Abstract
BACKGROUND:
Spontaneous clearance of hepatitis C virus (HCV) occurs in approximately 25% of individuals.
METHODS:
To better understand the characteristics associated with clearance, the present study evaluated HCV clearance in a community-based cohort study. The Community Health and Safety Evaluation project recruited 3553 individuals via community organizations and door-to-door canvassing of a random sample of single occupancy hotels in the community to monitor uptake of health services and to estimate the incidence of communicable infections. Cohort data were linked with longitudinal laboratory databases, including HCV antibody and polymerase chain reaction assay results.
RESULTS:
Overall, 762 individuals had HCV antibody and RNA testing performed between 1999 and 2005. Spontaneous HCV clearance was observed in 179 individuals (23.5%), while HCV persistence was observed in 583 individuals (76.5%). The ability to develop protective immunity against HCV, as demonstrated by viral clearance, occurred more often in individuals of Aboriginal ethnicity (adjusted OR [AOR] 2.9, 95% CI 2.0 to 4.3; P<0.001) and female individuals (AOR 1.6, 95% CI 1.1 to 2.4; P=0.01). The rate of spontaneous HCV clearance was reduced in individuals using any type of illicit drugs (AOR 0.54, 95% CI 0.29 to 1.00; P=0.05) and those with HIV coinfection (AOR 0.58, 95% CI 0.38 to 0.88; P=0.01). Of 218 HIV-infected subjects, 48 of 51 (94%) in whom the order of HCV and HIV infection was established were infected with HCV a median of 2.4 years (range 0.2 to 10 years) before becoming infected with HIV.
CONCLUSIONS:
Aboriginal ethnicity and female sex were associated with increased rates of HCV clearance, while HIV coinfection and illicit drug use were associated with increased HCV persistence.
Keywords: Aboriginal ethnicity, Female, Hepatitis C virus, HIV, Injection drug use
Abstract
HISTORIQUE :
La clairance spontanée du virus de l’hépatite C (VHC) se produit chez environ 25 % des personnes atteintes.
MÉTHODOLOGIE :
Pour mieux comprendre les caractéristiques associées à la clairance, la présente étude a évalué la clairance du VHC au sein d’une étude de cohorte communautaire. Le projet d’évaluation de la santé et de la sécurité communautaires a recruté 3 553 personnes auprès d’organismes communautaires et par la sollicitation à domicile d’un échantillon aléatoire provenant de maisons de chambres au sein de la collectivité afin de surveiller la mise en application des services de santé et d’estimer l’incidence d’infections transmissibles. Les données de cohorte étaient reliées aux bases de données longitudinales de laboratoire, y compris l’anticorps du VHC et les résultats des dosages de la réaction en chaîne de la polymérase.
RÉSULTATS :
Dans l’ensemble, 762 personnes ont subi des épreuves d’anticorps du VHC et d’ARN exécutées entre 1999 et 2005. On a observé la clairance spontanée du VHC chez 179 personnes (23,5 %) et la persistance du VHC chez 583 personnes (76,5 %). La capacité de développer une immunité protectrice contre le VHC, démontrée par la clairance virale, se produit plus souvent chez des personnes d’ethnie autochtone (rapport de cotes rajusté [RCR] 2,9, 95 % IC 2,0 à 4,3; P<0,001) et de sexe féminin (RCR 1,6, 95 % IC 1,1 à 2,4; P=0,01). Le taux de clairance spontanée du VHC était réduit chez les personnes qui utilisaient quelque type de drogues illicites que ce soit (RCR 0,54, 95 % IC 0,29 à 1,00; P=0,05) et chez celles qui étaient co-infectées par le VIH (RCR 0,58, 95 % IC 0,38 à 0,88; P=0,01). Des 218 sujets infectés par le VIH, 48 des 51 personnes (94 %) chez qui on avait établi l’ordre d’apparition de l’infection au VHC et au VIH avaient été infectées par le VIH, une médiane de 2,4 ans (fourchette de 0,2 à 10 ans) avant d’être infectés par le VIH.
CONCLUSIONS :
L’ethnie autochtone et le sexe féminin s’associaient à une augmentation des taux de clairance du VHC, tandis que la co-infection au VIH et l’usage illicite de drogues étaient reliés à la persistance accrue du VHC.
Illicit drug use is associated with high rates of hepatitis C virus (HCV) transmission in many urban centres. Of the estimated 170 million HCV prevalent cases in the world, over 50% occur among injection drug users (IDUs), and over 75% of incident infections occur in the IDU population (1). Acute infection with HCV is characterized by the detection of viremia, subsequent development of HCV-specific antibodies, a high likelihood of persistent viremia and chronic infection (2,3). Following acute infection, the overall rate of spontaneous viral clearance is estimated to be 25%, but appears to be dependent on the route of transmission, and other host and pathogen-related characteristics (4–9). Factors that have been associated with spontaneous clearance include female sex (10–14) and young age at the time of infection (15,16). Conversely, black ethnicity and coinfection with HIV (5,7,17,18) have been associated with reduced rates of HCV clearance.
In 1997, studies (19–21) from the Downtown Eastside of Vancouver, British Columbia, home to over 5000 IDUs, have reported annual HCV incidence rates of over 16 cases per 100 person-years between 1996 and 1999. The corresponding prevalences of HIV and HCV were 23% and 88%, respectively (20). This neighbourhood received international attention in the late 1990s due to increasing visible drug use, a significant rise in overdose-related deaths, and the declaration of a public health emergency due to epidemic rates of HIV and HCV infection. These outbreaks of HIV and HCV infection occurred despite the presence of needle-exchange programs and free access to medical treatment.
To increase our understanding of the HCV epidemic in this population, identifying the determinants of early clearance of viremia (generally understood to represent a ‘spontaneous cure’ of HCV) is essential. With this in mind, the present study measured the rate and characteristics of HCV clearance among infected individuals enrolled in a large, community-based cohort in Vancouver.
METHODS
Study population
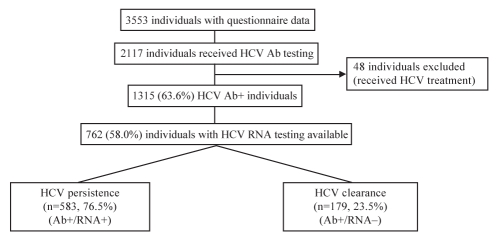
The Community Health And Safety Evaluation (CHASE) project is a prospective, open cohort study designed to evaluate health service use in the Downtown Eastside of Vancouver. Between January 2003 and June 2004, 3553 individuals were recruited via community organizations and door-to-door canvassing of a random sample of single occupancy hotels in the community, based on census information. Individuals were eligible for inclusion if they lived or used health services in the community. Study participants received a $10 stipend to complete a short, interviewer-administered questionnaire to collect information about demographics, health service utilization, HIV and HCV testing, and recent illicit drug use. Subjects were requested to provide a time-limited consent for the researchers to perform linkages with provincial health services databases. This included HCV, HIV and hepatitis B virus (HBV) testing performed at the British Columbia Centre for Disease Control (BCCDC) and the University of British Columbia virology department. Individuals who received treatment for HCV infection were excluded from the present study to eliminate the possibility of treatment-induced viral clearance as a confounding factor. Of the CHASE cohort participants, 762 were included in the study by demonstrating anti-HCV reactivity and having one or more commercial HCV RNA tests performed (Figure 1). The University of British Columbia’s and Providence Health Care’s (Vancouver, British Columbia) research ethics boards approved the present study.
Figure 1).
Subject disposition. Ab Antibody; HCV Hepatitis C virus
Laboratory testing
Samples from individuals with a confirmed positive test for anti-HCV antibodies were further evaluated for the presence of HCV RNA. HCV persistence was defined by the presence of one or more detectable HCV RNA tests following a positive test for anti-HCV. HCV clearance was defined by the presence of one or more undetectable HCV RNA tests following a positive test for anti-HCV. Individuals were suspected of having acute HCV infection based on a single HCV RNA-positive or -negative result within six months of their first anti-HCV-positive test, and were not considered in the present analysis because of the potential misclassification of spontaneous viral clearance.
All HCV antibody and RNA testing was performed at two certified provincial laboratories between 1992 and 2005. HCV antibody testing was performed using second- or third-generation enzyme immunoassays (EIAs) as follows: May 1992 to September 1993, UBI HCV EIA version 2.0 (Organon Teknika, USA); October 1993 to July 1994, UBI HCV EIA version 2.1 (Organon Teknika, USA); August 1994 to March 1997, UBI HCV EIA version 4.0 (Organon Teknika, USA); and April 1997 to present, AxSYM HCV version 3.0 (Abbott Diagnostics, USA). Specimens reactive for anti-HCV antibodies were retested by the second- or third-generation recombinant immunoblot assay (Chiron, USA) until 1999 for confirmation. Between April 1997 and July 1999, AxSYM HCV version 3.0 anti-HCV reactive specimens were retested by UBI HCV version 4.0, and from August 1999 to present, AxSYM HCV version 3.0 reactive samples were retested by Ortho EcI (Ortho-Clinical Diagnostics, Canada). Only specimens reactive by both manufacturer’s tests were considered to be anti-HCV reactive. HCV RNA testing was performed by the qualitative COBAS AMPLICOR HCV Test version 2.0 (Roche Diagnostic Systems, Canada) with a limit of detection of 50 IU/mL. HBV and HIV serology test results were abstracted as recorded in the BCCDC virology database by confidential record linkage.
Statistical analysis
Variables of interest in the present analysis included sex, estimated age at infection, ethnicity, housing status, recent treatment with methadone, recent jail time, HCV treatment history, alcohol use, injection drug use, noninjection illicit drug use, previous HBV infection and HIV status. The duration of HCV infection in HCV seroprevalent individuals was calculated using the date of their first recorded positive EIA test for HCV antibodies. In 658 patients who were seroprevalent on their first test, age at infection was estimated by random sampling from the age distribution of the incident cases. The duration of infection in 104 individuals with HCV seroconversion between 1992 and 2005 was estimated using the midpoint of the first positive and last negative HCV antibody tests. Aboriginal ethnicity included all people with an indigenous heritage, including the Inuit, First Nations, Native American, Alaskan Native and Métis people. Exposure to HBV was defined by current or historical anti-HBV total reactivity. Active HBV infection was defined by the detection of HBV surface antigen. HIV status was determined by either serological testing from the BCCDC HIV testing database or subject self-reporting for individuals diagnosed outside the province. Unstable housing was defined as living in a shelter or living on the street. Injection and noninjection illicit drug use and alcohol use in the previous six months was evaluated as ‘frequent use’ (everyday or most days) or ‘any use’ (subclassified as two to three times per week, two to three times per month or once a month). Specific drug use included injection and noninjection of cocaine, heroin and crystal methamphetamine use. Characteristics of individuals with and without HCV clearance were compared using two-sample t tests for quantitative variables and χ2 tests or Fisher’s exact tests, as appropriate, for testing differences between proportions. A multiple logistic regression model was then fit comprised of all variables and subsequently reduced using backwards elimination. Statistically significant differences were assessed at a significance level of 0.05. All reported P values were two-sided.
RESULTS
Of the 1315 HCV antibody-positive individuals enrolled in the CHASE cohort, a total of 762 individuals received testing for HCV RNA and were subsequently followed for a median period of 4.4 years. The mean number of HCV RNA tests per individual was 1.6 (range one to 10). No significant differences were observed in the demographics of HCV antibody-positive individuals who did and did not receive HCV RNA testing, including age (P=0.86), male sex (P=0.95), ethnicity (P=0.15), unstable housing (P=0.19), illicit drug use (P=0.42) and HIV infection (P=0.15). However, individuals who did not receive HCV RNA testing were more likely to engage in recent injection drug use (64.3% versus 56.6%, P=0.006). Overall, 583 individuals (76.5%) had persistent viremia, and 179 (23.5%) were determined to have spontaneous clearance of viremia (Figure 1). The demographic and behavioural characteristics of individuals with persistent viremia versus those with spontaneous clearance are shown in Tables 1 and 2. Overall, the mean age was 42 years and the estimated age at HCV infection was 32.2 years. There were no significant differences in the mean age (41.7 years versus 42.5 years; P=0.32) or the estimated age at infection (32.4 years versus 31.5 years; P=0.27) between individuals with persistent viremia and those with spontaneous clearance.
TABLE 1.
Characteristics of participants with persistent hepatitis C virus (HCV) versus those with HCV clearance
| Characteristic | HCV persistence (Ab+/RNA+) (N=583), n (%) | HCV clearance (Ab+/RNA–) (N=179), n (%) | OR (95% CI) | P* |
|---|---|---|---|---|
| Sex | ||||
| Male | 411 (70.5) | 102 (57.0) | – | – |
| Female | 172 (29.5) | 77 (43.0) | 1.8 (1.3–2.6) | 0.001 |
| Ethnicity | ||||
| Caucasian | 389 (66.7) | 82 (45.8) | – | – |
| Aboriginal | 145 (24.9) | 86 (48.0) | 2.8 (2.0–4.0) | <0.001 |
| Other | 49 (8.4) | 11 (6.2) | 1.1 (0.53–2.1) | 0.99 |
| Estimated age at infection† | ||||
| <30 years | 236 (40.6) | 81 (45.3) | – | – |
| ≥30 years | 345 (59.2) | 98 (54.7) | 0.83 (0.59–1.2) | 0.31 |
| Estimated age at infection | ||||
| ≤20 | 41 (7.1) | 16 (8.9) | – | – |
| 21 to 30 | 195 (33.6) | 65 (36.3) | 0.85 (0.45–1.6) | 0.75 |
| 31 to 40 | 208 (35.8) | 63 (35.2) | 0.78 (0.41–1.5) | 0.55 |
| 41 to 50 | 111 (19.1) | 27 (15.1) | 0.62 (0.31–1.3) | 0.27 |
| >50 | 26 (4.5) | 8 (4.5) | 0.79 (0.30–2.1) | 0.81 |
| Housing status | ||||
| Unstable | 434 (74.4) | 124 (69.3) | – | – |
| Stable | 149 (25.6) | 55 (30.7) | 1.3 (0.89–1.9) | 0.20 |
| Methadone treatment | ||||
| No | 368 (63.1) | 125 (69.8) | – | – |
| Yes | 215 (36.9) | 54 (30.2) | 0.74 (0.52–1.1) | 0.12 |
| Jail time | ||||
| No | 446 (76.5) | 138 (77.1) | – | – |
| Yes | 137 (23.5) | 41 (22.9) | 0.97 (0.65–1.4) | 0.95 |
| HBV coinfection | ||||
| No previous infection | 559 (95.9) | 170 (95.0) | – | – |
| Previous infection | 24 (4.1) | 9 (5.0) | 1.2 (0.56–2.7) | 0.67 |
| HIV-1 coinfection | ||||
| HIV-1− | 406 (69.6) | 138 (77.1) | – | – |
| HIV-1+ | 177 (30.4) | 41 (22.9) | 0.68 (0.46–1.0) | 0.06 |
Percentages indicate proportion in columns.
As determined by the χ2 or Fisher’s exact test as appropriate;
Age data were not obtained for two participants. – Negative; + Positive; Ab Antibody; HBV Hepatitis B virus
TABLE 2.
Characteristics of participants with persistent hepatitis C virus (HCV) versus those with HCV clearance over the previous six months
| Characteristic | HCV persistence (Ab+/RNA+) (N=583), n (%) | HCV clearance (Ab+/RNA–) (N=179), n (%) | OR (95% CI) | P* |
|---|---|---|---|---|
| Alcohol use | ||||
| None | 323 (55.4) | 94 (52.5) | − | – |
| Any | 260 (44.6) | 85 (47.5) | 1.1 (0.80–1.6) | 0.55 |
| Illicit drug use | ||||
| None | 42 (7.2) | 19 (10.6) | – | – |
| Any | 541 (92.8) | 160 (89.4) | 0.65 (0.37–1.2) | 0.19 |
| Injection drug use | ||||
| None | 243 (41.7) | 88 (49.1) | – | – |
| Any | 340 (58.3) | 91 (50.8) | 0.74 (0.53–1.0) | 0.09 |
| Injection cocaine use | ||||
| None | 306 (52.5) | 106 (59.2) | – | – |
| Any | 277 (47.5) | 73 (40.8) | 0.76 (0.54–1.1) | 0.14 |
| Injection heroin use | ||||
| None | 388 (66.6) | 128 (71.5) | – | – |
| Any | 195 (33.4) | 51 (28.5) | 0.79 (0.55–1.2) | 0.25 |
| Crack cocaine use | ||||
| None | 162 (27.8) | 56 (31.3) | – | – |
| Any | 421 (72.2) | 123 (68.7) | 0.85 (0.59–1.2) | 0.42 |
As determined by the χ2 or Fisher’s exact test as appropriate. – Negative; + Positive; Ab Antibody
In the univariate analysis, the ability to develop protective immunity to HCV, evident in the present study by spontaneous clearance of HCV infection, occurred more frequently among individuals of Aboriginal ethnicity (OR 2.8, 95% CI 2.0 to 4.0; P<0.001) and female sex (OR 1.8, 95% CI 1.3 to 2.6; P=0.001). Decreased rates of spontaneous HCV clearance were observed in individuals with HIV coinfection (OR 0.68, 95% CI 0.46 to 1.0; P=0.06). Estimated age at infection, housing status, previous methadone treatment, recent jail time, HBV infection, alcohol use, and illicit noninjection or injection drug use in the preceding six months were not associated with HCV persistence or clearance (Tables 1 and 2).
As shown in Table 3, after adjusting for confounding variables using multiple logistic regression analysis, the factors independently associated with spontaneous clearance of HCV included Aboriginal ethnicity (adjusted OR [AOR] 2.9, 95% CI 2.0 to 4.3; P<0.001) and female sex (AOR 1.6, 95% CI 1.1 to 2.4; P=0.01). Spontaneous clearance of HCV was inversely associated with the use (versus nonuse) of any illicit drugs (AOR 0.54, 95% CI 0.29 to 1.0; P=0.05) and HIV infection (AOR 0.58, 95% CI 0.38 to 0.88; P=0.01).
TABLE 3.
Multiple logistic regression of factors associated with clearance of hepatitis C virus infection
| Characteristic | Adjusted OR | 95% CI | P |
|---|---|---|---|
| Aboriginal ethnicity (versus Caucasian) | 2.9 | 2.0–4.3 | <0.001 |
| Female sex | 1.6 | 1.1–2.4 | 0.01 |
| HIV-1-positive | 0.58 | 0.38–0.88 | 0.01 |
| Any illicit drug use | 0.54 | 0.29–1.0 | 0.05 |
To identify whether HIV infection impacts HCV clearance or persistence, 51 subjects were identified in whom the order of HCV and HIV infections could be established based on documented timing of HCV and HIV seroconversion. In total, 48 of 51 individuals (94%) acquired HCV infection a median of 2.4 years (range 0.2 to 10 years) before being diagnosed with HIV.
DISCUSSION
We investigated the rate and characteristics associated with HCV clearance among inner-city residents in Vancouver by studying a large, community-based cohort consisting mainly of illicit drug users. We documented that 23.5% of individuals in whom testing for HCV antibodies and viremia were available spontaneously cleared their infection. This is consistent with previously reported clearance rates of 14% to 46% in non-IDUs (4–8). In our cohort, Aboriginal ethnicity and female sex were associated with an enhanced capacity to clear HCV infection. In contrast, HIV coinfection and illicit drug use were associated with increased persistence of HCV infection.
Our findings with respect to the Aboriginal race are consistent with data from other centres in Canada, suggesting that spontaneous HCV clearance may be higher in these individuals (22,23). A similar finding has recently been published in studies of Alaskan Natives (24). Interestingly, other data suggest that African Americans exhibit decreased rates of HCV clearance (5,7,18). The basis for the association between race and HCV clearance is not well understood.
We observed that female sex was associated with increased rates of HCV clearance. This is supported by previously published data (10–14) and a recent systematic review of 31 longitudinal studies evaluating the correlates of spontaneous HCV clearance (9). In a pooled analysis of 675 subjects with HCV clearance, the investigators determined that male subjects were significantly less likely (OR 0.43, 95% CI 0.36 to 0.53, P<0.001) to clear HCV spontaneously (19%) than female subjects (40%). It has been postulated that HCV clearance in female subjects may be facilitated by estrogen (11,25). However, this difference may also be attributed to genetic or immunological differences that have not yet been determined.
The association of sex and race with spontaneous HCV clearance speaks to possible roles for host genetics and immunity in viral control. Genetic polymorphisms in a number of immunological proteins involved in the regulation of cellular immune responses (such as interleukin [IL]-10, IL-19, IL-20 and tumour necrosis factor-alpha), as well as in both human leukocyte antigen class I and II molecules, are associated with reduced clearance (26–31). Immunological studies in Aboriginals suggest a lower genetic tendency to produce IL-10 than Caucasians and a reduced susceptibility to HCV protein-induced IL-10 immune responses, implicating a role for the immune system in this enhanced protection (32). It has recently been determined that inhibitory natural killer cells may be important in HCV clearance, and that differing activity of certain genes encoding interactions between human leukocyte antigen class I molecules and natural killer cells may play an important role in the efficacy of this process (33). However, further research is needed to understand the associations between host genetics or the immune system and HCV clearance.
HIV infection was associated with reduced rates of HCV clearance. Similar observations have been found in studies among United States veterans (18), hemophiliacs (16) and IDUs (7). The results from our study suggest that the majority of illicit drug users are infected with HCV a median of 2.4 years before HIV infection, which is consistent with reports from other groups (34). Therefore, given that HCV clearance generally occurs within the first six to 12 months of infection, HIV is most often impacting persistence of HCV rather than its initial clearance in this setting. HIV infection may decrease circulating HCV-specific CD4 and CD8 T cells that are generally present in higher levels in individuals who cleared HCV infection (35). This preservation of higher CD4 cell levels, which is associated with preserved anti-HCV lymphoproliferative responses, may be reduced or eliminated on HIV infection (35). Our cohort, as currently recruited, does not provide us with the statistical power to evaluate this hypothesis.
In addition, illicit drug use was associated with diminished capacity to resolve HCV infection. Relationships between specific drug use patterns and viral clearance were not detected, although this may reflect sample size. Alternatively, the effect may be of marginal significance, because the overall rate of HCV clearance in our cohort, which included a large proportion of IDUs, approximated that previously reported in non-IDU populations. The lower level of viral clearance among those who report injecting may also relate to a higher risk of reinfection. It is interesting to note that data from this cohort (36) and others (37) have demonstrated that HCV reinfection after HCV clearance occurs less frequently than de novo HCV infection in IDUs and non-IDUs alike, suggesting little or no effect of this behaviour on the virus-host relationship.
The present study has a number of limitations, mostly those inherent to observational cohorts. Testing for anti-HCV antibodies and HCV RNA occurred as clinically indicated. In practice, antibody testing would be performed periodically in individuals with such high risk of HCV infection, but less symptomatic patients (who may be more likely to resolve infection) may not be tested for HCV RNA and would be missing from our analysis. Moreover, the majority of HCV RNA testing was performed in recent years because of the increased availability of HCV treatment in this population. Viral clearance was, in some cases, confirmed by a single negative test, which may represent fluctuating low levels of viremia rather than true clearance. Also, given the level of detection of the assay used (less than 50 U/mL), some individuals, defined as spontaneously clearing HCV infection, may have had low but undetectable viremia. However, the overall rate of HCV clearance (24%) is consistent with other studies and reassures us that our conclusions are valid. Additionally, not all patients received HCV RNA testing, introducing a potential selection bias against individuals who may not have come forward for more comprehensive testing or who were not likely to receive follow-up care. Drug use was assessed only in the six months preceding the questionnaire, and historical drug use information was not available. However, similar demographics and HIV status between the two groups suggest a relative comparability of the groups and indicate similar testing patterns. An additional limitation with the present study is that many of the variables, such as illicit drug use behaviours, were based on patient self-report and may be prone to socially desirable responses.
Given the limited currently available data investigating HCV clearance in IDUs, the results of our study provide novel insights into this issue. Our results suggest that Aboriginal ethnicity and female sex may promote clearance (perhaps by enhanced immunological control), while HIV coinfection and illicit drug use may reduce the likelihood of clearance, presumably by a similar mechanism. Understanding the mechanisms underlying our observations may be helpful in the management of individuals with acute infection. Moreover, the understanding of factors that promote or hinder the generation of protective immunity may aid in the development of vaccines or improved treatment options for HCV infection. Further studies are required to confirm our observations from a clinical and pathophysiological perspective. This information will be an important step in refining our approach to HCV infection in medical practice.
Acknowledgments
The present research was partially supported by the Canadian Institutes of Health Research (Jesse D Raffa), the National Canadian Research Training Program in Hepatitis C (Jason Grebely) and Vancouver Coastal Health (Mark W Tyndall and Brian Conway). Mark W Tyndall is the recipient of a Senior Scholar Award from the Michael Smith Foundation for Health Research.
REFERENCES
- 1.Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5:558–67. doi: 10.1016/S1473-3099(05)70216-4. [DOI] [PubMed] [Google Scholar]
- 2.Cox AL, Netski DM, Mosbruger T, et al. Prospective evaluation of community-acquired acute-phase hepatitis C virus infection. Clin Infect Dis. 2005;40:951–8. doi: 10.1086/428578. [DOI] [PubMed] [Google Scholar]
- 3.Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol. 2005;5:215–29. doi: 10.1038/nri1573. [DOI] [PubMed] [Google Scholar]
- 4.Gerlach JT, Diepolder HM, Zachoval R, et al. Acute hepatitis C: High rate of both spontaneous and treatment-induced viral clearance. Gastroenterology. 2003;125:80–8. doi: 10.1016/s0016-5085(03)00668-1. [DOI] [PubMed] [Google Scholar]
- 5.Villano SA, Vlahov D, Nelson KE, Cohn S, Thomas DL. Persistence of viremia and the importance of long-term follow-up after acute hepatitis C infection. Hepatology. 1999;29:908–14. doi: 10.1002/hep.510290311. [DOI] [PubMed] [Google Scholar]
- 6.Seeff LB. Natural history of chronic hepatitis C. Hepatology. 2002;36(5 Suppl 1):S35–46. doi: 10.1053/jhep.2002.36806. [DOI] [PubMed] [Google Scholar]
- 7.Thomas DL, Astemborski J, Rai RM, et al. The natural history of hepatitis C virus infection: Host, viral, and environmental factors. JAMA. 2000;284:450–6. doi: 10.1001/jama.284.4.450. [DOI] [PubMed] [Google Scholar]
- 8.Jauncey M, Micallef JM, Gilmour S, et al. Clearance of hepatitis C virus after newly acquired infection in injection drug users. J Infect Dis. 2004;190:1270–4. doi: 10.1086/423943. [DOI] [PubMed] [Google Scholar]
- 9.Micallef JM, Kaldor JM, Dore GJ. Spontaneous viral clearance following acute hepatitis C infection: A systematic review of longitudinal studies. J Viral Hepat. 2006;13:34–41. doi: 10.1111/j.1365-2893.2005.00651.x. [DOI] [PubMed] [Google Scholar]
- 10.Kenny-Walsh E. Clinical outcomes after hepatitis C infection from contaminated anti-D immune globulin. Irish Hepatology Research Group. N Engl J Med. 1999;340:1228–33. doi: 10.1056/NEJM199904223401602. [DOI] [PubMed] [Google Scholar]
- 11.Alric L, Fort M, Izopet J, et al. Study of host- and virus-related factors associated with spontaneous hepatitis C virus clearance. Tissue Antigens. 2000;56:154–8. doi: 10.1034/j.1399-0039.2000.560207.x. [DOI] [PubMed] [Google Scholar]
- 12.Inoue G, Horiike N, Michitaka K, Onji M. Hepatitis C virus clearance is prominent in women in an endemic area. J Gastroenterol Hepatol. 2000;15:1054–8. doi: 10.1046/j.1440-1746.2000.02276.x. [DOI] [PubMed] [Google Scholar]
- 13.Yamakawa Y, Sata M, Suzuki H, Noguchi S, Tanikawa K. Higher elimination rate of hepatitis C virus among women. J Viral Hepat. 1996;3:317–21. doi: 10.1111/j.1365-2893.1996.tb00104.x. [DOI] [PubMed] [Google Scholar]
- 14.Bakr I, Rekacewicz C, El Hosseiny M, et al. Higher clearance of hepatitis C virus infection in females compared with males. Gut. 2006;55:1183–7. doi: 10.1136/gut.2005.078147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang M, Rosenberg PS, Brown DL, et al. Correlates of spontaneous clearance of hepatitis C virus among people with hemophilia. Blood. 2006;107:892–7. doi: 10.1182/blood-2005-07-2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Messick K, Sanders JC, Goedert JJ, Eyster ME. Hepatitis C viral clearance and antibody reactivity patterns in persons with haemophilia and other congenital bleeding disorders. Haemophilia. 2001;7:568–74. doi: 10.1046/j.1365-2516.2001.00559.x. [DOI] [PubMed] [Google Scholar]
- 17.Krajden M, Shivji R, Gunadasa K, et al. Evaluation of the core antigen assay as a second-line supplemental test for diagnosis of active hepatitis C virus infection. J Clin Microbiol. 2004;42:4054–9. doi: 10.1128/JCM.42.9.4054-4059.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piasecki BA, Lewis JD, Reddy KR, et al. Influence of alcohol use, race, and viral coinfections on spontaneous HCV clearance in a US veteran population. Hepatology. 2004;40:892–9. doi: 10.1002/hep.20384. [DOI] [PubMed] [Google Scholar]
- 19.Patrick DM, Tyndall MW, Cornelisse PG, et al. Incidence of hepatitis C virus infection among injection drug users during an outbreak of HIV infection. CMAJ. 2001;165:889–95. [PMC free article] [PubMed] [Google Scholar]
- 20.Strathdee SA, Patrick DM, Currie SL, et al. Needle exchange is not enough: Lessons from the Vancouver injecting drug use study. AIDS. 1997;11:F59–65. doi: 10.1097/00002030-199708000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Tyndall MW, Currie S, Spittal P, et al. Intensive injection cocaine use as the primary risk factor in the Vancouver HIV-1 epidemic. AIDS. 2003;17:887–93. doi: 10.1097/00002030-200304110-00014. [DOI] [PubMed] [Google Scholar]
- 22.Minuk GY, Zhang M, Wong SG, et al. Viral hepatitis in a Canadian First Nations community. Can J Gastroenterol. 2003;17:593–6. doi: 10.1155/2003/978162. [DOI] [PubMed] [Google Scholar]
- 23.Minuk GY, Uhanova J. Viral hepatitis in the Canadian Inuit and First Nations populations. Can J Gastroenterol. 2003;17:707–12. doi: 10.1155/2003/350175. [DOI] [PubMed] [Google Scholar]
- 24.Scott JD, McMahon BJ, Bruden D, et al. High rate of spontaneous negativity for hepatitis C virus RNA after establishment of chronic infection in Alaska Natives. Clin Infect Dis. 2006;42:945–52. doi: 10.1086/500938. [DOI] [PubMed] [Google Scholar]
- 25.Hayashi J, Kishihara Y, Ueno K, et al. Age-related response to interferon alfa treatment in women vs men with chronic hepatitis C virus infection. Arch Intern Med. 1998;158:177–81. doi: 10.1001/archinte.158.2.177. [DOI] [PubMed] [Google Scholar]
- 26.Thio CL, Goedert JJ, Mosbruger T, et al. An analysis of tumor necrosis factor alpha gene polymorphisms and haplotypes with natural clearance of hepatitis C virus infection. Genes Immun. 2004;5:294–300. doi: 10.1038/sj.gene.6364072. [DOI] [PubMed] [Google Scholar]
- 27.Thio CL, Gao X, Goedert JJ, et al. HLA-Cw*04 and hepatitis C virus persistence. J Virol. 2002;76:4792–7. doi: 10.1128/JVI.76.10.4792-4797.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thio CL, Thomas DL, Goedert JJ, et al. Racial differences in HLA class II associations with hepatitis C virus outcomes. J Infect Dis. 2001;184:16–21. doi: 10.1086/321005. [DOI] [PubMed] [Google Scholar]
- 29.Barrett S, Ryan E, Crowe J. Association of the HLA-DRB1*01 allele with spontaneous viral clearance in an Irish cohort infected with hepatitis C virus via contaminated anti-D immunoglobulin. J Hepatol. 1999;30:979–83. doi: 10.1016/s0168-8278(99)80249-9. [DOI] [PubMed] [Google Scholar]
- 30.Thursz M, Yallop R, Goldin R, Trepo C, Thomas HC. Influence of MHC class II genotype on outcome of infection with hepatitis C virus. The HENCORE group. Hepatitis C European Network for Cooperative Research. Lancet. 1999;354:2119–24. doi: 10.1016/s0140-6736(99)91443-5. [DOI] [PubMed] [Google Scholar]
- 31.Oleksyk TK, Thio CL, Truelove AL, et al. Single nucleotide polymorphisms and haplotypes in the IL10 region associated with HCV clearance. Genes Immun. 2005;6:347–57. doi: 10.1038/sj.gene.6364188. [DOI] [PubMed] [Google Scholar]
- 32.Aborsangaya KB, Dembinski I, Khatkar S, Alphonse MP, Nickerson P, Rempel JD. Impact of aboriginal ethnicity on HCV core-induced IL-10 synthesis: Interaction with IL-10 gene polymorphisms. Hepatology. 2007;45:623–30. doi: 10.1002/hep.21511. [DOI] [PubMed] [Google Scholar]
- 33.Khakoo SI, Thio CL, Martin MP, et al. HLA and NK cell inhibitory receptor genes in resolving hepatitis C virus infection. Science. 2004;305:872–4. doi: 10.1126/science.1097670. [DOI] [PubMed] [Google Scholar]
- 34.Thomas DL, Vlahov D, Solomon L, et al. Correlates of hepatitis C virus infections among injection drug users. Medicine (Baltimore) 1995;74:212–20. doi: 10.1097/00005792-199507000-00005. [DOI] [PubMed] [Google Scholar]
- 35.Kim A, Schulze Zur Wiesch J, Allen T, et al. Virus-specific T-cell responses and loss of spontaneous control of HCV in HIV+ individuals. 13th Conference on Retroviruses and Opportunistic Infections; Denver. February 5 to 8, 2006. [Google Scholar]
- 36.Grebely J, Conway B, Raffa JD, Lai C, Krajden M, Tyndall MW. Hepatitis C virus reinfection in injection drug users. Hepatology. 2006;44:1139–45. doi: 10.1002/hep.21376. [DOI] [PubMed] [Google Scholar]
- 37.Mehta SH, Cox A, Hoover DR, et al. Protection against persistence of hepatitis C. Lancet. 2002;359:1478–83. doi: 10.1016/S0140-6736(02)08435-0. [DOI] [PubMed] [Google Scholar]