Abstract
Tuberous sclerosis complex (TSC) is a relatively rare autosomal dominant disorder characterized by widespread benign tumor formation in a variety of organs. Mutations in either TSC1 or TSC2 tumor suppressor gene are responsible for TSC. The gene products of TSC1 and TSC2, also known as hamartin and tuberin, respectively, form a physical and functional complex and inhibit the mammalian target of rapamycin complex 1 (mTORC1) signaling. The mTORC1 pathway is an evolutionarily conserved growth promoting pathway. mTORC1 plays an essential role in a wide array of cellular processes including translation, transcription, trafficking and autophagy. In this review, we will discuss recent progresses in the TSC-mTOR field and their physiological functions and alterations of this pathway in pathophysiology.
INTRODUCTION
Tuberous sclerosis complex (TSC) is a multi-system disorder characterized by the formation of non-invasive benign tumors, which rarely develop to metastatic lesions, in many organs such as brain, lung, skin, heart and kidney (1). More than 80% of the people with tuberous sclerosis have central nervous system complications, such as severe and refractory seizures, and autism (2). Renal lesions are the most common lethal complication in patients with TSC (3). Angiomyolipoma, hamartoma and renal cysts are major renal tumors associated with TSC. Multiple and bilateral angiomyolipoma are found in around 80% of adult patients and the developed tumors with abnormal or immature blood vessels cause spontaneous bleeding (3,4). Multiple and large renal cysts often lead to end-stage renal failure with bacterial infections and severe hypertension. A few percent of TSC patients show pulmonary lymphangiomyomatosis, particularly those in premenopausal women (5). Pulmonary lymphangiomyomatosis is characterized by abnormal alveolar smooth muscle proliferation and lung parenchyma destruction and leads to chronic obstructive pulmonary disease. Currently, no effective therapy for progressive pulmonary lymphangiomyomatosis is available.
TSC is caused by the mutation of either TSC1 or TSC2 gene (6,7). The gene products of TSC1 and TSC2, also known as hamartin and tuberin, respectively, form a physical and functional complex in which TSC2 functions as the catalytic subunit to promote GTP hydrolysis of the small GTPase Rheb (8,9). Therefore, Rheb is the major if not the sole downstream target of TSC1/TSC2 complex. TSC1 interacts with TSC2 via its coiled–coiled domain and stabilizes TSC2.
Important cellular function of TSC proteins had been uncovered by genetic studies in Drosophila, which revealed that dTSC1 and dTSC2 play an essential role in the regulation of cell size control functioning downstream of insulin receptor (10–12). Extensive biochemical and genetic studies identified that the TSC complex inhibits rapamycin-sensitive mTOR signaling pathway, which is directly activated by Rheb, therefore suppresses cell growth (13). The functional characterization of TSC proteins as an intrinsic suppressor of rapamycin-sensitive mTOR signaling pathway immediately suggested a possible therapeutic value of rapamycin for TSC disease. Although various TSC phenotypes seen in patients may not be the sole outcome of mTOR activation, several reports suggested that rapamycin or its analogues have a beneficial effect in the treatment of TSC tumors (14–16). Furthermore, mTOR activation is observed in many types of cancer. Therefore, rapamycin or related mTOR inhibitors have received extensive attention as a common cancer drug.
DOWNSTREAM OF TSC
mTOR complexes and their substrates
Based on extensive genetic and biochemical studies, it is well established that TOR is a major downstream target inhibited by the TSC1/TSC2 complex. TSC1/TSC2 suppress mTORC1 by inhibiting Rheb. In contrast, TSC1/TSC2 do not directly inhibit mTORC2.
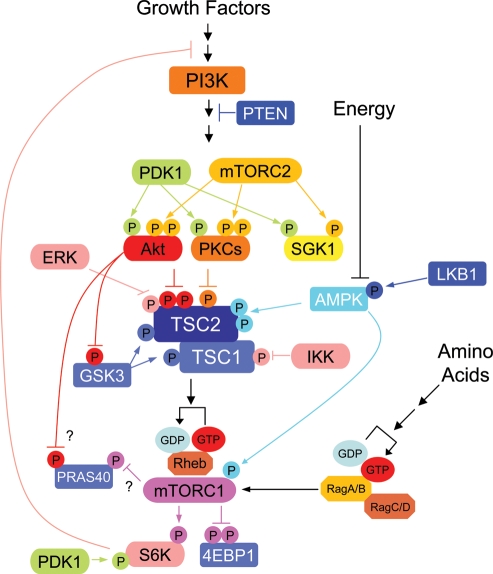
TOR is a serine/threonine kinase conserved from yeast to mammals. As seen in yeast, mTOR also exists in two distinct multi-protein complexes termed mTOR complex 1 (mTORC1) and mTORC2. mTORC1 is a rapamycin-sensitive complex and consists of Raptor, mLST8, PRAS40 and mTOR (17–19). Rapamycin suppresses phosphorylation of the mTORC1 substrates such as S6K1, S6K2, 4EBP1 and PRAS40 with high sensitivity (20–23). On the other hand, mTORC2 contains Rictor, mSin1, mLST8, PRR5 and mTOR (17,24–27), and directly phosphorylates S473 of the protein kinase B/Akt and S422 of the serum- and glucocorticoid-induced protein kinase1 (SGK1) in vitro and in vivo (28,29). Ablation of components in mTORC2 eliminates both Akt S473 (30) and SGK1 S422 phosphorylation (hydrophobic motif) (28). In addition, mTORC2 also stimulates turn motif phosphorylation in both Akt and conventional protein kinase C (PKC), and stabilizes protein expression of these kinases (31,32). The precise mechanism by which mTORC2 enhances the turn motif phosphorylation of Akt and PKC is yet unclear, since no evidence has shown that mTORC2 directly phosphorylates these sites. In contrast to mTORC1, the sensitivity of rapamycin to inhibit TORC2 function is low and the effect of rapamycin on mTORC2 activity is cell-type dependent, possibly due to indirect interference of mTORC1 (33).
Function of mTORC1
In TSC1 or TSC2 mutant cells, mTORC1 is constitutively active. We have learned a great deal about the function of mTORC1 because the availability of rapamycin potently and selectively inhibits mTORC1. In response to growth factors and nutrient availability such as amino acids and glucose, mTORC1 is activated and regulates a wide array of cellular processes, including protein translation, transcription, mRNA splicing, cell cycle and autophagy (34). The best-characterized mTORC1 function is its role in the protein synthesis, especially translation that associates with cell growth. Hyperactivation of mTORC1 pathway has also been postulated as an important contributing factor for tumorigenesis. mTORC1 regulates S6K1 and 4EBP1, two important translational regulators (Fig. 1). T389 in S6K1 and multiple serine/threonine in 4EBP1 are directly phosphorylated by mTORC1 but not by mTORC2. 4EBP1 is a translation initiation suppressor by competing with eIF4G for the formation of an eIF4E initiation complex (35). mTORC1-dependent phosphorylation of 4EBP1 decreases its affinity to interact with eIF4E, thereby activates eIF4E-dependent translation. S6K T389 phosphorylation tightly correlates with its kinase activity. Activated S6K by both mTORC1 and PDK1 then phosphorylates many target proteins such as S6, eIF4B, PDCD4, Skar, eEF2K and fragile X mental retardation protein that are mainly involved in the regulation of mRNA maturation and protein translation (36–40).
Figure 1.
A model of the TSC-mTOR signaling. Phosphorylation-dependent signaling cascades of the TSC-mTOR pathway are depicted. The kinases and their target phosphorylation are denoted with the same color.
Genetic ablation of mTOR or Raptor causes early embryonic death (mTOR e5.5–6.5; Raptor by e7), indicating that mTORC1 play an essential role in early development of embryo (30). Tissue-specific Raptor knockout models reveal that mTORC1 play a critical role in metabolism regulation in both adipose and muscle tissues. Adipose tissue-specific Raptor knockout (raptor ad−/−) mice display lean phenotype and protect diet-induced obesity and hypercholesterolemia (41). As expected, raptor−/− adipocytes are smaller than those of wild-type mice. Interestingly, raptor ad−/− mice show improved insulin sensitivity in muscle despite their low plasma adiponectin levels, suggesting that mTORC1 in adipose tissue may regulate unknown factors that could affect muscle insulin sensitivity. The lean phenotype of raptor ad−/− mice appears to be due to enhanced energy expenditure associated with largely increased UCP1 expression in the white adipose tissue. Again, UCP1 expression in muscle is also enhanced in raptor ad−/− mice. Since rapamycin treatment does not enhance UCP1 expression in differentiated 3T3-L1 cells, UCP1 expression in both muscle and adipose tissue may be regulated cell non-autonomously.
Skeletal muscle-specific ablation of raptor (raptor muscle−/−) exhibits dramatic loss in the number of mitochondria in muscles and induces muscle dystrophy (42). Despite of a reduced mitochondria number with glycogen accumulation in muscle, the expression of slow myosin heavy chain and troponin 1, makers for slow-twitch muscle fiber, are largely enhanced in both soleus and extensor digitorum longus muscles of raptor −/− mice. These data suggest that ablation of mTORC1 in skeletal muscle causes a shift of their metabolic properties from oxidative (slow) to glycolytic (fast). Overall, these genetic studies in mice have revealed that mTORC1 play an essential role in the regulation and maintenance for physiological functions of adipose tissue and skeletal muscles.
Regulation of TSC-mTORCs pathway
mTORC1 can be activated by both growth factors and nutrients. Growth factor such as insulin or IGF-1 stimulates Akt activity via the Phosphatidyl Inositide 3 kinase (PI3K) and PDK1, which phosphorylates the activation loop of Akt T308. The activated Akt can phosphorylate a wide array of substrates that involved in cell proliferation, growth and apoptosis. TSC2 is one of the Akt substrates and phosphorylation inactivates TSC2 GAP activity by an unknown mechanism (43–45). It has been postulated that phosphorylation of TSC2 may disrupt the interaction between TSC1 and TSC2 (44,46). Another model is that TSC2 phosphorylation attenuates membrane localization of TSC complex where TSC2 inhibits Rheb through its GAP activity (47). In addition to Akt, studies have identified several kinases such as PKC, ERK and IKK-β that can phosphorylate TSC2 or TSC1 and inhibit the TSC complex function thereby activating mTORC1 signaling (48–50) (Fig. 1). Therefore, TSC1/TSC2 can receive inputs from multiple signaling pathways. Recent studies also demonstrated alternative regulation of mTORC1 by growth factor. Upon growth factor stimulation, PRAS40 can be phosphorylated by Akt on T246 and mTORC1 on S183 and these phosphorylations reduce the affinity of PRAS40 to bind mTORC1 (18,19,23). Given the fact that PRAS40 is a substrate of mTORC1 in vivo and in vitro, the physiological role of PRAS40 in the regulation of mTORC1 integrity and kinase activity remains unclear.
In addition to growth factors, nutrients such as glucose and amino acids activate mTORC1 activity. The TSC complex also plays an important role in glucose-dependent mTORC1 activation. Upon glucose starvation, AMP-activated protein kinase (AMPK) is activated. Under this condition, AMPK and glycogen synthesis kinase 3 (GSK3) cooperatively phosphorylate TSC2 and enhance TSC complex activity thereby inhibiting mTORC1 activity (51,52). Consistent with this model, low glucose or intracellular ATP-depletion-induced mTORC1 inhibition is largely compromised in TSC deficient cells. However, a caveat of this model is that TSC null cells remain responsive to long-term glucose starvation, and the relationship between AMPK and TOR function is conserved in all eukaryotes such as a warm and budding yeast that lack TSC2 orthologs (53,54). Recent studies have demonstrated that AMPK is able to regulate mTORC1 directly (54). AMPK phosphorylates Raptor in its linker region and inhibits mTORC1 activity both in vivo and in vitro. Importantly, AMPK phosphorylation sites on Raptor are well conserved across all eukaryotes (54).
Amino acids are essential activator of mTORC1 signaling pathway. Amino acids depletion does suppress mTORC1 activity regardless TSC expression, suggesting that amino acid withdrawal inhibits mTORC1 activity in a manner independent of TSC1/TSC2 GAP activity (55). However, none of the stimuli including amino acids and growth factors is able to stimulate mTORC1 activity in the absence of Rheb in both Drosophila and mammalian cells, indicating that Rheb is an indispensable component in mTOR activation. Importantly, purified GTP-bound form of Rheb directly activates mTORC1 activity in vitro (18). It also has been shown that recombinant Rheb directly binds to mTOR in an amino acid sensitive manner (56). These studies suggest that amino acids may activate mTORC1 by regulating their spatial interaction between mTORC1 and active Rheb.
Recent studies have revealed that another small GTPase subfamily, Rags (A, B and C, D), plays an important role in the regulation of amino acid-induced mTORC1 activation (57,58). Rags are evolutionarily conserved GTPases from yeast to mammals (59). Human Rags are in two groups RagA, B and RagC, D. RagA or B forms heterodimer with RagC or D through its carboxyl-terminal region and stabilizes each other. In active heterodimer complex, for instance in the RagA/C complex, the RagA is in a GTP-bounded form, whereas the RagC is in a GDP-bounded form. The active Rag heteodimer directly interacts with Raptor and maintains high level of S6 kinase phosphorylation even under amino acids starvation condition (57,58). In contrast, GDP-bounded form of RagA or RagB suppresses mTORC1 activity under nutrient rich condition. Furthermore, Rag GTP levels are regulated by amino acid stimulation, suggesting that Rag complex may function as a sensor for nutrients to regulate mTORC1 activity in cultured cells.
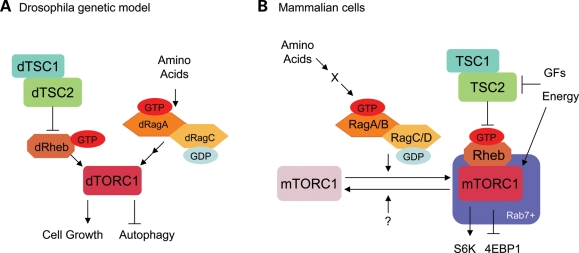
The role of Rag complex in the nutrient-dependent regulation of mTOR signaling was further supported by Drosophila genetic study. In the Drosophila fat body where nutrients play a critical role in cell growth control, expression of wild-type or active dRagA in the fat body has little effect on cell size under normal-fed condition (57). However, under the starved condition, constitutive active dRagA (dRagA QL mutant) but not wild-type RagA dramatically increased cell size. Consistent with these observations in the gain of function study, a dominant negative dRagA (dRagA TN mutant) or dRagC ablation significantly decreases fat body cell size under fed-condition but not starved-condition. These data indicate that dRags sense nutrient availability and play a critical role in cell growth control (57). As Rheb activity is critical for mTORC1 function, Rheb overexpression in fat body increases cell size in dRagC null background. Moreover, the increase in cell size in response to active dRagA is diminished in Rheb hypomorphic mutant background. These genetic observations indicate that Rags act in parallel to or upstream of Rheb to stimulate cell growth (Fig. 2A).
Figure 2.
Regulation of the mTORC1 pathway by two distinct small GTPases. (A) Drosophila genetic study indicates that Rags function parallel to or upstream of Rheb to regulate mTORC1 activity. (B) Rags regulate mTORC1 localization in response to amino acid availability.
The question that arises from these observations is how these small GTPases, Rags and Rheb coordinately regulate mTORC1 activity. Sabatini and colleagues (58) investigated mTOR localization in the presence or absence of amino acids and found that mTOR is diffusely cytoplasmic in the absence of amino acids, but translocates to Rab7 positive compartments upon amino acid stimulation in a manner depending on both Rags and Raptor. Interestingly, Rheb is constitutively associated with Rab7 positive compartments even in the absence of amino acids. As expected, active RagB (RagB QL mutant) induces mTOR to be constitutively in the Rab7 compartments. These results suggest that Rags play a crucial role to determine mTOR localization by interacting with Raptor in response to amino acids, therefore bring mTORC1 to the Rab7 compartment where mTORC1 is activated by Rheb (Fig. 2B). This elegant model of spatial regulation of mTORC1 by amino acids is consistent with the results of Drosophila genetic studies. These new findings significantly advanced our understanding of amino acid signaling in the TORC1 pathway.
In addition to Rag in amino acid-induced mTORC1 activation, it has been postulated that two kinases, type III Phosphatidylinositol-3 kinase/VPS34 and germinal center kinase-related kinase/MAP4K3 are involved in the regulation of mTORC1 activation in response to amino acids (55,60,61). VPS34 has been well established to have a key role in endocytic trafficking, endosomal sorting and fusion, and is implicated in the initiation of autophagy (62,63). Interestingly, the hVPS34 lipid kinase activity is regulated by extracellular amino acid availability possibly via intracellular calcium concentration (55,64). hVPS34 knockdown significantly reduced amino acids-induced mTORC1 activity. Overexpression of hVPS34 enhances mTORC1 activity in mammalian cells. It is intriguing to speculate that hVPS34 may play a role in mTORC1 localization in response to amino acids. It should be noted that genetic ablation of Drosophila vps34 has little effect on dTORC1 signaling although the same mutation affects autophagy (65). Therefore, additional studies are needed to unequivocally confirm the function of VPS34 in amino acid signaling to TORC1 activation.
RNAi screen searching for kinases necessary for dS6K phosphorylation in Drosophila identified MAP4K3, a Ste20 family kinase, as an activator for dTORC1 signaling (61). Interestingly, MAP4K3 activity is reported to be up-regulated by amino acids but not insulin. Overexpression of MAP4K3 activates and ablation of MAP4K3 significantly reduces mTORC1 activity in a PI3K independent manner. However, the effect of MAP4K3 on mTOR activity is rather modest, raising an issue of the relative importance of MAP4K3 in amino acid signaling. Nevertheless, a function of MAP4K3 in amino acids-induced mTORC1 activation is suggested.
GENETIC MODELS FOR TSC FUNCTION
Conventional knockout of either TSC1 or TSC2 causes early embryonic lethality in mice (66). Physiological role of the TSC complex using conditional knockout approach has been investigated in several tissues including brain, pancreatic β cells and blood cells. Lack of TSC1 or TSC2 induces extopic axons in vitro and in vivo (67). mTORC1-dependent up-regulation of the neuronal polarity SAD kinase appears to be important for the formation and growth of axon, but not those of dendrites. In addition to axonal formation, TSC1 ablation in adult mice protects retinal ganglion cell death and promotes axon regeneration after optic nerve injury, suggesting that mTORC1 plays a critical role in the axon regeneration (68).
Mice with TSC1 ablation in most differentiated neurons using synapcin1-cre develop several pathogenic features seen in TSC disease, including enlarged and dysplastic neurons and reduced myelination with high expression of phosphorylated S6 proteins (69). Furthermore, these mice display hyperactive, development of seizures, poor weight gains and short life span (30 days). Specific mTORC1 inhibitors (rapamycin and RAD001) ameliorate the majority of pathological phenotypes except for dysplastic neuronal features, suggesting that other function of TSC-Rheb plays a role for dysplasia of neuron. It is also likely that all dysplastic neuronal features have been established and irreversible when the mice are treated with mTORC1 inhibitors. Interestingly, mice treated with mTORC1 inhibitors for a short period (postnatal day 7–30) showed a persistent improvement in phenotype with an increased life span (30–80 days) (70). Although cell size and signaling profiles revert to their pretreatment patterns after rapamyain withdraw, myelination remains intact, indicating that most of the clinical neurological features of TSC may be due to loss of myelination by mTORC1 activation. The mechanism by which mTORC1 disrupts normal myelination is unknown.
TSC-mTOR pathway also plays a critical role in the function, development and survival of pancreatic β cells. Constitutive mTORC1 activation due to loss of TSC2 gene in pancreatic β cells leads to increased islet mass attributable in large part to enlargement of the individual β cell in rapamycin sensitive manner (71,72). These mice also display hyperinsulinemia and hyoglycemia, indicating that overproduction and secretion of insulin from β cells and the development of insulin resistance. Interestingly, continuous mTORC1 activation in the β cells causes progressive hyperglycemia and hypoinsulinemia due to a decrease in the number of β cells in later stage (71). It is intriguing to speculate that uncontrolled mTORC1 activity in the β cells may have a role in the loss of β cells observed in type2 diabetes. In this regard, specific attenuation of mTORC1 activity in the pancreatic β cells in type2 diabetes may rescue β cells death and could maintain β cells function.
Recent studies have implicated a role of the TSC-mTORC1 pathway in the regulation of stem cell self-renewal and senescence. Ablation of TSC1 in hematopoietic stem cells (HSCs) causes a marked reduction of hematopoiesis and self-renewal of HSCs (73,74). As seen in PTEN-deficient HSCs, HSC cell cycling is initially increased but later HSCs are progressively depleted (75). As expected, rapamycin treatment restored the reduction of TSC1-deficient HSCs. Importantly, TSC1 depletion in HSCs does not cause leukemia or other malignancies. These studies indicate that HSCs require TSC1 to maintain quiescence and self-renew over time. The mechanism by which TSC complex maintains HSCs population is that TSC complex may suppresses mTORC1-dependent reactive oxygen species production that has a significant harmful effect on HSCs maintenance (74). To support this idea, Chen et al. showed that N-acetylcystine (NAC), a potent anti-oxidant, restored TSC1-deficient HSC population. These studies demonstrate a function of TSC-mTORC1 in stem cell biology.
PERSPECTIVE
Extensive genetic and biochemical studies have revealed the exciting pathway by which mTORC1 is inhibited by TSC1/TSC2 and how this pathway is regulated by nutrients (energy and amino acids) and growth factors. It is clear that constitutive activation of mTORC1 is a major pathological consequence of mutation in TSC1 or TSC2. Furthermore, the mTOR pathway is activated in many human cancers. Therefore, interrogation of this pathway and specific inhibitors of mTOR could be valuable tools for cancer treatment. However, many key questions remain to be addressed. Previous study in Drosophila has suggested TCTP as a GEF for Rheb (76). However, recent reports dispute this conclusion (77,78). Then, what is the GEF for Rheb? A role of Rag GTPases in amino acid signaling appears to be convincing. How do the Rag proteins sense amino acids? What are the Rag GEFs and GAPs? Another key issue is how mTORC1 is activated by Rheb. It seems to involve a rather complex mechanism, including Rheb and Rag binding and spatial regulation. Identification of trafficking and sorting regulations of mTORC1 into certain cellular compartments in response to amino acids will be important for understanding the mechanism of mTORC1 activation. In addition to these biological and biochemical questions, physiological and pathological roles of TSC-mTOR signaling pathway in a variety of organs should be addressed. Such studies will reveal tissue-specific function of TSC-mTOR pathway and provide relevant information to explore new therapeutic approaches for cancers and metabolic diseases.
Conflict of Interest statement. None declared.
FUNDING
This work is supported by grants from National Institutes of Health and Department of Defense (K.-L.G.).
REFERENCES
- 1.Gomez M.R. Phenotypes of the tuberous sclerosis complex with a revision of diagnostic criteria. Ann. N Y Acad. Sci. 1991;615:1–7. doi: 10.1111/j.1749-6632.1991.tb37742.x. [DOI] [PubMed] [Google Scholar]
- 2.Curatolo P., Cusmai R., Cortesi F., Chiron C., Jambaque I., Dulac O. Neuropsychiatric aspects of tuberous sclerosis. Ann. N Y Acad. Sci. 1991;615:8–16. doi: 10.1111/j.1749-6632.1991.tb37743.x. [DOI] [PubMed] [Google Scholar]
- 3.Shepherd C.W., Gomez M.R., Lie J.T., Crowson C.S. Causes of death in patients with tuberous sclerosis. Mayo Clin. Proc. 1991;66:792–796. doi: 10.1016/s0025-6196(12)61196-3. [DOI] [PubMed] [Google Scholar]
- 4.Rakowski S.K., Winterkorn E.B., Paul E., Steele D.J., Halpern E.F., Thiele E.A. Renal manifestations of tuberous sclerosis complex: incidence, prognosis, and predictive factors. Kidney Int. 2006;70:1777–1782. doi: 10.1038/sj.ki.5001853. [DOI] [PubMed] [Google Scholar]
- 5.Hancock E., Osborne J. Lymphangioleiomyomatosis: a review of the literature. Respir. Med. 2002;96:1–6. doi: 10.1053/rmed.2001.1207. [DOI] [PubMed] [Google Scholar]
- 6.The European Chromosome 16 Tuberous Sclerosis Consortium. Identification and characterization of the tuberous sclerosis gene on chromosome 16. Cell. 1993;75:1305–1315. doi: 10.1016/0092-8674(93)90618-z. [DOI] [PubMed] [Google Scholar]
- 7.van Slegtenhorst M., de Hoogt R., Hermans C., Nellist M., Janssen B., Verhoef S., Lindhout D., van den Ouweland A., Halley D., Young J., et al. Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science. 1997;277:805–808. doi: 10.1126/science.277.5327.805. [DOI] [PubMed] [Google Scholar]
- 8.van Slegtenhorst M., Nellist M., Nagelkerken B., Cheadle J., Snell R., van den Ouweland A., Reuser A., Sampson J., Halley D., van der Sluijs P. Interaction between hamartin and tuberin, the TSC1 and TSC2 gene products. Hum. Mol. Genet. 1998;7:1053–1057. doi: 10.1093/hmg/7.6.1053. [DOI] [PubMed] [Google Scholar]
- 9.Aspuria P.J., Tamanoi F. The Rheb family of GTP-binding proteins. Cell Signal. 2004;16:1105–1112. doi: 10.1016/j.cellsig.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 10.Gao X., Pan D. TSC1 and TSC2 tumor suppressors antagonize insulin signaling in cell growth. Genes Dev. 2001;15:1383–1392. doi: 10.1101/gad.901101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tapon N., Ito N., Dickson B.J., Treisman J.E., Hariharan I.K. The Drosophila tuberous sclerosis complex gene homologs restrict cell growth and cell proliferation. Cell. 2001;105:345–355. doi: 10.1016/s0092-8674(01)00332-4. [DOI] [PubMed] [Google Scholar]
- 12.Potter C.J., Huang H., Xu T. Drosophila Tsc1 functions with Tsc2 to antagonize insulin signaling in regulating cell growth, cell proliferation, and organ size. Cell. 2001;105:357–368. doi: 10.1016/s0092-8674(01)00333-6. [DOI] [PubMed] [Google Scholar]
- 13.Huang J., Manning B.D. The TSC1–TSC2 complex: a molecular switchboard controlling cell growth. Biochem. J. 2008;412:179–190. doi: 10.1042/BJ20080281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kenerson H., Dundon T.A., Yeung R.S. Effects of rapamycin in the Eker rat model of tuberous sclerosis complex. Pediatr. Res. 2005;57:67–75. doi: 10.1203/01.PDR.0000147727.78571.07. [DOI] [PubMed] [Google Scholar]
- 15.Bissler J.J., McCormack F.X., Young L.R., Elwing J.M., Chuck G., Leonard J.M., Schmithorst V.J., Laor T., Brody A.S., Bean J., et al. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N. Engl. J. Med. 2008;358:140–151. doi: 10.1056/NEJMoa063564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goncharova E.A., Goncharov D.A., Lim P.N., Noonan D., Krymskaya V.P. Modulation of cell migration and invasiveness by tumor suppressor TSC2 in lymphangioleiomyomatosis. Am. J. Respir. Cell Mol. Biol. 2006;34:473–480. doi: 10.1165/rcmb.2005-0374OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loewith R., Jacinto E., Wullschleger S., Lorberg A., Crespo J.L., Bonenfant D., Oppliger W., Jenoe P., Hall M.N. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol. Cell. 2002;10:457–468. doi: 10.1016/s1097-2765(02)00636-6. [DOI] [PubMed] [Google Scholar]
- 18.Sancak Y., Thoreen C.C., Peterson T.R., Lindquist R.A., Kang S.A., Spooner E., Carr S.A., Sabatini D.M. PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol. Cell. 2007;25:903–915. doi: 10.1016/j.molcel.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 19.Vander Haar E., Lee S.I., Bandhakavi S., Griffin T.J., Kim D.H. Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat. Cell. Biol. 2007;9:316–323. doi: 10.1038/ncb1547. [DOI] [PubMed] [Google Scholar]
- 20.Chung J., Kuo C.J., Crabtree G.R., Blenis J. Rapamycin-FKBP specifically blocks growth-dependent activation of and signaling by the 70 kD S6 protein kinases. Cell. 1992;69:1227–1236. doi: 10.1016/0092-8674(92)90643-q. [DOI] [PubMed] [Google Scholar]
- 21.Gingras A.C., Kennedy S.G., O'Leary M.A., Sonenberg N., Hay N. 4E-BP1, a repressor of mRNA translation, is phosphorylated and inactivated by the Akt(PKB) signaling pathway. Genes Dev. 1998;12:502–513. doi: 10.1101/gad.12.4.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee-Fruman K.K., Kuo C.J., Lippincott J., Terada N., Blenis J. Characterization of S6K2, a novel kinase homologous to S6K1. Oncogene. 1999;18:5108–5114. doi: 10.1038/sj.onc.1202894. [DOI] [PubMed] [Google Scholar]
- 23.Oshiro N., Takahashi R., Yoshino K., Tanimura K., Nakashima A., Eguchi S., Miyamoto T., Hara K., Takehana K., Avruch J., et al. The proline-rich Akt substrate of 40 kDa (PRAS40) is a physiological substrate of mammalian target of rapamycin complex 1. J. Biol. Chem. 2007;282:20329–20339. doi: 10.1074/jbc.M702636200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woo S.Y., Kim D.H., Jun C.B., Kim Y.M., Haar E.V., Lee S.I., Hegg J.W., Bandhakavi S., Griffin T.J., Kim D.H. PRR5, a novel component of mTOR complex 2, regulates platelet-derived growth factor receptor beta expression and signaling. J. Biol. Chem. 2007;282:25604–25612. doi: 10.1074/jbc.M704343200. [DOI] [PubMed] [Google Scholar]
- 25.Frias M.A., Thoreen C.C., Jaffe J.D., Schroder W., Sculley T., Carr S.A., Sabatini D.M. mSin1 is necessary for Akt/PKB phosphorylation, and its isoforms define three distinct mTORC2s. Curr. Biol. 2006;16:1865–1870. doi: 10.1016/j.cub.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 26.Sarbassov D.D., Ali S.M., Kim D.H., Guertin D.A., Latek R.R., Erdjument-Bromage H., Tempst P., Sabatini D.M. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr. Biol. 2004;14:1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 27.Pearce L.R., Huang X., Boudeau J., Pawlowski R., Wullschleger S., Deak M., Ibrahim A.F., Gourlay R., Magnuson M.A., Alessi D.R. Identification of Protor as a novel Rictor-binding component of mTOR complex-2. Biochem. J. 2007;405:513–522. doi: 10.1042/BJ20070540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garcia-Martinez J.M., Alessi D.R. mTOR complex 2 (mTORC2) controls hydrophobic motif phosphorylation and activation of serum- and glucocorticoid-induced protein kinase 1 (SGK1) Biochem. J. 2008;416:375–385. doi: 10.1042/BJ20081668. [DOI] [PubMed] [Google Scholar]
- 29.Sarbassov D.D., Guertin D.A., Ali S.M., Sabatini D.M. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 30.Guertin D.A., Stevens D.M., Thoreen C.C., Burds A.A., Kalaany N.Y., Moffat J., Brown M., Fitzgerald K.J., Sabatini D.M. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev. Cell. 2006;11:859–871. doi: 10.1016/j.devcel.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 31.Ikenoue T., Inoki K., Yang Q., Zhou X., Guan K.L. Essential function of TORC2 in PKC and Akt turn motif phosphorylation, maturation and signalling. EMBO J. 2008;27:1919–1931. doi: 10.1038/emboj.2008.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Facchinetti V., Ouyang W., Wei H., Soto N., Lazorchak A., Gould C., Lowry C., Newton A.C., Mao Y., Miao R.Q., et al. The mammalian target of rapamycin complex 2 controls folding and stability of Akt and protein kinase C. EMBO J. 2008;27:1932–1943. doi: 10.1038/emboj.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sarbassov dos D., Ali S.M., Sengupta S., Sheen J.H., Hsu P.P., Bagley A.F., Markhard A.L., Sabatini D.M. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol. Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 34.Wullschleger S., Loewith R., Hall M.N. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 35.Gingras A.C., Raught B., Sonenberg N. eIF4 initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation. Annu. Rev. Biochem. 1999;68:913–963. doi: 10.1146/annurev.biochem.68.1.913. [DOI] [PubMed] [Google Scholar]
- 36.Jeno P., Ballou L.M., Novak-Hofer I., Thomas G. Identification and characterization of a mitogen-activated S6 kinase. Proc. Natl Acad. Sci. USA. 1988;85:406–410. doi: 10.1073/pnas.85.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dorrello N.V., Peschiaroli A., Guardavaccaro D., Colburn N.H., Sherman N.E., Pagano M. S6K1- and betaTRCP-mediated degradation of PDCD4 promotes protein translation and cell growth. Science. 2006;314:467–471. doi: 10.1126/science.1130276. [DOI] [PubMed] [Google Scholar]
- 38.Raught B., Peiretti F., Gingras A.C., Livingstone M., Shahbazian D., Mayeur G.L., Polakiewicz R.D., Sonenberg N., Hershey J.W. Phosphorylation of eucaryotic translation initiation factor 4B Ser422 is modulated by S6 kinases. EMBO J. 2004;23:1761–1769. doi: 10.1038/sj.emboj.7600193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Narayanan U., Nalavadi V., Nakamoto M., Thomas G., Ceman S., Bassell G.J., Warren S.T. S6K1 phosphorylates and regulates fragile X mental retardation protein (FMRP) with the neuronal protein synthesis-dependent mammalian target of rapamycin (mTOR) signaling cascade. J. Biol. Chem. 2008;283:18478–18482. doi: 10.1074/jbc.C800055200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Richardson C.J., Broenstrup M., Fingar D.C., Julich K., Ballif B.A., Gygi S., Blenis J. SKAR is a specific target of S6 kinase 1 in cell growth control. Curr. Biol. 2004;14:1540–1549. doi: 10.1016/j.cub.2004.08.061. [DOI] [PubMed] [Google Scholar]
- 41.Polak P., Cybulski N., Feige J.N., Auwerx J., Ruegg M.A., Hall M.N. Adipose-specific knockout of raptor results in lean mice with enhanced mitochondrial respiration. Cell Metab. 2008;8:399–410. doi: 10.1016/j.cmet.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 42.Bentzinger C.F., Romanino K., Cloetta D., Lin S., Mascarenhas J.B., Oliveri F., Xia J., Casanova E., Costa C.F., Brink M., et al. Skeletal muscle-specific ablation of raptor, but not of rictor, causes metabolic changes and results in muscle dystrophy. Cell Metab. 2008;8:411–424. doi: 10.1016/j.cmet.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 43.Manning B.D., Tee A.R., Logsdon M.N., Blenis J., Cantley L.C. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol. Cell. 2002;10:151–162. doi: 10.1016/s1097-2765(02)00568-3. [DOI] [PubMed] [Google Scholar]
- 44.Inoki K., Li Y., Zhu T., Wu J., Guan K.L. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat. Cell. Biol. 2002;4:648–657. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- 45.Dan H.C., Sun M., Yang L., Feldman R.I., Sui X.M., Yeung R.S., Halley D.J., Nicosia S.V., Pledger W.J., Cheng J.Q. PI3K/AKT pathway regulates TSC tumor suppressor complex by phosphorylation of tuberin. J. Biol. Chem. 2002;11:11. doi: 10.1074/jbc.M205838200. [DOI] [PubMed] [Google Scholar]
- 46.Potter C.J., Pedraza L.G., Xu T. Akt regulates growth by directly phosphorylating Tsc2. Nat. Cell. Biol. 2002;4:658–665. doi: 10.1038/ncb840. [DOI] [PubMed] [Google Scholar]
- 47.Cai S.L., Tee A.R., Short J.D., Bergeron J.M., Kim J., Shen J., Guo R., Johnson C.L., Kiguchi K., Walker C.L. Activity of TSC2 is inhibited by AKT-mediated phosphorylation and membrane partitioning. J. Cell. Biol. 2006;173:279–289. doi: 10.1083/jcb.200507119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tee A.R., Anjum R., Blenis J. Inactivation of the tuberous sclerosis complex-1 and -2 gene products occurs by phosphoinositide 3-kinase (PI3K)/Akt-dependent and -independent phosphorylation of tuberin. J. Biol. Chem. 2003;278:37288–37296. doi: 10.1074/jbc.M303257200. [DOI] [PubMed] [Google Scholar]
- 49.Ma L., Chen Z., Erdjument-Bromage H., Tempst P., Pandolfi P.P. Phosphorylation and functional inactivation of TSC2 by Erk implications for tuberous sclerosis and cancer pathogenesis. Cell. 2005;121:179–193. doi: 10.1016/j.cell.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 50.Lee D.F., Kuo H.P., Chen C.T., Hsu J.M., Chou C.K., Wei Y., Sun H.L., Li L.Y., Ping B., Huang W.C., et al. IKK beta suppression of TSC1 links inflammation and tumor angiogenesis via the mTOR pathway. Cell. 2007;130:440–455. doi: 10.1016/j.cell.2007.05.058. [DOI] [PubMed] [Google Scholar]
- 51.Inoki K., Zhu T., Guan K.L. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 52.Inoki K., Ouyang H., Zhu T., Lindvall C., Wang Y., Zhang X., Yang Q., Bennett C., Harada Y., Stankunas K., et al. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell. 2006;126:955–968. doi: 10.1016/j.cell.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 53.Hahn-Windgassen A., Nogueira V., Chen C.C., Skeen J.E., Sonenberg N., Hay N. Akt activates the mammalian target of rapamycin by regulating cellular ATP level and AMPK activity. J. Biol. Chem. 2005;280:32081–32089. doi: 10.1074/jbc.M502876200. [DOI] [PubMed] [Google Scholar]
- 54.Gwinn D.M., Shackelford D.B., Egan D.F., Mihaylova M.M., Mery A., Vasquez D.S., Turk B.E., Shaw R.J. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol. Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nobukuni T., Joaquin M., Roccio M., Dann S.G., Kim S.Y., Gulati P., Byfield M.P., Backer J.M., Natt F., Bos J.L., et al. Amino acids mediate mTOR/raptor signaling through activation of class 3 phosphatidylinositol 3OH-kinase. Proc. Natl Acad. Sci. USA. 2005;102:14238–14243. doi: 10.1073/pnas.0506925102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Long X., Ortiz-Vega S., Lin Y., Avruch J. Rheb binding to mTOR is regulated by amino acid sufficiency. J. Biol. Chem. 2005;280:23433–23436. doi: 10.1074/jbc.C500169200. [DOI] [PubMed] [Google Scholar]
- 57.Kim E., Goraksha-Hicks P., Li L., Neufeld T.P., Guan K.L. Regulation of TORC1 by Rag GTPases in nutrient response. Nat. Cell Biol. 2008;10:935–945. doi: 10.1038/ncb1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sancak Y., Peterson T.R., Shaul Y.D., Lindquist R.A., Thoreen C.C., Bar-Peled L., Sabatini D.M. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science. 2008;320:1496–1501. doi: 10.1126/science.1157535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hirose E., Nakashima N., Sekiguchi T., Nishimoto T. RagA is a functional homologue of S. cerevisiae Gtr1p involved in the Ran/Gsp1-GTPase pathway. J. Cell Sci. 1998;111(Pt 1):11–21. doi: 10.1242/jcs.111.1.11. [DOI] [PubMed] [Google Scholar]
- 60.Byfield M.P., Murray J.T., Backer J.M. hVps34 is a nutrient-regulated lipid kinase required for activation of p70 S6 kinase. J. Biol. Chem. 2005;280:33076–33082. doi: 10.1074/jbc.M507201200. [DOI] [PubMed] [Google Scholar]
- 61.Findlay G.M., Yan L., Procter J., Mieulet V., Lamb R.F. A MAP4 kinase related to Ste20 is a nutrient-sensitive regulator of mTOR signalling. Biochem. J. 2007;403:13–20. doi: 10.1042/BJ20061881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Backer J.M. The regulation and function of Class III PI3Ks: novel roles for Vps34. Biochem. J. 2008;410:1–17. doi: 10.1042/BJ20071427. [DOI] [PubMed] [Google Scholar]
- 63.Kihara A., Noda T., Ishihara N., Ohsumi Y. Two distinct Vps34 phosphatidylinositol 3-kinase complexes function in autophagy and carboxypeptidase Y sorting in Saccharomyces cerevisiae. J. Cell Biol. 2001;152:519–530. doi: 10.1083/jcb.152.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gulati P., Gaspers L.D., Dann S.G., Joaquin M., Nobukuni T., Natt F., Kozma S.C., Thomas A.P., Thomas G. Amino acids activate mTOR complex 1 via Ca2+/CaM signaling to hVps34. Cell Metab. 2008;7:456–465. doi: 10.1016/j.cmet.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Juhasz G., Hill J.H., Yan Y., Sass M., Baehrecke E.H., Backer J.M., Neufeld T.P. The class III PI(3)K Vps34 promotes autophagy and endocytosis but not TOR signaling in Drosophila. J. Cell Biol. 2008;181:655–666. doi: 10.1083/jcb.200712051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kwiatkowski D.J. Tuberous Sclerosis: from Tubers to mTOR. Ann. Hum. Genet. 2003;67:87–96. doi: 10.1046/j.1469-1809.2003.00012.x. [DOI] [PubMed] [Google Scholar]
- 67.Choi Y.J., Di Nardo A., Kramvis I., Meikle L., Kwiatkowski D.J., Sahin M., He X. Tuberous sclerosis complex proteins control axon formation. Genes Dev. 2008;22:2485–2495. doi: 10.1101/gad.1685008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Park K.K., Liu K., Hu Y., Smith P.D., Wang C., Cai B., Xu B., Connolly L., Kramvis I., Sahin M., et al. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322:963–966. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Meikle L., Talos D.M., Onda H., Pollizzi K., Rotenberg A., Sahin M., Jensen F.E., Kwiatkowski D.J. A mouse model of tuberous sclerosis: neuronal loss of Tsc1 causes dysplastic and ectopic neurons, reduced myelination, seizure activity, and limited survival. J. Neurosci. 2007;27:5546–5558. doi: 10.1523/JNEUROSCI.5540-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Meikle L., Pollizzi K., Egnor A., Kramvis I., Lane H., Sahin M., Kwiatkowski D.J. Response of a neuronal model of tuberous sclerosis to mammalian target of rapamycin (mTOR) inhibitors: effects on mTORC1 and Akt signaling lead to improved survival and function. J. Neurosci. 2008;28:5422–5432. doi: 10.1523/JNEUROSCI.0955-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shigeyama Y., Kobayashi T., Kido Y., Hashimoto N., Asahara S., Matsuda T., Takeda A., Inoue T., Shibutani Y., Koyanagi M., et al. Biphasic response of pancreatic beta-cell mass to ablation of tuberous sclerosis complex 2 in mice. Mol. Cell Biol. 2008;28:2971–2979. doi: 10.1128/MCB.01695-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rachdi L., Balcazar N., Osorio-Duque F., Elghazi L., Weiss A., Gould A., Chang-Chen K.J., Gambello M.J., Bernal-Mizrachi E. Disruption of Tsc2 in pancreatic beta cells induces beta cell mass expansion and improved glucose tolerance in a TORC1-dependent manner. Proc. Natl Acad. Sci. USA. 2008;105:9250–9255. doi: 10.1073/pnas.0803047105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gan B., Sahin E., Jiang S., Sanchez-Aguilera A., Scott K.L., Chin L., Williams D.A., Kwiatkowski D.J., DePinho R.A. mTORC1-dependent and -independent regulation of stem cell renewal, differentiation, and mobilization. Proc. Natl Acad. Sci. USA. 2008;105:19384–19389. doi: 10.1073/pnas.0810584105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen C., Liu Y., Liu R., Ikenoue T., Guan K.L., Liu Y., Zheng P. TSC-mTOR maintains quiescence and function of hematopoietic stem cells by repressing mitochondrial biogenesis and reactive oxygen species. J. Exp. Med. 2008;205:2397–2408. doi: 10.1084/jem.20081297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yilmaz O.H., Valdez R., Theisen B.K., Guo W., Ferguson D.O., Wu H., Morrison S.J. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 2006;441:475–482. doi: 10.1038/nature04703. [DOI] [PubMed] [Google Scholar]
- 76.Hsu Y.C., Chern J.J., Cai Y., Liu M., Choi K.W. Drosophila TCTP is essential for growth and proliferation through regulation of dRheb GTPase. Nature. 2007;445:785–788. doi: 10.1038/nature05528. [DOI] [PubMed] [Google Scholar]
- 77.Rehmann H., Bruning M., Berghaus C., Schwarten M., Kohler K., Stocker H., Stoll R., Zwartkruis F.J., Wittinghofer A. Biochemical characterisation of TCTP questions its function as a guanine nucleotide exchange factor for Rheb. FEBS Lett. 2008;582:3005–3010. doi: 10.1016/j.febslet.2008.07.057. [DOI] [PubMed] [Google Scholar]
- 78.Wang X., Fonseca B.D., Tang H., Liu R., Elia A., Clemens M.J., Bommer U.A., Proud C.G. Re-evaluating the roles of proposed modulators of mammalian target of rapamycin complex 1 (mTORC1) signaling. J. Biol. Chem. 2008;283:30482–30492. doi: 10.1074/jbc.M803348200. [DOI] [PMC free article] [PubMed] [Google Scholar]