Abstract
The present report describes a young woman with no previous gastrointestinal complaints who was initially diagnosed with postinfective irritable bowel syndrome (IBS) after a confirmed case of Campylobacter jejuni enteritis. However, because of persistent diarrhea, new-onset bloating and the development of iron and vitamin deficiencies, serological markers for celiac disease (CD) were evaluated. A positive tissue transglutaminase immunoglobulin A antibody test and repeat endoscopy with duodenal biopsy showing a Marsh IIIa lesion confirmed the diagnosis of CD. Infectious gastroenteritis is a well-established risk factor for the development of IBS, and there is recent evidence that it could play a role in the initiation and exacerbation of inflammatory bowel disease. The present case suggests that the clinical expression of CD can be unmasked by an acute gastrointestinal infection and supports the hypothesis that environmental factors other than gliadin may play a role in the clinical onset of CD in a genetically susceptible host. The increasing availability of serological testing and upper endoscopy has led to increasingly frequent diagnoses of CD and recognition that it may mimic IBS. The present case findings suggest that CD should be considered in the differential diagnosis of persistent IBS-like symptoms after an episode of infectious gastroenteritis.
Keywords: Celiac disease, Postinfective irritable bowel syndrome
Abstract
Le présent rapport décrit le cas d’une jeune femme sans antécédents de troubles gastro-intestinaux chez qui on avait d’abord diagnostiqué un syndrome du côlon irritable (SCI) postinfectieux après un cas confirmé d’en-térite à Campylobacter jejuni. Cependant, en raison d’une diarrhée persistante, de gonflements de novo et de l’apparition de carences en fer et en vitamines, on a évalué les marqueurs sérologiques de la maladie cœliaque (MC). Un test positif de dépistage des anticorps antitransglutaminases tissulaires de l’immunoglobuline A et une reprise de l’endoscopie accompagnée d’une biopsie duodénale ont révélé une lésion Marsh IIIa et confirmé le diagnostic de MC. La gastroentérite infectieuse est un facteur de risque établi de SCI, et d’après des données récentes, elle pourrait contribuer à l’apparition ou l’exacerbation d’une maladie inflammatoire de l’intestin. Selon le présent cas, l’expression clinique de la MC peut être démasquée par une infection gastro-intestinale aiguë, ce qui étaye l’hypothèse selon laquelle d’autres facteurs environnementaux que la gliadine peuvent favoriser l’apparition clinique d’une MC chez un hôte génétiquement susceptible. La disponibilité croissante des tests sérologiques et de l’endoscopie supérieure suscite des diagnostics de plus en plus fréquents de MC et permet d’établir que cette maladie imite le SCI. Les présentes observations indiquent que la MC pourrait être envisagée dans le diagnostic différentiel de symptômes pseudo-SCI persistants après un épisode de gastroentérite aiguë.
CASE PRESENTATION
A case of a 29-year-old East Indian woman with an anxious personality who was diagnosed with celiac disease (CD) after a six-year history of postinfective irritable bowel syndrome (IBS) is presented.
In September 1999, she was admitted to the gastrointestinal (GI) service due to an acute episode of fever and bloody diarrhea. She presented with abdominal pain, cramps, explosive diarrhea accompanied by 10 to 15 bowel movements per day over the previous five days and a high temperature. She had no previous history of abdominal pain or irregular bowel movements. She denied any overseas travel, antibiotic treatment or contact with pets. There was no family history of inflammatory bowel disease, colorectal cancer or other GI disorder.
The patient was moderately overweight; she appeared to be nontoxic and was normotensive, although she was tachycardic with a body temperature of 38°C. On abdominal examination, mild tenderness was elicited in the right lower quadrant with guarding and reduced bowel sounds.
Laboratory tests, including hemoglobin and serum iron concentrations, were normal, except for a white cell blood count of 12.4×109/L with neutrophilia. An abdominal x-ray showed a few air fluid levels but was not consistent with intestinal obstruction. Sigmoidoscopy findings were compatible with infective colitis, which was confirmed by stool culture positive for Campylobacter jejuni.
Four weeks later, due to the persistence of diarrhea, a colonoscopy was performed to evaluate intestinal inflammation. Intestinal biopsies revealed acute and chronic inflammation in cecal biopsies, and features of melanosis coli. The changes were considered to be nonspecific and a consequence of previous GI infection. Stool culture and stool tests for ova, parasites and Clostridium difficile toxins were negative. A presumptive diagnosis of postinfective IBS was made.
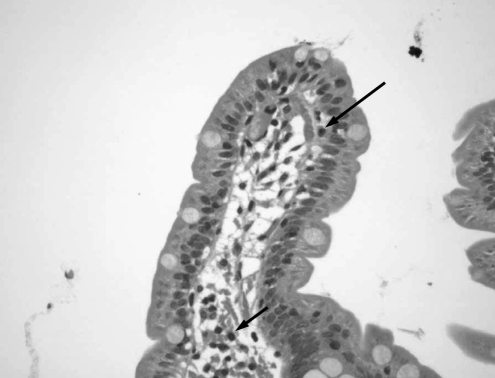
Six months later, as the patient continued to complain of abdominal pain, bloating, nausea and diarrhea, further studies were performed to exclude organic causes. Stool examination and abdominal ultrasound results were normal. In March 2000, an upper GI endoscopy with duodenal biopsies was performed to rule out CD. Duodenal biopsies showed normal mucosa with isolated subepithelial and lamina propria lymphocytes (Figure 1). A small bowel meal study showed no abnormality suggestive of Crohn’s disease. One month later, she presented with low serum urea concentrations, low vitamin B12, folate and ferritin levels, as well as a low lymphocyte count. Other results including erythrocyte sedimentation rate, C-reactive protein, thyroid stimulating hormone and serum immunoglobulin (Ig) A, IgG and IgE concentrations were normal. Because her lactose hydrogen breath test was positive, she was diagnosed with postinfective IBS and secondary lactose deficiency, and was treated with a lactose-free diet. Her vitamin deficiencies were treated with parenteral vitamin B12, oral folate and oral iron.
Figure 1).
First duodenal biopsy. Duodenal section showing normal villous structure. Isolated subepithelial (long arrow) and lamina propria (short arrow) lymphocytes were observed
Over the next five years, her symptoms persisted, with an average of four loose bowel movements per day accompanied by bloating and abdominal cramps. Despite supplemental therapy with iron, her ferritin levels remained low. In May 2002, she presented with low serum zinc and urea levels, as well as a low lymphocyte count.
In 2005, serological markers for CD were evaluated because her symptoms remained unchanged. Tissue transglutaminase IgA antibodies were positive for CD (83 U), and gliadin IgA and IgG antibodies were normal. Subsequent upper GI endoscopy showed no macroscopic abnormality. However, duodenal biopsies revealed the presence of mild, partial villous atrophy (Figure 2A) and increased intraepithelial lymphocytes (Marsh IIIa) suggestive of CD (Figure 2B).
Figure 2).
Second duodenal biopsy. A Duodenal section showing partial villous atrophy. B Intense lymphocytic infiltrate in the lamina propria and in the intraepithelial compartment (arrow)
The patient was treated with a gluten-free diet, with which she experienced a significant symptomatic recovery. In 2006, serological markers for CD (tissue transglutaminase IgA antibodies, and gliadin IgA and IgG antibodies) were normal.
DISCUSSION
We describe a patient who developed symptomatic CD after a confirmed case of Campylobacter jejuni enteritis. In the absence of any other specific findings, there was no obvious reason to change the initial diagnosis of postinfective IBS and secondary lactose intolerance, particularly because the first duodenal biopsy had been normal. However, re-evaluation after six years revealed a positive tissue transglutaminase IgA antibody result that suggested CD and prompted a second duodenal biopsy, despite a normal biopsy report six years previously.
CD is a common inflammatory GI disorder with a prevalence in the United States of one in 113 for individuals who are not considered to be at risk (1). Environmental, genetic and immunological factors play a role in the pathogenesis of CD. The only well-documented environmental trigger is gliadin, a protein with a very high glutamine and proline content. It is possible that the clinical expression of CD is modulated by environmental factors such as infection. Anecdotal evidence exists to support this, because patients often attribute the onset of classic symptoms to a stressful episode or gastroenteritis (2). Cases of CD have been reported as presenting as persistent traveller’s diarrhea when no infectious cause could be documented (3,4). However, to date, there have not been reports of clinically significant CD developing after a confirmed case of infective gastroenteritis.
The mechanisms whereby infection can contribute to clinical expression of CD include molecular mimicry or immune modulation. Based on the 12-amino acid homology sequence of adenovirus type 12 E1B protein and alpha-gliadin, it has been suggested that exposure to adenovirus type 12 E1B may sensitize individuals to gliadin and trigger CD. However, it is difficult to establish a causative relationship between adenovirus type 12 E1B and gliadin, because adenovirus type 12 E1B is also highly prevalent in the duodenal tissue of normal individuals (5). CD is epidemiologically associated with other viral infections, such as chronic hepatitis C, nonviral disorders including insulin-dependent diabetes, thyroid disease and cardiomyopathy, and HIV (6,7). This suggests that the association involves chronic immune stimulation, which in turn triggers an autoimmune reaction. One study (8) also showed a significant higher prevalence of Helicobacter pylori infection in patients with CD. Our case suggests, in conjunction with previous anecdotal evidence, that environmental factors other than gliadin may also be important in the clinical onset of CD. GI infection may trigger or facilitate the onset of clinical CD, either by increasing intestinal permeability (9) and, hence, the uptake of antigen in a genetically susceptible host, or by amplifying the immune response to gliadin.
Most cases of CD go unrecognized, and there is a spectrum of clinical presentation ranging from mild GI symptoms resembling IBS to frank malabsorption (10). The average delay from onset of symptoms to diagnosis of CD is 11 years, and the likelihood of delay is especially high when the patient’s symptoms are consistent with IBS and the clinical picture is not one of classic CD (11). Infective gastroenteritis is now a well-established risk factor for the development of IBS (12), and there is evidence that infectious gastroenteritis represents a risk factor for the development of chronic intestinal diseases such as inflammatory bowel disease (13). Recent epidemiological studies indicate that CD is, in fact, a very common disorder, and its mimicry of IBS is becoming increasingly recognized. The findings described in the present study should encourage clinicians to consider CD in the differential diagnosis of patients who have persistent IBS-like symptoms after a confirmed episode of gastroenteritis. Moreover, it should be recognized that an initial negative biopsy may be insufficient to rule out organic small intestinal disease; follow-up may be required, because sampling errors may lead to a false-negative diagnosis at the outset or the classic histological lesions may take time to develop.
CONCLUSION
The present case suggests that although postinfective IBS is a common condition, persistent IBS-like symptoms after an episode of gastroenteritis may also be indicative of CD. In patients with persistent symptoms, the accuracy of celiac serology is sufficiently high and, thus, it should be considered as a first-line screening test for unexplained GI tract symptoms.
REFERENCES
- 1.Fasano A, Catassi C. Current approaches to diagnosis and treatment of celiac disease: An evolving spectrum. Gastroenterology. 2001;120:636–51. doi: 10.1053/gast.2001.22123. [DOI] [PubMed] [Google Scholar]
- 2.Green PH, Jabri B. Coeliac disease. Lancet. 2003;362:383–91. doi: 10.1016/S0140-6736(03)14027-5. [DOI] [PubMed] [Google Scholar]
- 3.Landzberg BR, Connor BA. Persistent diarrhea in the returning traveler: Think beyond persistent infection. Scand J Gastroenterol. 2005;40:112–4. doi: 10.1080/00365520410009366. [DOI] [PubMed] [Google Scholar]
- 4.Mendelson RM, Wright SG, Tomkins AM. Coeliac disease presenting as malabsorption from the tropics. Gut. 1978;19:992. [Google Scholar]
- 5.Lawler M, Humphries P, O’Farrelly C, et al. Adenovirus 12 E1A gene detection by polymerase chain reaction in both normal and celiac duodenum. Gut. 1994;35:1226–32. doi: 10.1136/gut.35.9.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fine KD, Ogunji F, Saloum Y, Beharry S, Crippin J, Weinstein J. Celiac sprue: Another autoimmune disorder associated with hepatitis C. Am J Gastroenterol. 2001;96:138–45. doi: 10.1111/j.1572-0241.2001.03464.x. [DOI] [PubMed] [Google Scholar]
- 7.Bizzaro N, Villalta D, Tonutti E, Tampoia M, Bassetti D, Tozzoli R. Association of celiac disease with connective tissue diseases and autoimmune diseases of the digestive tract. Autoimmun Rev. 2003;2:358–63. doi: 10.1016/s1568-9972(03)00055-7. [DOI] [PubMed] [Google Scholar]
- 8.Konturek PC, Karczewska E, Dieterich W, Hahn EG, Schuppan D. Increased prevalence of Helicobacter pylori infection in patients with celiac disease. Am J Gastroenterol. 2000;95:3682–3. doi: 10.1111/j.1572-0241.2000.03421.x. [DOI] [PubMed] [Google Scholar]
- 9.DeMeo MT, Mutlu EA, Keshavarzian A, Tobin MC. Intestinal permeation and gastrointestinal disease. J Clin Gastroenterol. 2002;34:385–96. doi: 10.1097/00004836-200204000-00003. [DOI] [PubMed] [Google Scholar]
- 10.National Institutes of Heath Consensus Development Program. NIH consensus statement on celiac disease. <http://consensus.nih.gov/2004/2004CeliacDisease118PDF.pdf> (Version current at March 22, 2007).
- 11.Green PHR, Stavropoulos SN, Panagi SG, et al. Characteristic of adult celiac disease in USA: Results of a national survey. Am J Gastroenterol. 2001;96:126–31. doi: 10.1111/j.1572-0241.2001.03462.x. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez LA, Ruigomez A. Increased risk of irritable bowel syndrome after bacterial gastroenteritis: Cohort study. BMJ. 1999;318:565–6. doi: 10.1136/bmj.318.7183.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia Rodriguez LA, Ruizgomez A, Panes J. Acute gastroenteritis is followed by an increased risk of inflammatory bowel disease. Gastroenterology. 2006;130:1588–94. doi: 10.1053/j.gastro.2006.02.004. [DOI] [PubMed] [Google Scholar]