopac AcqKnowledge software.
Cardiovascular reactivity tasks
Two cardiovascular reactivity tasks were administered. A 2-minute mirror tracing task required participants to trace the image of a star as quickly and accurately as possible while only seeing a mirror image of their hand and the star. The mirror tracing device (Stoelting Co., Chicago, IL) provided auditory feedback when the metal stylus lost contact with the star. A speech task required participants to prepare a speech on a specific topic (i.e., defending themselves against a traffic ticket), and to deliver the speech. The preparation and delivery phases of the speech task were each 2 minutes. To further increase evaluation apprehension during the speech task, an experimental observer was present in the room and silently took notes on the participant’s behavior. Tasks were followed by 2-minute recovery periods, during which participants were instructed to sit quietly.
Procedure
Participants were first assessed for height and weight with a fixed steel tape and a beam scale. Waist circumference was measured in cm at the level of the umbilicus. Next, the experimenter attached the cardiovascular equipment. Participants were seated comfortably in a small recording room. The experimenter noted the presence of an unobtrusive, ceiling-mounted camera and informed the participants that they would be monitored throughout the protocol. Participants then viewed a neutral travelogue film for a 10-minute acclimation and baseline assessment. The speech and mirror tracing tasks then were administered in counterbalanced order, separated by a ten minute rest period. After the second task, participants completed the BDI-II and the BAI, sensors were removed, and participants were paid, debriefed, and thanked.
Data Recording and Processing
BP recordings were taken during the 6th, 8th, and 10th minute of the 10 minute rest period and ECG and impedance cardiography was recorded continuously during the last 5 minutes. BP was obtained during each minute of the speech preparation, speech, and mirror tracing tasks. During the recovery phases, one BP readings was recorded during the second minute. Impedance and ECG data were collected continuously during the task and recovery phases.
Heart rate (HR) and the impedance-derived measures of pre-ejection period (PEP) and cardiac output (CO) were obtained using MindWare IMP 2.56 software (MindWare Technologies, Ltd., Gahanna, OH). The ECG and dZ/dt signals were ensemble-averaged over 60-second epochs. The data were screened for artifact by visual inspection. Mean arterial pressure (MAP) was calculated as (SBP + (2 * DBP))/3. Total peripheral resistance (TPR) was estimated using the formula TPR = (MAP/CO) * 80 in dyne-sec/cm5.
Baseline, task and recovery values for each measure were computed by averaging the available values for each phase, except for recovery BP, which included only one assessment. Reactivity scores were calculated as the arithmetic difference between task and baseline averages. Recovery scores were calculated as the arithmetic difference between recovery and baseline values, such that smaller values indicate greater recovery.
Data Analyses
We first examined baseline levels of SBP, DBP and HR for significant group differences using Analyses of Covariance (ANCOVA) controlling for gender. To examine group differences in reactivity, we conducted ANCOVAs with group (control, MDD) as the between-subjects factor and the speech preparation, speech delivery and mirror tracing task change scores as dependent measures, controlling for gender.
To examine group differences in appraisals, we conducted independent samples t-tests with pre-task appraisal scores as the dependent measures. To test for mediation, we followed steps outlined by Baron and Kenny (1986). We first identified which reactivity analyses and which appraisals t-tests indicated a significant group difference. Next, we examined if appraisals were related to reactivity measures for the corresponding task. In cases where all three conditions for mediation were met, we conducted hierarchical regression analyses regressing gender, group and appraisal scores onto reactivity measures and examined if group continued to predict reactivity while controlling for appraisals.
To examine group differences in recovery, we conducted ANCOVAs on the recovery change scores while controlling for gender and corresponding reactivity change score. For the speech task, reactivity covariates were computed from averaged preparation and delivery values minus baseline averages. We examined if appraisals mediated the effect of group on recovery using the procedures outlined above. Slight variations in degrees of freedom reflect missing data for some cardiovascular variables due to inadequate signals from movement artifact.
Before testing the hypotheses, we first conducted preliminary analyses to examine the potential role of medication and anxiety comorbidity. Preliminary analyses of medications used only participants not taking psychiatric medication and the overall pattern of findings was very similar to those reported below. Similarly, preliminary analyses of anxiety were conducted using only participants without an anxiety disorder and again the overall pattern of findings was remarkably similar to those reported below. In light of these preliminary results, these variables were not considered further.
Results
Demographic and Physical Variables
Table 1 presents the demographic, physical and clinical characteristics of the sample. As reported elsewhere (Rottenberg, et al., 2007), the two groups did not differ on age, income, body mass index, or waist circumference. Depressed participants reported more depressive symptoms and more anxiety symptoms than healthy control participants.
Baseline Levels
A significant difference in resting HR between depressed participants and healthy controls emerged, F(1, 47) = 17.31, p < .001, η2 = .269. Depressed participants exhibited a higher resting HR on average than control participants. As displayed in Table 2, no significant group differences in baseline levels of SBP or DBP were found.
Table 2.
Mean Resting Cardiovascular Level by Group.
| Group
|
||
|---|---|---|
| Measure | MDD | Control |
| SBP (mmHg) | 115.84 (1.96) | 113.93 (1.96) |
| DBP (mmHg) | 70.94 (1.45) | 69.12 (1.45) |
| HR* (bpm) | 80.63 (2.10) | 68.21 (2.10) |
Note:
Significant effect of group, p < .05, using ANCOVA controlling for gender.
Numbers reported are covariate adjusted Means (M) and standard error of the mean (SEM), bpm = beats per minute.
Reactivity
For speech preparation, significant effects of group were found for SBP (F(1, 47) = 4.37, p < .05, η2 = .085), HR (F(1, 46) = 4.64, p < .05, η2 = .092), and CO (F(1, 46) = 10.69, p <.01, η2 = .189) reactivity. Depressed participants exhibited less SBP, HR, and CO reactivity than healthy controls. No other significant differences in speech preparation reactivity were found.
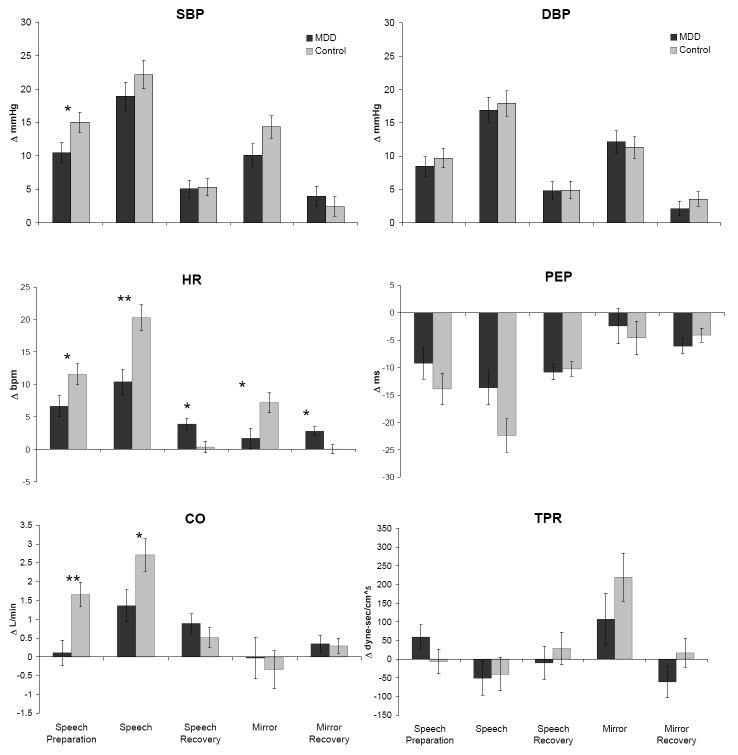
For speech delivery reactivity, significant effects of group were found for HR (F(1, 47) = 12.37, p < .01, η2 = .208) and CO (F(1, 47) = 4.80, p < .05, η2 = .093) reactivity. Depressed participants exhibited less HR and CO reactivity. For mirror tracing reactivity, a significant effect of group was found for HR reactivity, F(1, 46) = 6.15, p < .05, η2 = .118). No other significant differences in mirror tracing reactivity were found. Figure 1 presents means for reactivity by group.
Figure 1.
Mean Reactivity and Recovery Change Scores by Group and Phase. (Note: Significant effect of group indicated by *p < .05 or **p < .01 using ANCOVA. Bars reflect +/- 1 standard error of the mean. Means for speech preparation, speech, and mirror are adjusted for gender. Means for recovery phases are adjusted for gender and task reactivity. Δ = change in, mmHg = millimeters of mercury, bpm = beats per minute, L/min = liters per minute, dyne-sec/cm5 refers to peripheral resistance units)
Appraisals
Table 2 reports the means and standard deviations for task appraisal items and raw challenge-threat ratios. Depressed participants appraised the upcoming speech task as more demanding, threatening, stressful, and reported less ability to cope, than healthy controls. Depressed participants also had smaller challenge-threat ratios, suggesting that healthy controls were more challenged than depressed participants. Results were similar for threatening, stressful, and coping ability appraisals and challenge-threat ratios for the mirror tracing task.
Mediation analyses
Significant effects were found between the predictor, group, and reactivity (speech preparation SBP, HR, and CO; speech delivery HR and CO, mirror tracing HR) meeting the first criteria for mediation. Significant effects were found between the predictor and mediator appraisal variables (speech demand, threat, stress, ability to cope, and challenge-threat ratio; mirror tracing threat, stress, ability to cope, and challenge-threat ratio), meeting the second criteria for mediation. Therefore, we examined the correlations between the reactivity measures and appraisals noted above for each task to identify potential mediators. Speech HR reactivity was significantly correlated with pre-speech demand (partial r(46) = -.30), threat (partial r(46) = -.36), ability to cope (partial r(46) = .34) and challenge-threat ratio (partial r(46) = .41) controlling for gender (all p’s < .05). No other significant partial correlations were found.
In all four regression models testing mediation (see data analysis section), the effect of group on speech delivery HR reactivity remained significant after entering the appraisal item (all t’s > |2.13|, p’s < .04). Further, the appraisal items were not significant predictors of speech delivery HR reactivity when entered into the model (all t’s < |1.49|, all p’s > .14). Therefore, although the groups differed on reactivity and appraisals, and some appraisals were related to reactivity, appraisals did not mediate the effect of group on reactivity.
Recovery
Significant effects of group on HR recovery were found for the speech task (F(1, 45) = 7.08, p < .05, η2 = .136) and the mirror tracing task (F(1, 45) = 7.29, p = .01, η2 = .139). Depressed participants exhibited less HR recovery after the tasks compared to healthy controls. No significant effects were found for other cardiovascular recovery variables. The means for recovery are presented in Figure 1. Also, pre-task appraisals were not related to HR recovery for either the speech or mirror tracing tasks (p’s > .36), controlling for gender and reactivity. Therefore, appraisals could not mediate the effect of group on recovery.
Discussion
We sought to examine differences in sympathetically-mediated cardiovascular reactivity and recovery between individuals diagnosed with MDD and healthy controls. The present study is unique in that we compared a group with diagnosed MDD to a virtually symptom-free group in a community-based sample. Further, in contrast to most prior studies, our participants were screened for the absence of cardiovascular disease, allowing us to examine depression and reactivity in relatively healthy sample. Our results indicate that depressed persons exhibit less SBP, HR, and CO reactivity than controls. These findings are consistent with a growing number of studies showing attenuated reactivity as a function of depressed mood (e.g., Carroll et al., 2007; York et al., 2007) or of MDD (Straneva-Meuse et al., 2004). They are also consistent with our previous analysis of respiratory sinus arrhythmia (RSA) which demonstrated that depressed participants exhibited less RSA reactivity and impaired RSA recovery compared to controls (Rottenberg et al., 2007). However, these findings are inconsistent with the hypothesis that that depression confers risk for CVD through exaggerated cardiovascular reactivity.
Given the finding that depression confers risk for CVD, and the now replicated finding that depression can attenuate reactivity in some contexts, it is tempting to speculate that attenuated reactivity is a pathway that explains depressed persons’ elevated risk for CVD. McEwen has proposed that multiple patterns of stress responsivity reflect allostatic load, and thus, disease risk. One suggested pattern is non-responsiveness in one physiological stress system that may indicate compensatory responses in other physiological stress systems (McEwen, 1998). Allostatic load is an attractive idea in the context of MDD, as individuals with this condition suffer from chronic depressed mood and a host of other physical and psychological symptoms. The attenuated reactivity associated with depression may reflect the non-response pattern of allostatic load, one that indicates an over-activation of the hypothalamic-pituitary-adrenocortical axis or other compensatory responses. For example, depression has been associated with chronic activation of the hypothalamic-pituitary-adrenocortical axis (Joynt, et al., 2003). Thus, one may tentatively speculate that the attenuated cardiovascular reactivity seen among depressed individuals serves as a marker for CVD risk through compensatory mechanisms. The tenability of this hypothesis remains to be tested.
Mood may serve as a source of information when appraising task demand. When faced with a difficult task, those in a negative mood may perceive the demands of a task as too high for their abilities and may not mobilize effort resulting in attenuated cardiovascular reactivity for difficult tasks (Gendolla & Krusken, 2001). Accordingly, depressed participants appraised the upcoming tasks as more demanding and stressful, perceived that they were less able to cope, and perceived the task as less challenging than healthy controls. Further, greater perceived demand and threat, and lesser perceived coping ability and challenge appraisal were related to less HR reactivity. These findings resonate with findings from nonclinical samples indicating that challenge appraisals are associated with enhanced cardiac reactivity (Tomaka et al., 1993;Tomaka et al., 1997). Importantly, these appraisals did not mediate the relationship between depression and reactivity. Thus, the attenuated reactivity seen in MDD may be due to physiological factors, or to psychological factors other than appraisals.
We also examined recovery from the tasks and found that depressed individuals exhibited less HR recovery compared to controls. Although depressed participants exhibited less reactivity and a higher resting HR, both of which would require less of a decrease in HR for recovery, they continued to exhibit elevated HR during the recovery period. Impaired recovery has been identified as a CVD risk factor that is independent of reactivity (Hocking Schuler & O’Brien, 1997; Steptoe & Marmot, 2006). Specifically, impaired HR recovery has been related to lack of physical fitness (Hocking Schuler & O’Brien, 1997) a positive family history of hypertension (Trieber et al., 2001), and has been found to predict increases in resting blood pressure (Steptoe & Marmot, 2005) and HR (Stewart et al., 2006). Further, inadequate recovery is also commensurate with the idea of allostatic load, as a prolonged response pattern (McEwen, 1998). To our knowledge, we are the first to report impaired cardiovascular recovery among medically-healthy diagnosed depressed individuals. Our recovery findings suggest that depression may confer CVD risk through impaired recovery, independent of reactivity. As others have suggested (e.g., Brosschot et al., 2006) impaired recovery may be more pernicious for the course of disease given that these cardiovascular elevations persist longer than acute stress reactivity. Further, blunted reactivity followed by impaired recovery has been linked to chronic stress (Matthews, Gump & Owens, 2001), suggesting that MDD serves as a chronic stressor or is associated with ongoing background stress. This suggests that impaired recovery is an epiphenomenon of blunted reactivity that may, as a specific response pattern, indicate risk. Perhaps those who are chronically stressed lack the psychological or physiological means to mount a large reactivity response, but perseverative processes then cause the response to persist after the stressor itself has ended. Although compelling, further research is needed to determine whether impaired recovery and blunted recovery are independent or reflect a coupled response pattern.
Depression was not related to BP recovery, although many studies point to delayed BP recovery as an indicator of risk (Hocking Schuler & O’Brien, 1997; Steptoe & Marmot, 2005; Steptoe & Marmot, 2006; Stewart et al., 2006; Trieber et al., 2001). However, our recovery period was relatively short, lasting only two minutes. HR may recover more quickly than blood pressure as HR is under both sympathetic and parasympathetic control and parasympathetic control has near instantaneous effects on HR (Levy, Yang & Wallick, 1993). Further, vagal rebound occurs quickly after cessation of a stressor, suggesting the predominance of parasympathetic mechanisms (Mezzacappa, Kelsey, Katkin & Sloan, 2001; Rottenberg, et al., 2007). Thus, the recovery period may have been too short to allow for variability in recovery of other cardiovascular responses.
The present study is not without limitations. The sample size was relatively small, partially a function of the challenge of recruiting participants who met diagnostic criteria for MDD and were in a current depressive episode, a condition that affects 10% of adults in any given year (Kessler et al., 1994). Also, it should be borne in mind that the absence of cardiovascular disease was established via self-report methods only. Although this practice is commensurate with prior work (e.g., Light, et al., 1998), we cannot rule out the possibility that our study may have included participants with undiagnosed or mis-reported CVD. Mitigating this concern, the negative predictive validity of self-reported cardiovascular diseases is generally quite high; with commonly reported estimates ranging from 89% to 99% (Martin, Leff, Calonge, Garrett & Nelson, 2000; St.Sauver et al., 2005). Given that this sample contained many younger participants, the self-reported absence of cardiovascular disease is likely to accurately reflect not being diagnosable.
The naturalistic design of the study precluded a conclusive analysis of medication or comorbid anxiety disorder as moderators of the reported findings. A fair proportion of the MDD participants were taking one or more medications and small cell sizes precluded analysis of individual medications. Similarly, a proportion of the MDD participants had comorbid anxiety disorders, which were again heterogeneous in the type and number. We conducted parallel analyses focusing only on unmedicated participants and on non-anxious participants. Many of the effects remained statistically significant despite the reduced power (data not reported). Further, the pattern of findings was the same; MDD participants exhibited higher resting HR, attenuated reactivity and impaired recovery relative to healthy controls. Thus, despite the limitations of a naturalistic design, there were no indications that the reported effects were driven by medication or the presence of co-morbid anxiety.
As noted earlier, few studies have examined reactivity among individuals with diagnosed MDD. Our recruitment strategy excluded participants with minor or subclinical depression as evidenced by the range of BDI scores among our healthy and depressed groups. This prevented examination of reactivity and recovery across a range of depressed mood. Future research would benefit from explicit comparison of psychiatrically healthy, mildly depressed, and MDD diagnosed participants. Also, a longer post-task period would allow for a more detailed analysis of recovery. Finally, we did not assess participants’ reports of rumination or positive affect during recovery, which could prove useful for explaining impaired recovery in the MDD group.
Major depressive disorder may confer prospective risk for CVD through impaired recovery from stress. If prolonged post-stress activation is the result of psychological processes associated with depression, treatments aimed at reducing rumination and increasing positive affect may ameliorate this risk. The blunted reactivity exhibited by depressed individuals may also indicate risk if the non-response pattern represents allostatic load that signals compensatory responses in other physiological systems. It may also indicate that depressed participants do not always exhibit exaggerated responses to stress if the stressor is perceived as overly demanding and threatening, and that impaired recovery may be a more consistent marker of risk.
Table 3.
Mean Pre-Task Cognitive Appraisals.
| MDD | Control | t | p | |
|---|---|---|---|---|
| Speech Appraisals | ||||
| Demanding | 3.80 (1.08) | 2.72 (1.02) | 3.63 | .001 |
| Threatening | 3.40 (1.12) | 2.12 (1.17) | 3.96 | < .001 |
| Stressful | 3.80 (1.12) | 2.56 (1.23) | 3.73 | < .001 |
| Able to Cope | 3.12 (0.88) | 4.28 (0.89) | -4.63 | < .001 |
| Challenge-Threat Ratio | 1.14 (0.94) | 2.81 (1.70) | -4.28 | < .001 |
| Mirror Tracing Appraisals | ||||
| Demanding | 3.32 (0.99) | 2.80 (1.08) | 1.78 | .082 |
| Threatening | 2.16 (1.07) | 1.48 (1.01) | 2.32 | .025 |
| Stressful | 2.84 (1.07) | 1.64 (0.81) | 4.48 | < .001 |
| Able to Cope | 4.00 (0.87) | 4.48 (0.82) | -2.01 | .050 |
| Challenge-Threat Ratio | 2.51 (1.58) | 3.72 (1.38) | -2.87 | .006 |
Note: Standard deviations are in parentheses. Independent samples t-tests were used to examine group differences.
Acknowledgments
The authors would like to thank Lauren Bylsma, Sarah Bolden Gunderson, & Katherine Rieger and for their assistance in data collection.
The first and last authors were supported by University of South Florida New Researcher Grants and National Institutes of Health grant MH077669.
References
- Allen NB, Trinder J, Brennen C. Affective startle modulation in clinical depression: Preliminary findings. Biological Psychiatry. 1999;46:542–550. doi: 10.1016/s0006-3223(99)00025-6. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 1994. [Google Scholar]
- Aromaa A, Raitasalo R, Reunanen A, Impivaara O, Heliövaara M, Knekt P, et al. Depression and cardiovascular diseases. Acta Psychiatrica Scandinavia Supplement. 1994;377:77–82. doi: 10.1111/j.1600-0447.1994.tb05807.x. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic and statistical considerations. Journal of Personality & Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: Psychometric properties. Journal of Consulting & Clinical Psychology. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the BDI-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Brosschot J, Gerin W, Thayer J. The perseverative cognition hypothesis: A review of worry, prolonged stress-related physiological activation, and health. Journal of Psychosomatic Research. 2006;60:113–124. doi: 10.1016/j.jpsychores.2005.06.074. [DOI] [PubMed] [Google Scholar]
- Carroll D, Phillips AC, Hunt K, Der G. Symptoms of depression and cardiovascular reactions to acute psychological stress: Evidence from a population study. Biological Psychiatry. 2007;75:68–74. doi: 10.1016/j.biopsycho.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Dawson ME, Schell AM, Catania JJ. Autonomic correlates of depression and clinical improvement following electroconvulsive shock therapy. Psychophysiology. 1977;14:569–578. doi: 10.1111/j.1469-8986.1977.tb01201.x. [DOI] [PubMed] [Google Scholar]
- Delehanty SG, Dimsdale JE, Mills P. Psychosocial correlates of reactivity in black and white men. Journal of Psychosomatic Research. 1991;35:451–460. doi: 10.1016/0022-3999(91)90040-u. [DOI] [PubMed] [Google Scholar]
- Dimsdale JE, Siegler M, Mills P, Delahanty SG, Berry C. Effects of salt, race, and hypertension on reactivity to stressors. Hypertension. 1990;16:573–580. doi: 10.1161/01.hyp.16.5.573. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW. User’s guide for the structured clinical interview for DSM-IV Axis I disorders (SCID-I, Version 2.0, October 1995 Final Version) 1995 [Google Scholar]
- Ford DE, Mead LA, Chang PP, Cooper-Patrick L, Wang NY, Klag MJ. Depression is a risk factor for coronary artery disease in men: the precursors study. Archives of Internal Medicine. 1998;158:1422–1425. doi: 10.1001/archinte.158.13.1422. [DOI] [PubMed] [Google Scholar]
- Gendolla GHE, Krusken J. The joint impact of mood state and task difficulty on cardiovascular and electrodermal reactivity in active coping. Psychophysiology. 2001;38:548–556. doi: 10.1017/s0048577201000622. [DOI] [PubMed] [Google Scholar]
- Glynn LM, Christenfeld N, Gerin W. The role of rumination in recovery from reactivity: Cardiovascular consequences of emotional states. Psychosomatic Medicine. 2002;64:714–726. doi: 10.1097/01.psy.0000031574.42041.23. [DOI] [PubMed] [Google Scholar]
- Guinjoan SM, Bernabó JL, Cardinali DP. Cardiovascular tests of autonomic function and sympathetic skin responses in patients with major depression. Journal Neurology, Neurosurgery, & Psychiatry. 1995;59:299–302. doi: 10.1136/jnnp.59.3.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gump BB, Matthews KA, Eberly LE, Chang YF MRFIT Research Group. Depressive symptoms and mortality in men: results from the Multiple Risk Factor Intervention Trial. Stroke. 2005;36:98–102. doi: 10.1161/01.STR.0000149626.50127.d0. [DOI] [PubMed] [Google Scholar]
- Hamer M, Tanaka G, Okamura H, Tsuda A, Steptoe A. The effects of depressive symptoms on cardiovascular and catecholamine responses to the induction of depressive mood. Biological Psychology. 2007;74:20–25. doi: 10.1016/j.biopsycho.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Hocking Schuler JL, O’Brien W. Cardiovascular recovery from stress and hypertension risk factors: A meta-analytic review. Psychophysiology. 1997;34:649–659. doi: 10.1111/j.1469-8986.1997.tb02141.x. [DOI] [PubMed] [Google Scholar]
- Jonas BS, Lando JF. Negative affect as a prospective risk factor for hypertension. Psychosomatic Medicine. 2000;62:188–196. doi: 10.1097/00006842-200003000-00006. [DOI] [PubMed] [Google Scholar]
- Joynt KE, Whellan DJ, O’Connor CM. Depression and cardiovascular disease: Mechanisms of interaction. Biological Psychiatry. 2003;54:248–261. doi: 10.1016/s0006-3223(03)00568-7. [DOI] [PubMed] [Google Scholar]
- Kasch KL, Rottenberg J, Arnow BA, Gotlib IH. Behavioral activation and inhibition and the severity and course of depression. Journal of Abnormal Psychology. 2002;111:589–597. doi: 10.1037//0021-843x.111.4.589. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Walters EE. Prevalence, severity, and comorbidity of 12 month DSM-IV Disorders in the National Comorbidity Survery Replication. Archives of General Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, et al. Lifetime and 12-Month Prevalence of DSM-III-R Psychiatric Disorders in the United States: Results From the National Comorbidity Survey. Archives of General Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Kibler JL, Ma M. Depressive symptoms and cardiovascular reactivity to laboratory behavioral stress. International Journal of Behavioral Medicine. 2004;11:81–87. doi: 10.1207/s15327558ijbm1102_3. [DOI] [PubMed] [Google Scholar]
- Knight BG, McCallum TJ. Heart rate reactivity and depression in African-American and white dementia caregivers: Reporting bias or positive coping. Aging & Mental Health. 1998;2:212–221. [Google Scholar]
- Krantz DS, Manuck SB. Acute psychophysiologic reactivity and risk of cardiovascular disease: a review and methodologic critique. Psychological Bulletin. 1984;96:435–464. [PubMed] [Google Scholar]
- Kuper H, Marmot M, Hemingway H. Systematic review of prospective cohort studies of psychosocial factors in the etiology and prognosis of coronary heart disease. Seminars in Vascular Medicine. 2002;2:267–314. doi: 10.1055/s-2002-35401. [DOI] [PubMed] [Google Scholar]
- Levy MN, Yang T, Wallick DW. Assessment of beat-by-beat control of heart rate by the autonomic nervous system: Molecular biology techniques are necessary, but not sufficient. Journal of Cardiovascular Electrophysiology. 1993;4:183–193. doi: 10.1111/j.1540-8167.1993.tb01222.x. [DOI] [PubMed] [Google Scholar]
- Lovallo WR, Gerin W. Psychophysiological reactivity: Mechanisms and pathways to cardiovascular disease. Psychosomatic Medicine. 2003;65:36–45. doi: 10.1097/01.psy.0000033128.44101.c1. [DOI] [PubMed] [Google Scholar]
- Lyubormirsky S, Tucker KL, Caldwell ND, Berg K. When poor ruminators are poor problem solvers: Clues from the phenomenology of dysphoric rumination. Journal of Personality & Social Psychology. 1999;77:1041–1060. doi: 10.1037//0022-3514.77.5.1041. [DOI] [PubMed] [Google Scholar]
- Martin LM, Leff M, Calonge N, Garrett C, Nelson DE. Valication of self-reported chronic conditions and health services in a managed care population. American Journal of Preventative Medicine. 2000;18:215–218. doi: 10.1016/s0749-3797(99)00158-0. [DOI] [PubMed] [Google Scholar]
- Matthews SC, Nelsen RA, Dimsdale JE. Depressive symptoms are associated with increased systemic vascular resistance to stress. Psychosomatic Medicine. 2005;67:509–513. doi: 10.1097/01.psy.0000160467.78373.d8. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Gump BB, Owens JF. Chronic stress influences cardiovascular and neuroendocrine responses during acute stress and recovery, especially in men. Health Psychology. 2001;20:403–410. [PubMed] [Google Scholar]
- Matthews KA, Weiss SM, Detre T, Dembroski TM, Falkner B, Manuck SB, et al. Handbook of stress, reactivity and cardiovascular disease. New York: Wiley; 1986. [Google Scholar]
- McEwen B. Stress, adaptation and disease: Allostasis and allostatic load. Annals of the New York Academy of Sciences. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- Mezzacappa ES, Kelsey RM, Katkin ES, Sloan RP. Vagal rebound and recovery from psychological stress. Psychosomatic Medicine. 2001;63:650–657. doi: 10.1097/00006842-200107000-00018. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. The role of rumination in depressive disorders and mixed anxiety/depressive symptoms. Journal of Abnormal Psychology. 2000;109:504–511. [PubMed] [Google Scholar]
- Pratt LA, Ford DE, Crum RM, Armenian HK, Gallo JJ, Eaton WW. Depression, psychotropic medication, and risk of myocardial infarction: prospective data from the Baltimore ECA follow-up. Circulation. 1996;94:3123–3129. doi: 10.1161/01.cir.94.12.3123. [DOI] [PubMed] [Google Scholar]
- Rottenberg J, Clift A, Bolden S, Salomon K. RSA fluctuation in major depression. Psychophysiology. 2007;44:450–458. doi: 10.1111/j.1469-8986.2007.00509.x. [DOI] [PubMed] [Google Scholar]
- Rugulies R. Depression as a Predictor for Coronary Heart Disease A Review and Meta-Analysis. American Journal of Preventative Medicine. 2002;23:51–61. doi: 10.1016/s0749-3797(02)00439-7. [DOI] [PubMed] [Google Scholar]
- Shapiro D, Jamner LD, Lane JD, Light KC, Myrtek M, Sawada Y, et al. Blood pressure publication guidelines. Psychophysiology. 1996;33:1–12. doi: 10.1111/j.1469-8986.1996.tb02103.x. [DOI] [PubMed] [Google Scholar]
- Sherwood A, Allen MT, Fahrenberg J, Kelsey RM, Lovallo WR, Van Dooren LJP. Methodological guidelines for impedance cardiography. Psychophysiology. 1990;27:1–23. doi: 10.1111/j.1469-8986.1990.tb02171.x. [DOI] [PubMed] [Google Scholar]
- Spasojevic J, Alloy LB. Rumination as a common mechanism relating depressive risk factors to depression. Emotion. 2001;1:25–37. doi: 10.1037/1528-3542.1.1.25. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Donald AE, O’Donnell K, Marmot M, Deanfield JE. Delayed blood pressure recovery after psychological stress is associated with carotid intima-media thickness: Whitehall psychobiology study. Arteriosclerosis & Thrombotic Vascular Biology. 2006;26:2547–2551. doi: 10.1161/01.ATV.0000242792.93486.0d. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Marmot M. Impaired cardiovascular recovery following stress predicts 3-year increases in blood pressure. Journal of Hypertension. 2005;23:529–536. doi: 10.1097/01.hjh.0000160208.66405.a8. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Marmot M. Psychosocial, hemostatic, and inflammatory correlates of delayed poststress blood pressure recovery. Psychosomatic Medicine. 2006;68:531–537. doi: 10.1097/01.psy.0000227751.82103.65. [DOI] [PubMed] [Google Scholar]
- Stewart JC, Janicki DL, Kamarck TW. Cardiovascular reactivity to and recovery from psychological challenge as predictors of 3-year change in blood pressure. Health Psychology. 2006;25:111–118. doi: 10.1037/0278-6133.25.1.111. [DOI] [PubMed] [Google Scholar]
- Straneva-Meuse PA, Light KC, Allen MT, Golding M, Girdler SS. Bupropion and paroxetine differentially influence cardiovascular and neuroendocrine responses to stress in depressed patients. Journal of Affective Disorders. 2004;79:51–61. doi: 10.1016/S0165-0327(02)00352-X. [DOI] [PubMed] [Google Scholar]
- St Sauver JL, Hagen PT, Cha SS, Bagniewski SM, Mandrekar JN, Curoe AM, et al. Agreement between patient reports of cardiovascular disease and patient medical records. Mayo Clinic Proceedings. 2005;80:203–210. doi: 10.4065/80.2.203. [DOI] [PubMed] [Google Scholar]
- Taylor CB, Conrad A, Wilhelm FH, Neri E, DeLorenzo A, Kramer MA, et al. Psychophysiological and cortisol responses to psychological stress in depressed and nondepressed older men and women with elevated cardiovascular disease risk. Psychosomatic Medicine. 2006;68:538–546. doi: 10.1097/01.psy.0000222372.16274.92. [DOI] [PubMed] [Google Scholar]
- Tugade MM, Frederickson BL. Resilient individuals use positive emotions to bounce back from negative emotional experiences. Journal of Personality & Social Psychology. 2004;86:320–333. doi: 10.1037/0022-3514.86.2.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton EW, Hallas CN. Affective status following myocardial infarction can predict long-term heart rate variability and blood pressure reactivity. British Journal of Health Psychology. 1999;4:231–245. [Google Scholar]
- Tomaka J, Blascovich J, Kelsey RM, Leitten CL. Subjective, physiological, and behavioral effects of threat and challenge appraisal. Journal of Personality & Social Psychology. 1993;68:616–624. [Google Scholar]
- Tomaka J, Blascovich J, Kibler J, Ernst JM. Cognitive and physiological antecedents of threat and challenge appraisal. Journal of Personality & Social Psychology. 1997;73:63–72. doi: 10.1037//0022-3514.73.1.63. [DOI] [PubMed] [Google Scholar]
- Trieber FA, Musante L, Kapuku G, Davis C, Litaker M, Davis H. Cardiovascular (CV) responsivity and recovery to acute stress and future CV functioning in youth with family history of CV disease: A 4-year longitudinal study. International Journal of Psychophysiology. 2001;41:65–74. doi: 10.1016/s0167-8760(00)00183-5. [DOI] [PubMed] [Google Scholar]
- Wuslin LR, Singal BM. Do Depressive Symptoms Increase the Risk for the Onset of Coronary Disease? A Systematic Quantitative Review. Psychosomatic Medicine. 2003;65:201–210. doi: 10.1097/01.psy.0000058371.50240.e3. [DOI] [PubMed] [Google Scholar]
- York KM, Hassan M, Li Q, Li H, Fillingim RB, Sheps DS. Coronary artery disease and depression: Patients with more depressive symptoms have lower cardiovascular reactivity during laboratory-induced mental stress. Psychosomatic Medicine. 2007;69:521–528. doi: 10.1097/PSY.0b013e3180cc2601. [DOI] [PubMed] [Google Scholar]