Abstract
OBJECTIVES:
Current guidelines, based on expert opinion, recommend that suspected 1 cm to 2 cm hepatocellular carcinoma (HCC) detected on screening be biopsied and, if positive, treated (eg, resection or transplantation). Alternative strategies are immediate treatment or observation until disease progression occurs.
METHODS:
A Markov decision model was developed that compared three management strategies – immediate resection, biopsy and resection if positive, and ultrasound surveillance every three months until disease progression – for a single 1 cm to 2 cm liver nodule suspicious for HCC following ultrasound screening and computed tomography confirmation. The cohort included 55-year-old patients with compensated cirrhosis and no significant comorbidities. The model used in the present study incorporated the probabilities of false-positive and false-negative results, needle-track seeding, HCC recurrence, cirrhosis progression and death. The quality-adjusted life expectancy (LE) and the unadjusted LE were evaluated and the model’s strength was assessed with sensitivity analyses.
RESULTS:
In the base case analysis, biopsy, resection and surveillance yielded an unadjusted LE of 60.5, 59.7 and 56.6 months, respectively, and a quality-adjusted LE of 46.6, 45.6 and 43.8 months, respectively. In probabilistic sensitivity analyses, biopsy was the preferred strategy 69.5% of the time, resection 30.5% of the time and surveillance never. Resection was the optimal decision if the sensitivity of biopsy was very low (less than 0.45) or if the accuracy of the imaging tests resulted in a high percentage of HCC-positive patients (greater than 76%) in the screened cohort, as with expert interpretation of triphasic computed tomography.
CONCLUSIONS:
The present model suggests that biopsy is the preferred management strategy for these patients. When postimaging probability of HCC is high or pathology expertise is lacking, resection is the best alternative. Surveillance is never the optimal strategy.
Keywords: Decision analysis, Hepatic resection, Hepatocellular carcinoma, Liver cirrhosis
Abstract
OBJECTIFS :
Les lignes directrices actuelles, fondées sur l’avis d’experts, recommandent que les carcinomes hépatocellulaires (CHC) présumé de 1 cm à 2 cm décelés au dépistage fassent l’objet d’une biopsie et soient traités lorsqu’ils sont positifs (p. ex., résection ou greffe). D’autres stratégies consistent à procéder à un traitement immédiat ou à poursuivre l’observation jusqu’à ce que la maladie évolue.
MÉTHODOLOGIE :
On a mis au point un modèle décisionnel de Markov pour comparer trois stratégies de prise en charge (résection immédiate, biopsie suivie d’une résection en présence de résultats positifs et surveillance par échographie) tous les trois mois jusqu’à l’apparition d’un seul nodule hépatique de 1 cm à 2 cm susceptible d’être un CHC après confirmation par échographie et tomodensitométrie. La cohorte était formée de patients de 55 ans atteints d’une cirrhose compensée, sans comorbidités importantes. Le modèle utilisé dans la présente étude intégrait les probabilités de résultats faux positifs et faux négatifs, d’ensemencement par piqûre d’aiguille, de récurrence du CHC, d’évolution de la cirrhose et de décès. On a évalué l’espérance de vie (EV) pondérée par la qualité de l’existence et l’EV non rajustée ainsi que la solidité du modèle au moyen d’analyses de sensibilité.
RÉSULTATS :
Dans l’analyse du scénario de référence, la biopsie, la résection et la surveillance ont donné lieu à une EV non rajustée de 60,5, 59,7 et 56,6 mois, respectivement, et à une EV pondérée par la qualité de l’existence de 46,6, 45,6 et 43,8 mois, respectivement. Dans les analyses probabilistes de sensibilité, la biopsie était la stratégie favorisée dans 65, 9% des cas, la résection, dans 30,5 % des cas et la surveillance, dans aucun cas. La résection était la décision optimale lorsque la sensibilité de la biopsie était très faible (moins de 0,45) ou que les tests d’imagerie donnaient un fort pourcentage de patients positifs au CHC (plus de 76 %) au sein de la cohorte dépistée, tout comme l’interprétation de la tomodensitométrie triphasique par des experts.
CONCLUSIONS :
D’après le présent modèle, la biopsie est la stratégie de prise en charge favorisée pour ces patients. Lorsque la probabilité de CHC est élevée après l’imagerie ou en l’absence de compétences en pathologie, la résection devient la meilleure solution. La surveillance n’est jamais la stratégie optimale.
Hepatocellular carcinoma (HCC) is the fifth most common cancer in the world and its incidence correlates with the prevalence of hepatitis B virus (HBV) and hepatitis C virus (HCV) infections (1). Over 80% of patients with HCC have liver cirrhosis (2,3) and HCC is the leading cause of death among patients with cirrhosis (4).
Unfortunately, by the time clinical symptoms are evident, HCC is often untreatable because of tumour size or number, extensive involvement of the liver, invasion of the portal or hepatic veins, or advanced liver cirrhosis (5–8). For this reason, surveillance by ultrasound (US) of patients with compensated cirrhosis followed by confirmation using computed tomography (CT) or magnetic resonance imaging of a suspicious mass has become an accepted practice among hepatologists (5,9–11). Published guidelines are available to guide the investigation of any solid lesion that is not a hemangioma (10,12,13). Lesions smaller than 1 cm are unlikely to be HCC and should be observed at intervals until they either disappear or enlarge. Lesions larger than 2 cm can be confidently diagnosed as HCC if they exhibit typical vascularity during the arterial phase of a dynamic imaging procedure such as a CT scan or magnetic resonance imaging (12–15). Nodules of 1 cm to 2 cm may be early and well-differentiated HCC but they often have imaging characteristics similar to some cirrhotic and dysplastic nodules, making them difficult to diagnose (10). Therapy of these small lesions may produce survival advantages over treatment of larger HCCs (16).
The guidelines for the management of HCC issued by the European Association for the Study of the Liver (EASL) in 2001 (12) suggest that lesions between 1 cm and 2 cm should be biopsied and resection should be offered if the biopsy confirms HCC. However, this recommendation was supported by expert opinion rather than evidence. Liver biopsy has a high false-negative rate, especially in a cirrhotic liver (8,17), and also carries risks of mortality and morbidity, including tumour seeding in the needle track (18). The guidelines also state that liver resection is the treatment of choice in most centres for patients with preserved liver function (Child-Pugh class A cirrhosis). A more recent set of guidelines published by the American Association for the Study of Liver Diseases (AASLD) (13) indicated that for patients with a lesion smaller than 2 cm with preserved liver function, the chosen treatment should be resection. Radiofrequency ablation (RFA) is reserved for patients whose disease is too extensive for resection or whose liver function will not allow surgery. Because there was no clear consensus on the management of 1 cm to 2 cm liver nodules, a decision analysis (19) was used to compare the outcomes of three strategies for the management of 1 cm to 2 cm liver nodules suspicious for HCC: needle biopsy and hepatic resection of positive nodules, resection without biopsy, and continued surveillance with imaging followed by resection of nodules with growth suggestive of HCC.
METHODS
A decision analysis incorporating the Markov model, a method used to represent the natural history of conditions with ongoing risk (20,21), was developed. The model estimates the prognosis of a cohort of hypothetical patients by describing transitions between discrete health states during specified cycles. The model was terminated when less than 0.001% of the cohort remained alive and then the average unadjusted and quality-adjusted life expectancies were calculated under each strategy. DATA 4.0 software was used to construct the model (22).
The initial cohort consisted of 55-year-old men and women with compensated cirrhosis (Child-Pugh class A) and no serious comorbidities that would decrease their life expectancy (LE) or represent a contraindication to surgery. All patients had a solitary liver nodule, 1 cm to 2 cm in diameter, which was suspicious for HCC based on US and CT findings. The model incorporated the diagnostic test characteristics, the prevalence of HCC, the natural history of cirrhosis, HCC development, recurrence and treatment, and mortality due to liver disease, HCC, treatment or causes unrelated to liver disease.
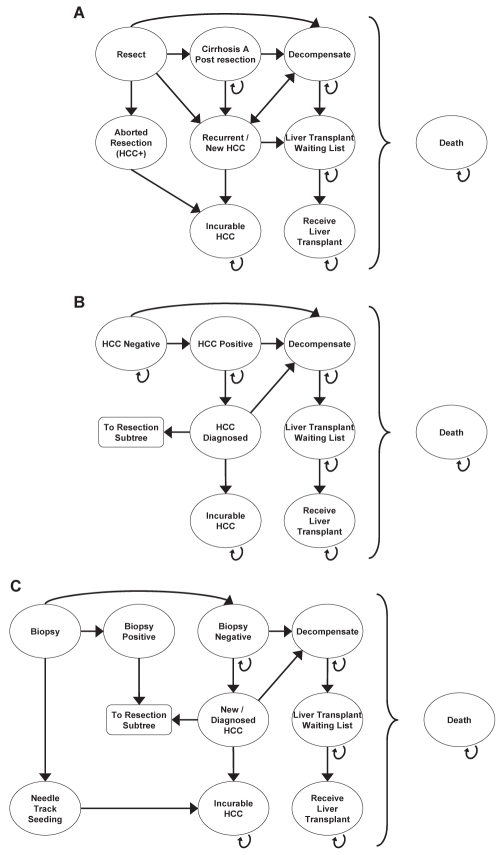
Three strategies were evaluated: immediate resection, continued surveillance until growth suggestive of malignancy occurred, and biopsy with resection if positive. Patients assigned to the immediate resection strategy (Figure 1A) underwent liver resection unless the surgery was aborted due to the intraoperative finding of extensive disease such that cure was not possible. The assumption was made that such findings indicated the original nodule was truly HCC and that after surgery these patients would receive palliative care. Patients who underwent successful resection had follow-up sessions of US surveillance every three months, and those who developed a newly detected nodule that grew steadily and had imaging characteristics typical of HCC were candidates for orthotopic liver transplantation (OLT). Patients who developed decompensated cirrhosis discontinued HCC surveillance and were candidates for OLT for their cirrhosis. Those who had or developed a contraindication to OLT, such as progression of HCC beyond the usual transplant criteria (13,23) while waiting for a donor organ, underwent no further treatment except palliation.
Figure 1).
Schematic diagrams of the Markov model for the decisions to resect without biopsy (A), to continue surveillance (B) or to biopsy (C). Note that all health states can proceed directly to death. Arrows are omitted for simplicity. HCC Hepatocellular carcinoma; + Positive
The second group of patients had follow-up sessions of US surveillance every three months, unless they developed liver decompensation (Figure 1B). Any patient with compensated cirrhosis whose nodule grew steadily and had imaging characteristics typical of HCC was considered for liver resection. These patients then followed the course of patients with HCC in the resection strategy. However, with each monthly cycle, patients risked developing liver decompensation or unresectable HCC. Patients whose nodules were benign and did not grow continued US surveillance and remained at risk for developing another nodule, which would be followed with surveillance. Their prognosis depended on the growth of the nodule, resectability of HCC, progression of cirrhosis, tumour recurrence, suitability for OLT and mortality from surgery, cirrhosis, HCC and nonspecific causes. Patients who developed liver decompensation discontinued US surveillance for HCC and were considered for OLT.
For the biopsy strategy (Figure 1C), all patients with positive biopsy results underwent liver resection. The prognosis of patients with true positive results was the same as that of HCC-positive patients in the immediate resection strategy, providing that they did not experience biopsy-related mortality or seeding of tumour cells along the needle track. Patients with false-positive results had unnecessary hepatic resection, putting them at risk for perioperative mortality or liver decompensation. Survivors of resection would continue HCC surveillance and would be candidates for OLT, not repeat resection, for the treatment of subsequent HCC. If they developed liver decompensation, US surveillance was discontinued and the patients were considered for OLT. Patients with negative biopsy results, (either true-negative or false-negative), received no immediate therapy but continued US surveillance every three months. Their prognosis would be similar to that of patients in the surveillance strategy. Patients with false-negative biopsies would be at risk of developing unresectable HCC or liver decompensation before HCC detection.
The output from the model was expressed as quality-adjusted life expectancy (QALE) and LE, in months, for each of the strategies.
Summary of data used in the model
Table 1 shows the rates, probabilities and utilities, with their plausible low and high values, for the important parameters in the model. All given rates and probabilities were converted into monthly probabilities for use in the model, assuming an exponential distribution for timed outcomes where necessary (24).
TABLE 1.
Model variables: Test characteristics, probabilities and utilities with base case values and ranges
| Variable | Base case | Low | High | References | ||||
|---|---|---|---|---|---|---|---|---|
| HCC incidence and screening test characteristics | ||||||||
| Prevalence of HCC in cirrhosis | 0.05 | 0.02 | 0.08 | (3,4,6,12,30–34) | ||||
| Prevalence of 1 cm to 2 cm HCC | 0.68 | 0.50 | 0.90 | (31,35) | ||||
| Sensitivity of ultrasound in screening | 0.75 | 0.60 | 0.90 | (31,34,37) | ||||
| Specificity of ultrasound | 0.88 | 0.70 | 0.95 | (6,31,32,34,38) | ||||
| Sensitivity of dual-phase CT | 0.80 | 0.60 | 0.90 | (32,41–44) | ||||
| Specificity of dual-phase CT | 0.90 | 0.80 | 0.95 | (32,42–44) | ||||
| Sensitivity of triphasic CT | 0.88 | 0.80 | 0.95 | (43,45–47) | ||||
| Specificity of triphasic CT | 0.99 | 0.90 | 1.00 | (43,45–47) | ||||
| Sensitivity of needle biopsy | 0.70 | 0.50 | 0.95 | (8,15,17,18,30,48) | ||||
| Specificity of needle biopsy | 0.90 | 0.80 | 1.00 | (8,17) | ||||
| Survival | ||||||||
| Compensated cirrhosis | ||||||||
| Five-year survival | 0.75 | 0.65 | 0.85 | (6,7,67) | ||||
| Decompensated cirrhosis | ||||||||
| Five-year survival | 0.3 | 0.2 | 0.4 | (6,7,67) | ||||
| Death from needle biopsy | 0.0004 | 0.0001 | 0.001 | (32,49–51) | ||||
| Death within 30 days of resection | 0.01 | 0 | 0.03 | (11,30,58–66) | ||||
| Death from small HCC | ||||||||
| Three-year survival | 0.5 | 0.4 | 0.6 | (6) | ||||
| Three-month mortality | 0.08 | (69) | ||||||
| Annual mortality rate | 0.2 | 0.05 | 0.45 | (6,90–92,98) | ||||
| Death from incurable HCC | ||||||||
| One-year survival | 0.2 | 0.1 | 0.4 | (6,72) | ||||
| Cirrhosis and HCC | ||||||||
| Liver decompensation | ||||||||
| Annual probability | 0.04 | 0.02 | 0.1 | (6,7,67,72) | ||||
| Development of HCC | 0.003 | 0.001 | 0.005 | (3,4,6,12,31–34) | ||||
| Postresection recurrence of HCC | ||||||||
| Annual recurrence rate | 0.2 | 0.1 | 0.3 | (32,67) | ||||
| Five-year recurrence rate | 0.7 | 0.5 | 0.85 | (5,12,68) | ||||
| Growth of HCC | ||||||||
| Mean doubling time (days) | 180 | 30 | 360 | (6,90,92,99–102) | ||||
| Probability of growth per six months | 0.3 | 0.2 | 0.4 | |||||
| Needle-track seeding after biopsy | 0.015 | 0.005 | 0.05 | (8,18,50,53,54,57) | ||||
| Liver transplantation | ||||||||
| For HCC, per year | 0.07 | 0.02 | 0.1 | (71,72) | ||||
| For decompensated cirrhosis, per year | 0.033 | 0.015 | 0.05 | |||||
| Utilities | ||||||||
| Compensated cirrhosis | 0.8 | 0.5 | 0.92 | (32,73,76–80,103) | ||||
| Decompensated cirrhosis | 0.6 | 0.2 | 0.8 | (32,73,76–80) | ||||
| HCC with compensated cirrhosis | 0.72 | 0.1 | 0.8 | (32,73,76–78,103) | ||||
| HCC with decompensated cirrhosis | 0.57 | 0.1 | 0.8 | (32,73,76–78,103) | ||||
| Incurable HCC | 0.4 | 0.2 | 0.6 | (75,83) | ||||
| Four weeks postresection | 0.7 | 0.4 | 0.9 | (32) | ||||
| Liver transplant survivor | 0.75 | 0.55 | 0.9 | (32,75,83) | ||||
CT Computed tomography; HCC Hepatocellular carcinoma
The cohort
The latency period from HBV or HCV infection to the development of HCC is usually between 10 and 30 years (25,26) but can be much longer. In North America and Europe, HCV is the major cause of cirrhosis and it occurs later in life, so most North American and European patients with HCC are 55 years of age or older (15,27,28). Therefore, the starting age of the initial cohort was 55 years but a range of 40 to 75 years was included in sensitivity analysis. Age-specific mortality rates were calculated from standard life tables (29).
Incidence and natural history
The annual incidence of HCC is 2% to 6% in HBV patients with cirrhosis, and 3% to 8% in patients with HCV and cirrhosis (3,4,12,30). In the present model, the base case value was 5%, with a range of 2% to 8% (3,4,6,12,31–34).
HCC nodules detected during surveillance of asymptomatic cirrhotic patients are of varying sizes. The proportion of small nodules depends on the population being screened and the frequency of surveillance. One study (17) reported that only 25% of HCCs detected during surveillance were smaller than 2 cm but five other studies (31,35) reported that 68% to 75% of HCC nodules were single and smaller than 3 cm. The assumption was made that the prevalence of 1 cm to 2 cm HCC nodules that were not biopsy-proven would be fairly high in these relatively young asymptomatic patients with compensated cirrhosis; therefore, a base case value was set at 68% and the range was extended from 50% to 90%.
The literature on the natural history of cirrhosis and HCC was reviewed to obtain data on rates of liver decompensation, tumour growth and survival with cirrhosis and HCC, as shown in Table 1. The monthly transition probabilities and plausible ranges were calculated for use in the model from these data.
Diagnostic test characteristics
Patients entering the model had cirrhosis, and US and CT results suspicious for HCC. The probability of a patient in the cohort actually having HCC was calculated according to Bayes’ theorem (36) and was dependent on the prevalence of HCC in cirrhotic patients, the proportion of 1 cm to 2 cm nodules and the characteristics of the imaging tests (Appendix). Variations in these parameters result in changes in the true prevalence of HCC among the cohort entering the model.
The sensitivity of US as a screening test in HCC surveillance is reported to range from 70% to 80% (31,34,37), and a baseline value of 75% was chosen. The specificity of US was 88% in the base case analysis, with a range of 70% to 95% (6,31,32,34,38). CT was used as the diagnostic test in the model. Recent advances in technology have increased the accuracy of CT in confirming the diagnosis of HCC in suspicious nodules (39,40) but the latest techniques are not universally available at this time. The sensitivity of dual-phase CT is reported to range from 60% to 88% and its specificity from 85% to 99% (32,41–44). Triphasic CT permits the detection of venous ‘washout’ during the early or delayed phase, a valuable diagnostic criterion for HCC (13,43). The sensitivity of triphasic CT is reported to range from approximately 85% to 94%, with a specificity of 99% (43,45–47). For the base case, it was assumed that dual-phase CT would be more widely available and the sensitivity and specificity of CT were set at 80% and 90%, respectively. The model was also run using a sensitivity of 88% and specificity of 99% to reflect the use of triphasic CT. For both US and CT, higher sensitivity and specificity resulted in a higher proportion of the cohort being truly HCC-positive when entering the model.
Needle biopsy has a 20% to 40% false-negative rate, because the needle may miss the cancerous cells, or the cells may be incorrectly diagnosed as normal cirrhotic liver cells (8,15,17,18,30,48). Seventy per cent was used as the base case sensitivity of needle biopsy (range 50% to 95% in sensitivity analysis). The base case specificity of biopsy was 90% (range 80% to 100%) (8,17).
Biopsy and treatments
Needle biopsy has a very small risk of immediate death, reported to range from one to 37 cases per 100,000 (18,49–51). It also carries the risk of spreading HCC along the needle track, which often turns a potentially curable disease into an incurable one (8,14,18,31). Although in some cases local excision of the implanted tumour is successful (8), in other cases more extensive treatments are required (52–54) and are ultimately not successful (54–56). Reported risks of tumour seeding vary from as low as 0.005% (49,56) to as high as 5% (53). In recent large studies, needle-track seeding occurred in 1.6% (8), 2.7% (54) and 3.4% (57) of patients. The true incidence of seeding and its outcome are not well documented and likely depend on the type of needle used, the size of the tumour and the duration of follow-up. Based on these limited data, 1.5% was used as the base case probability of seeding and 0.05% to 5% as the plausible range. It was assumed that needle-track seeding resulted in incurable HCC. This represents the worst-case scenario for the outcome after needle-track seeding and biases the model against the biopsy strategy. Sensitivity analysis was used to examine the scenarios in which needle-track seeding never occurred, and in which it was very high (5% to 10%).
Most of the data from the past five years reported that the 30-day perioperative mortality following liver resection ranges from 0% to 3% (11,30,58–65), but at our centre even a 1% mortality is considered high. In the model, a base case value of 1% and a range of 0% to 3% for the probability of death within 30 days of resection was used. Perioperative mortality in carefully selected elderly patients (70 to 82 years of age) was reported to be nearly double that of younger patients, although the difference was not statistically significant in the small sample (66). Therefore, the excess perioperative mortality was increased by 1% for every 15 years of age over 55 years of age, until mortality reached a maximum value of 3% for patients 85 years and older.
The main problem associated with hepatic resection is that it is not always curative and the HCC recurrence rate is approximately 50% after two to three years and at least 70% by five years (5,12,67,68). OLT is one of the recommended treatments for postresection recurrence of HCC (26,68–70) and the only treatment for patients with decompensated cirrhosis (12). In the United States, between January 1995 and February 2004, approximately 60% of HCC patients on the organ waiting list received liver transplants, while approximately 23% died or became too sick before a liver was available (71). Among patients with decompensated cirrhosis, the annual probability of getting a liver transplant was estimated to be 3.3%, with a plausible range of 1.7% to 4.9% (72).
Quality of life weights (utilities)
Utilities were obtained from published studies for the health states represented in the model (Table 1). For consistency, utilities reported by Chong et al (73), that were obtained directly from 193 patients with HCV using the standard gamble technique, were used for the base case values when available. The entire ranges of reported utilities were used in sensitivity analysis.
The base case utility for compensated cirrhosis from Chong et al (73) was 0.80. Reported utilities from patients and nonpatients ranged from 0.50 (74) to 0.92 (75), with most other values in the range of 0.75 to 0.85 (32,75–80). Most of the reported utilities for decompensated cirrhosis were approximately 0.60, as reported by Chong et al (73) and others (32,76–80), but they ranged from 0.20 (74) to 0.80 (79). Utilities for HCC were obtained from the Chong et al study, in which 10 (71%) of the HCC patients were Child-Pugh class A and the remaining four (29%) were Child-Pugh class B (73). These ambulatory patients provided utilities ranging from 0.55 to 0.72 for their own health. Based on these data, base case values of 0.72 were used for HCC with compensated cirrhosis and 0.57 for HCC with decompensated cirrhosis. These values were varied from 0.1 to 0.80, the ranges reported in the literature (32,76–79,81,82). Reported utilities for incurable or fatal HCC ranged from 0.2 (75) to 0.6 (83) and a base case value of 0.4 was used in the present study.
Additional utility values used in the model are reported in Table 1.
Model assumptions
The following assumptions were made in the model: small, undetected tumours were asymptomatic and were not associated with an increased risk of death unless the tumour grew or liver decompensation occurred; the probabilities of decompensation, and HCC development, growth and recurrence were constant over time; and compliance with surveillance was 100%.
Sensitivity analysis
One-way sensitivity analyses were performed to determine how varying the value of each individual parameter within its plausible range affected the decision. The model is sensitive to a parameter if the optimal decision changes within the range of that parameter’s plausible values (84). Probabilistic sensitivity analysis was also performed, in which each parameter was given a probability distribution defined by its mean and plausible range, and values from each distribution were randomly selected in Monte Carlo trials (85). Beta distributions were used for all of the parameters.
RESULTS
Base case analysis
Biopsy was the preferred strategy in the base case model but both active strategies were favoured over surveillance (Table 2). Biopsy yielded an average LE of 60.5 months, compared with 59.7 months with resection and only 56.6 months with surveillance. In terms of QALE, biopsy resulted in 46.6 quality-adjusted life months (QALMs), while resection and surveillance resulted in 45.6 and 43.8 QALMs, respectively. Therefore, the benefits of the biopsy strategy over the resection strategy were modest (0.8 months and 1.0 QALM), compared with its benefits over surveillance (3.9 months and 2.8 QALMs).
TABLE 2.
Results of base case analysis
| Decision | Life expectancy (months) | Quality-adjusted life expectancy (months) |
|---|---|---|
| Biopsy | 60.5 | 46.6 |
| Resection | 59.7 | 45.6 |
| Surveillance | 56.6 | 43.8 |
Survival rates for the HCC-positive patients were also calculated using the decision model. The model predicted a two-year survival rate of 74% and a five-year survival rate of 35% for HCC-positive patients who were resected without biopsy or who had a true-positive biopsy. The predicted two-year and five-year survival rates for patients who were initially untreated due to false-negative biopsies or surveillance were 58% and 27%, respectively. These predicted rates were similar to reported five-year postresection survival rates of approximately 30% to 60% (66,68,86–89) and two-year survival rates of 44% and 56% in patients with small untreated HCC (some of whom had advanced liver disease) (90–92).
The probability of a patient having HCC following positive US screening and dual-phase CT confirmation was 0.638. Therefore, 63.8% of the cohort had HCC upon entering the model. The decision to biopsy resulted in 19% of biopsied patients having initially undiagnosed HCC (due to false-negative results) and 3.6% undergoing unnecessary resection (due to false-positive results). However, 36.2% of the patients in the resection strategy had unnecessary resections and 63.8% of the patients in the surveillance strategy had initially untreated HCC. Therefore, the biopsy strategy resulted in the fewest diagnostic and treatment errors.
The model was also analyzed using the test characteristics of triphasic CT (Table 1). With its superior diagnostic accuracy, 95% of the cohort entering the model was HCC-positive. Resection therefore became the best strategy, yielding a LE of 53.6 months, compared with 51.3 months for the biopsy strategy. Corresponding QALMs were 40.9 for resection and 39.2 for biopsy. The outcomes with surveillance were the least favourable at only 45.1 months and 34.5 QALMs.
Sensitivity analysis
One-way sensitivity analyses showed that the decision to biopsy was sensitive to two parameters: the probability of being HCC-positive (ie, the percentage of the cohort that was HCC-positive) based on the US and CT results (36) (Appendix) and the sensitivity of biopsy. Resection became the preferred decision with variations in these parameters, but continued surveillance after the appearance of a suspicious lesion was never the best decision.
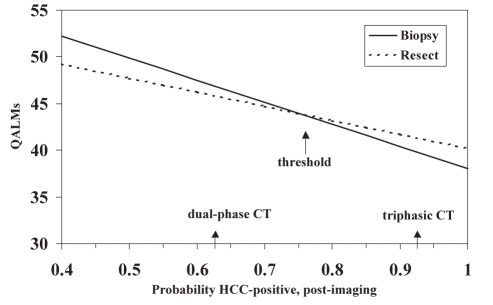
Figure 2 and Table 3 show how variations in the percentage of the cohort being HCC-positive affect the results of the model. The postimaging probability of patients being HCC-positive had to be less than 0.11, corresponding to unrealistically low values for the specificity of US and CT, for surveillance to be the preferred strategy. As the percentage of HCC-positive patients in the cohort increased, and all other model parameters stayed constant, biopsy became the preferred strategy. When more than 76% of the cohort was HCC-positive, there were fewer unnecessary resections in the resection strategy, the percentage of missed HCC increased in the biopsy strategy and resection was determined to be the best strategy. Figure 2 shows the results of the one-way sensitivity analysis and indicates the outcomes associated with dual-phase and triphasic CT.
Figure 2).
One-way sensitivity analysis on the post-imaging probability of the cohort being hepatocellular carcinoma (HCC)-positive. The arrows indicate the threshold value and the results when dual-phase and triphasic computed tomography (CT) are used. QALMs Quality-adjusted life months
TABLE 3.
Effects of variations in postimaging hepatocellular carcinoma (HCC)-positive probability in the cohort and biopsy sensitivity on the output of the model
| Parameter | Value | Resection – unnecessary resections (%) |
Biopsy |
Preferred decision | QALMs | |
|---|---|---|---|---|---|---|
| Unnecessary resections (%) | Missed HCC (%) | |||||
| HCC-positive per ultrasound and computed tomography
(% of cohort) |
0.11 | 89.0 | 8.9 | 3.3 | Surveillance = biopsy* | 59.2 |
| 0.40 | 60.0 | 6.0 | 12.0 | Biopsy | 52.2 | |
| 0.50 | 50.0 | 5.0 | 15.0 | Biopsy | 49.8 | |
| 0.76 | 24.5 | 2.5 | 22.6 | Resect = biopsy* | 43.8 | |
| 0.90 | 10.0 | 1.0 | 27.0 | Resect | 41.6 | |
| 0.95 | 5.0 | 0.05 | 28.0 | Resect | 40.9 | |
| Biopsy sensitivity | 0.40 | 36.2 | 6.4 | 38.4 | Resect | 45.6 |
| 0.45 | 36.2 | 6.0 | 32.0 | Resect = biopsy* | 45.6 | |
| 0.70 | 36.2 | 6.4 | 29.0 | Biopsy | 46.6 | |
| 0.80 | 36.2 | 6.4 | 12.8 | Biopsy | 47.0 | |
| 0.90 | 36.2 | 6.4 | 6.4 | Biopsy | 47.4 | |
Threshold value at which two options provide equivalent health outcomes (quality-adjusted life months [QALMs])
Increases in the sensitivity of biopsy, when all other parameters remained constant, resulted in more favourable outcomes for the biopsy strategy because there were fewer missed cases of HCC (Table 3). The sensitivity of biopsy had to be very low (less than 0.45), resulting in 32% of the HCC-positive patients being misdiagnosed, for resection to be the better strategy.
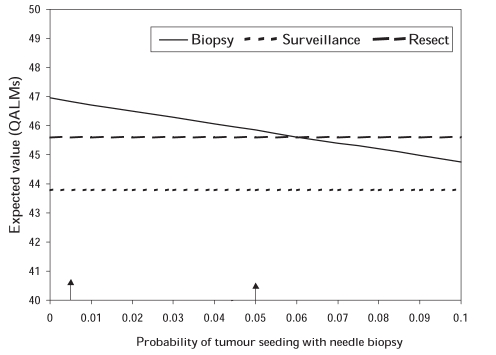
The effects of tumour seeding with needle biopsy were also examined. Due to the paucity of data from large studies, the actual probability of needle seeding or its consequences was uncertain so its probability was varied from 0 to 0.1, outside of the plausible range of 0.005 to 0.05, in sensitivity analysis. In the model, the assumption was made that needle seeding resulted in incurable HCC, which represented the worst-case scenario. However, even with this assumption, the threshold value for the probability of tumour seeding was 0.06, above the plausible range (Figure 3).
Figure 3).
Effects of tumour seeding with needle biopsy on the output of the model. Values between the vertical arrows are within the plausible range of values. QALMs Quality-adjusted life months
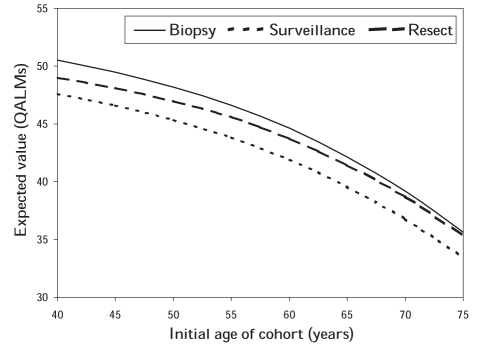
The decision was not sensitive to the initial age of the patient cohort, but QALE decreased as initial age increased. Also, the gains in QALE with the biopsy and resection strategies relative to surveillance became smaller, as did the differences in QALE between the two active strategies (Figure 4).
Figure 4).
Quality-adjusted life months (QALMs) for cohorts of different initial ages as predicted by the model
In the probabilistic sensitivity analysis, biopsy was the preferred strategy in 69.5% of the Monte Carlo simulations, resection was preferred in 30.5% and continued surveillance was never optimal. Table 4 describes the outputs from 1000 Monte Carlo simulations of the model. The mean ± SD difference in QALMs between the biopsy and resection strategies was 1.0±2.1, ranging from −7.8 to +8.0. The difference in favour of biopsy was greater than one month in 49.4% of the simulations. The mean ± SD difference between biopsy and surveillance was 3.0±1.2 QALMs, ranging from 0.1 to 7.1. The difference was greater than three months in 46% of the simulations.
TABLE 4.
Results of 1000 Monte Carlo simulations
|
Expected value (QALMs) |
|||
|---|---|---|---|
| Biopsy | Resect | Surveillance | |
| Mean | 47.7 | 46.7 | 44.7 |
| Standard deviation | 4.5 | 4.6 | 4.5 |
| Minimum | 33.6 | 33.4 | 31.7 |
| Median | 47.5 | 46.5 | 44.5 |
| Maximum | 64.5 | 60.9 | 63.1 |
| % optimal | 69.5 | 30.5 | 0.0 |
QALMs Quality-adjusted life months
DISCUSSION
In our decision analysis, biopsy was determined to be the optimal strategy for patients with 1 cm to 2 cm liver nodules suspicious for HCC following US screening and confirmatory diagnosis with dual-phase CT imaging. Biopsy yielded 1.0 QALM or 0.8 months longer survival than immediate resection and 2.8 QALMs or 3.9 months longer survival than surveillance. Differences of two months or more in LE are generally considered to be clinically significant (84,93). Therefore, the choice between biopsy and resection might be considered a close call, but both strategies are significantly better than surveillance.
The decision was sensitive to the postimaging probability of the cohort being HCC-positive and the sensitivity of biopsy. Resection was the preferred decision when the US screening and CT confirmation imaging tests were accurate enough that more than 75.5% of the cohort entering the model were truly HCC-positive. When dual-phase CT was used, the percentage of HCC-positive patients was this high in only 58 (5.8%) of 1000 Monte Carlo simulation trials. When we adjusted the base model with the reported sensitivity and specificity of triphasic CT, 95% of the initial cohort was HCC-positive and resection became the optimal strategy.
This finding is relevant to the practice guidelines for the management of HCC recently published by the AASLD (13). The criteria for radiological diagnosis of HCC have been revised for triphasic CT such that the presence of ‘washout’ in the venous phase confers specificity additional to that of arterial hypervascularity. The current recommendation is that if the typical appearances are found on two dynamic studies in a 1 cm to 2 cm lesion, the diagnosis of HCC can be considered to be firm. However, state-of-the-art equipment and experienced radiologists are required to interpret these findings (13). The model in the present paper demonstrated that a more accurate radiological diagnosis would reduce the need for biopsy but did not alter the finding that observation is the least preferred strategy.
Of late, fewer resections are being performed for small HCC. Many centres are treating them with local ablation by RFA or ethanol (13). This was not considered as a treatment option in the model used in the present study because neither the AASLD nor the EASL guidelines suggest that this is the most appropriate therapy. Although RFA can be used for lesions smaller than 2 cm with a high rate of complete ablation (16), it is still not clear that ablation produces a better overall outcome than resection. The only randomized controlled trial that compared resection with RFA showed no difference in four-year survivals between the two treatment groups (94). The recurrence rate was higher in the ablated group but the resected group had a higher rate of postoperative morbidity. These results indicate that over a longer follow-up period the group that received RFA will likely experience a higher mortality than the resected group.
We also did not consider liver transplantation as an initial treatment for small HCC. Despite its documented efficacy in selected patients (13,23), liver transplantation is seldom offered as first-line therapy for patients who have compensated cirrhosis and a single resectable HCC smaller than 2 cm (15) due to a shortage of organs and long wait times. Patients with such small lesions do not get any additional priority for liver transplantation. Such patients would then be on a standard waiting list, the length of which varies considerably from centre to centre. The additional complexity required to model these differences was beyond what we were trying to demonstrate in this study.
Our model was limited to patients with no significant comorbidity and we did not model the development or progression of comorbid disease with time. Economic factors, such as the relative time, costs and resources associated with biopsy and surgery were not included, which may have been important when the model results indicated a ‘close call’ between the two decisions.
Despite these limitations, we believe that our analysis represents a useful contribution to the literature. It follows patients with compensated cirrhosis from the appearance of a suspicious small nodule to death, incorporating events such as liver decompensation and its treatment, and continued surveillance and treatment for new or recurring HCC. Other published decision analyses concerning HCC screening (6,32) or treatment (7,67,83,95) modelled only a window in this chain of events. The one published decision analysis (96) that modelled biopsy and treatment of the small liver nodule used three-year survival estimates from the literature as outcomes for resection, local ablation, OLT or untreated HCC, with or without prior biopsy. The results of this analysis indicated that immediate OLT, without biopsy, was the preferred strategy for nodules diagnosed as HCC following imaging and serum alpha-fetoprotein. However, the long wait time for OLT limited its advantage over resection. Furthermore, the model did not include rescreening for patients with initial false-negative biopsies so, these patients had untreated HCC. In an earlier version of our model, in which patients with false-negative biopsies underwent no further surveillance or intervention, immediate resection yielded higher QALMs and LE than biopsy (97). These results are in accordance with the recommendation that a negative biopsy of a visible nodule should not be used as a criterion to rule out malignancy (12).
Our model identified several factors on which the decision to biopsy relies. These include the initial prevalence of HCC in the screened population and the accuracy of the imaging tests and biopsy. Needle track seeding of the tumour did not modify the decision to biopsy within the plausible range of its probability. Our model indicated that patients with compensated cirrhosis, small HCC and no serious comorbidity benefit from early confirmation of their diagnosis and prompt treatment. The model emphatically indicates that the least effective strategy is to simply observe suspicious nodules until growth occurs.
CONCLUSION
The results indicate that patients who have small lesions found on HCC screening should undergo appropriate radiological investigation and biopsy if the radiological appearances are atypical, and undergo treatment if the diagnosis is confirmed by radiology or biopsy. These results support the EASL and AASLD guidelines (12,13) for the diagnosis and management of small HCC.
APPENDIX
The probability of a patient in the cohort actually having HCC was calculated as:
| (sensitivity of ultrasound × sensitivity of CT ×
[prevalence of HCC in cirrhosis × prevalence of 1 cm to 2 cm HCC]) / (sensitivity of ultrasound × sensitivity of CT × [prevalence of HCC × prevalence of 1 cm to 2 cm HCC] + ([1 – specificity of ultrasound] × [1 – specificity of CT] × [1 – prevalence of HCC × prevalence of 1 cm to 2 cm HCC]) |
Footnotes
SUPPORT: Ms Bremner is supported by an operating grant from the Canadian Institutes for Health Research. Dr Krahn is supported by an Investigator Award from the Canadian Institutes for Health Research and the F Norman Hughes Chair in Pharmacoeconomics, Faculty of Pharmacy, University of Toronto.
REFERENCES
- 1.Bosch FX, Ribes J, Borras J. Epidemiology of primary liver cancer. Semin Liver Dis. 1999;19:271–85. doi: 10.1055/s-2007-1007117. [DOI] [PubMed] [Google Scholar]
- 2.Schafer DF, Sorrell MF. Hepatocellular carcinoma. Lancet. 1999;353:1253–7. doi: 10.1016/S0140-6736(98)09148-X. [DOI] [PubMed] [Google Scholar]
- 3.Bolondi L, Sofia S, Siringo S, et al. Surveillance programme of cirrhotic patients for early diagnosis and treatment of hepatocellular carcinoma: A cost effectiveness analysis. Gut. 2001;48:251–9. doi: 10.1136/gut.48.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colombo M, de Franchis R, Del Ninno E, et al. Hepatocellular carcinoma in Italian patients with cirrhosis. N Engl J Med. 1991;325:675–80. doi: 10.1056/NEJM199109053251002. [DOI] [PubMed] [Google Scholar]
- 5.Bruix J, Llovet JM. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology. 2002;35:519–24. doi: 10.1053/jhep.2002.32089. [DOI] [PubMed] [Google Scholar]
- 6.Sarasin FP, Giostra E, Hadengue A. Cost-effectivenes of screening for detection of small hepatocellular carcinoma in western patients with Child-Pugh class A cirrhosis. Am J Med. 1996;101:422–34. doi: 10.1016/S0002-9343(96)00197-0. [DOI] [PubMed] [Google Scholar]
- 7.Sarasin FP, Giostra E, Mentha G, Hadengue A. Partial hepatectomy or orthotopic liver transplantation for the treatment of resectable hepatocellular carcinoma? A cost-effectiveness perspective. Hepatology. 1998;28:436–42. doi: 10.1002/hep.510280222. [DOI] [PubMed] [Google Scholar]
- 8.Durand F, Regimbeau JM, Belghiti J, et al. Assessment of the benefits and risks of percutaneous biopsy before surgical resection of hepatocellular carcinoma. J Hepatol. 2001;35:254–8. doi: 10.1016/s0168-8278(01)00108-8. [DOI] [PubMed] [Google Scholar]
- 9.Sherman M. Surveillance for hepatocellular carcinoma. Semin Oncol. 2001;28:450–9. doi: 10.1016/s0093-7754(01)90138-1. [DOI] [PubMed] [Google Scholar]
- 10.Sherman M. Pathogenesis and screening for hepatocellular carcinoma. Clin Liver Dis. 2004;8:419–43. doi: 10.1016/j.cld.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Song TJ, Ip EW, Fong Y. Hepatocellular carcinoma: Current surgical management. Gastroenterology. 2004;127:S248–60. doi: 10.1053/j.gastro.2004.09.039. [DOI] [PubMed] [Google Scholar]
- 12.Bruix J, Sherman M, Llovet JM, et al. EASL Panel of Experts on HCC. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona – 2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–30. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 13.Bruix J, Sherman M, Practice Guidelines Committee, American Association for the Study of Liver Diseases Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–36. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 14.Sherman M. Hepatocellular carcinoma: Beyond screening. J Hepatol. 2003;39:269–71. doi: 10.1016/s0168-8278(03)00249-6. [DOI] [PubMed] [Google Scholar]
- 15.Llovet JM, Beaugrand M. Hepatocellular carcinoma: Present status and future prospects. J Hepatol. 2003;38:S136–49. doi: 10.1016/s0168-8278(02)00432-4. [DOI] [PubMed] [Google Scholar]
- 16.Sala M, Llovet JM, Vilana R, et al. Barcelona Clinic Liver Cancer Group. Initial response to percutaneous ablation predicts survival in patients with hepatocellular carcinoma. Hepatology. 2004;40:1352–60. doi: 10.1002/hep.20465. [DOI] [PubMed] [Google Scholar]
- 17.Borzio M, Borzio F, Macchi R, et al. The evaluation of fine-needle procedures for the diagnosis of focal liver lesions in cirrhosis. J Hepatol. 1994;20:117–21. doi: 10.1016/s0168-8278(05)80477-5. [DOI] [PubMed] [Google Scholar]
- 18.Torzilli G, Minagawa M, Takayama T, et al. Accurate preoperative evaluation of liver mass lesions without fine-needle biopsy. Hepatology. 1999;30:889–93. doi: 10.1002/hep.510300411. [DOI] [PubMed] [Google Scholar]
- 19.Detsky AS, Naglie G, Krahn MD, Naimark D, Redelmeier DA. Primer on medical decision analysis: Part 1 – getting started. Med Decis Making. 1997;17:123–5. doi: 10.1177/0272989X9701700201. [DOI] [PubMed] [Google Scholar]
- 20.Naimark D, Krahn MD, Naglie G, Redelmeier DA, Detsky AS. Primer on medical decision analysis: Part 5 – working with Markov processes. Med Decis Making. 1997;17:152–9. doi: 10.1177/0272989X9701700205. [DOI] [PubMed] [Google Scholar]
- 21.Sonnenberg FA, Beck JR. Markov models in medical decision making: A practical guide. Med Decis Making. 1993;13:322–38. doi: 10.1177/0272989X9301300409. [DOI] [PubMed] [Google Scholar]
- 22.TreeAge software Inc DATA 4.0<www.treeage.com> (Version current at June 25, 2007).
- 23.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–9. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 24.Beck JR, Pauker SG. The Markov process in medical prognosis. Med Decis Making. 1983;3:419–58. doi: 10.1177/0272989X8300300403. [DOI] [PubMed] [Google Scholar]
- 25.Zhu AX. Hepatocellular carcinoma: Are we making progress? Cancer Invest. 2003;21:418–28. doi: 10.1081/cnv-120018233. [DOI] [PubMed] [Google Scholar]
- 26.Donckier V, van Laethem JL, van Gansbeke D, et al. New considerations for an overall approach to treat hepatocellular carcinoma in cirrhotic patients. J Surg Oncol. 2003;84:36–44. doi: 10.1002/jso.10281. [DOI] [PubMed] [Google Scholar]
- 27.Dohmen K, Shigematsu H, Irie K, Ishibashi H. Comparison of the clinical characteristics among hepatocellular carcinoma of hepatitis B, hepatitis C and non-B non-C patients. Hepatogastroenterology. 2003;50:2022–7. [PubMed] [Google Scholar]
- 28.Liu JH, Chen PW, Asch SM, Busuttil RW, Ko CY. Surgery for hepatocellular carcinoma: Does it improve survival? Ann Surg Oncol. 2004;11:298–303. doi: 10.1245/aso.2004.03.042. [DOI] [PubMed] [Google Scholar]
- 29.Statistics Canada Life Tables, Canada, Provinces and Territories, 1995–1997<www.statcan.ca/english/freepub/84-537-XIE/84-537-XIE1997001.htm> (Version current at June 25, 2007). [Google Scholar]
- 30.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–17. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 31.Collier J, Sherman M. Screening for hepatocellular carcinoma. Hepatology. 1998;27:273–8. doi: 10.1002/hep.510270140. [DOI] [PubMed] [Google Scholar]
- 32.Arguedas MR, Chen VK, Eloubeidi MA, Fallon MB. Screening for hepatocellular carcinoma in patients with hepatitis C cirrhosis: A cost-utility analysis. Am J Gastroenterol. 2003;98:679–90. doi: 10.1111/j.1572-0241.2003.07327.x. [DOI] [PubMed] [Google Scholar]
- 33.Aguayo A, Patt YZ. Liver cancer. Clin Liver Dis. 2001;5:479–507. doi: 10.1016/s1089-3261(05)70175-6. [DOI] [PubMed] [Google Scholar]
- 34.Pateron D, Ganne N, Trinchet JC, et al. Prospective study of screening for hepatocellular carcinoma in Caucasian patients with cirrhosis. J Hepatol. 1994;20:65–71. doi: 10.1016/s0168-8278(05)80468-4. [DOI] [PubMed] [Google Scholar]
- 35.Francanzani AL, Burdick L, Borzio M, et al. Contrast-enhanced doppler ultrasonography in the diagnosis of hepatocellular carcinoma and premalignant lesions in patients with cirrhosis. Hepatology. 2001;34:1109–12. doi: 10.1053/jhep.2001.29373. [DOI] [PubMed] [Google Scholar]
- 36.Sox HC, Blatt MA, Higgins MC, Marton KI. Medical Decision Making. Boston: Butterworths; 1988. [Google Scholar]
- 37.Sherman M, Peltekian KM, Lee C. Screening for hepatocellular carcinoma in chronic carriers of hepatitis B virus: Incidence and prevalence of hepatocellular carcinoma in a North American urban population. Hepatology. 1995;22:432–8. [PubMed] [Google Scholar]
- 38.Bennett GL, Krinsky GA, Abitol RJ, Kim SY, Theise ND, Teperman LW. Sonographic detection of hepatocellular carcinoma and dysplastic nodules in cirrhosis: Correlation of pretransplantation sonography and liver explant pathology in 200 patients. AJR Am J Roentgenol. 2002;179:75–80. doi: 10.2214/ajr.179.1.1790075. [DOI] [PubMed] [Google Scholar]
- 39.Oliver JH, III, Baron RL. Helical biphasic contrast-enhanced CT of the liver: Techniques, indications, interpretation, and pitfalls. Radiology. 1996;201:1–14. doi: 10.1148/radiology.201.1.8816509. [DOI] [PubMed] [Google Scholar]
- 40.Baron RL, Brancatelli G. Computed tomographic imaging of hepatocellular carcinoma. Gastroenterology. 2004;127:S133–43. doi: 10.1053/j.gastro.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 41.Iannaccone R, Laghi A, Catalano C, et al. Hepatocellular carcinoma: Role of unenhanced and delayed phase multi-detector row helical CT in patients with cirrhosis. Radiology. 2005;234:460–7. doi: 10.1148/radiol.2342031202. [DOI] [PubMed] [Google Scholar]
- 42.Gambarin-Gelwan M, Wolf DC, Shapiro R, Schwartz ME, Min AD. Sensitivity of commonly available screening tests in detecting hepatocellular carcinoma in cirrhotic patients undergoing liver transplantation. Am J Gastroenterol. 2000;95:1535–8. doi: 10.1111/j.1572-0241.2000.02091.x. [DOI] [PubMed] [Google Scholar]
- 43.Lim JH, Choi D, Kim SH, et al. Detection of hepatocellular carcinoma: Value of adding delayed phase imaging to dual-phase helical CT. AJR Am J Roentgenol. 2002;179:67–73. doi: 10.2214/ajr.179.1.1790067. [DOI] [PubMed] [Google Scholar]
- 44.Saab S, Ly D, Nieto J, et al. Hepatocellular carcinoma screening in patients waiting for liver transplantation: A decision analytic model. Liver Transpl. 2003;9:672–81. doi: 10.1053/jlts.2003.50120. [DOI] [PubMed] [Google Scholar]
- 45.Laghi A, Iannaccone R, Rossi P, et al. Hepatocellular carcinoma: Detection with triple-phase multi-detector row helical CT in patients with chronic hepatitis. Radiology. 2002;226:543–9. doi: 10.1148/radiol.2262012043. [DOI] [PubMed] [Google Scholar]
- 46.Jang HJ, Lim JH, Lee SJ, Park CK, Park HS, Do YS. Hepatocellular carcinoma: Are combined CT during arterial portography and CT hepatic arteriography in addition to triple-phase helical CT all necessary for preoperative evaluation? Radiology. 2000;215:373–80. doi: 10.1148/radiology.215.2.r00ma30373. [DOI] [PubMed] [Google Scholar]
- 47.Kim SK, Lim JH, Lee WJ, et al. Detection of hepatocellular carcinoma: Comparison of dynamic three-phase computed tomography images and four-phase computed tomography images using multidetector row helical computed tomography. J Comput Assist Tomogr. 2002;26:691–8. doi: 10.1097/00004728-200209000-00005. [DOI] [PubMed] [Google Scholar]
- 48.Ng KKC, Poon RTP, Lo CM, et al. Impact of preoperative fine-needle aspiration cytologic examination on clinical outcome in patients with hepatocellular carcinoma in a tertiary referral center. Arch Surg. 2004;139:193–200. doi: 10.1001/archsurg.139.2.193. [DOI] [PubMed] [Google Scholar]
- 49.Caturelli E, Ghittoni G, Roselli P, De Palo M, Anti M. Fine needle biopsy of focal liver lesions: The hepatologist’s point of view. Liver Transpl. 2004;10:S26–9. doi: 10.1002/lt.20037. [DOI] [PubMed] [Google Scholar]
- 50.Bravo AA, Sunil G, Sheth G, Chopra S. Liver biopsy. N Engl J Med. 2001;344:495–500. doi: 10.1056/NEJM200102153440706. [DOI] [PubMed] [Google Scholar]
- 51.Piccinino F, Sagnelli E, Pasquale G, Giusti G. Complications following percutaneous liver biopsy. A multicentre retrospective study on 68,276 biopsies. J Hepatol. 1986;2:165–73. doi: 10.1016/s0168-8278(86)80075-7. [DOI] [PubMed] [Google Scholar]
- 52.Navarro F, Taourel P, Michel J, et al. Diaphragmatic and subcutaneous seeding of hepatocellular carcinoma following fine-needle aspiration biopsy. Liver. 1998;18:251–4. doi: 10.1111/j.1600-0676.1998.tb00161.x. [DOI] [PubMed] [Google Scholar]
- 53.Takamori R, Wong LL, Dang C, Wong L. Needle-tract implantation from hepatocellular cancer: is needle biopsy of the liver always necessary? Liver Transpl. 2000;6:67–72. doi: 10.1002/lt.500060103. [DOI] [PubMed] [Google Scholar]
- 54.Chapoutot C, Perney P, Fabre D, et al. [Needle-tract seeding after ultrasound-guided puncture of hepatocellular carcinoma. A study of 150 patients] Gastroenterol Clin Biol. 1999;23:552–6. [PubMed] [Google Scholar]
- 55.Frilling A, Broelsch CE. Letters to the Editor (Resection of hepatocellular carcinoma with out preoperative tumor biopsy) Ann Surg. 2002;235:604. doi: 10.1097/00000658-200204000-00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.John TG, Garden OJ. Needle track seeding of primary and secondary liver carcinoma after percutaneous liver biopsy. HPB Surg. 1993;6:199–203. doi: 10.1155/1993/39539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim SH, Lim HK, Lee WJ, Cho JM, Jang HJ. Needle-tract implantation in hepatocellular carcinoma: Frequency and CT findings after biopsy with a 19.5-gauge automated biopsy gun. Abdom Imaging. 2000;25:246–50. doi: 10.1007/s002610000025. [DOI] [PubMed] [Google Scholar]
- 58.Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Long-term survival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function: Implications for a strategy of salvage transplantation. Ann Surg. 2002;235:373–82. doi: 10.1097/00000658-200203000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lang BH, Poon RT, Fan ST, Wong J. Perioperative and long-term outcome of major hepatic resection for small solitary hepatocellular carcinoma in patients with cirrhosis. Arch Surg. 2003;138:1207–13. doi: 10.1001/archsurg.138.11.1207. [DOI] [PubMed] [Google Scholar]
- 60.Shimozawa N, Hanazaki K. Longterm prognosis after hepatic resection for small hepatocellular carcinoma. J Am Coll Surg. 2004;198:356–65. doi: 10.1016/j.jamcollsurg.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 61.Zhou XD, Tang ZY, Yang BH, et al. Experience of 1000 patients who underwent hepatectomy for small hepatocellular carcinoma. Cancer. 2001;91:1479–86. doi: 10.1002/1097-0142(20010415)91:8<1479::aid-cncr1155>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 62.Montorsi M, Santambrogio R, Bianchi P, et al. Survival and recurrences after hepatic resection or radiofrequency for hepatocellular carcinoma in cirrhotic patients: A multivariate analysis. J Gastrointest Surg. 2005;9:62–7. doi: 10.1016/j.gassur.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 63.Wu CC, Chen JT, Ho WL, et al. Liver resection for hepatocellular carcinoma in octogenarians. Surgery. 1999;125:332–8. [PubMed] [Google Scholar]
- 64.Grazi GL, Ercolani G, Pierangeli F, et al. Improved results of liver resection for hepatocellular carcinoma on cirrhosis give the procedure added value. Ann Surg. 2001;234:71–8. doi: 10.1097/00000658-200107000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Poon RT, Fan ST, Lo CM, et al. Improving survival results after resection of hepatocellular carcinoma: A prospective study of 377 patients over 10 years. Ann Surg. 2001;234:63–70. doi: 10.1097/00000658-200107000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Poon RT, Fan ST. Hepatectomy for hepatocellular carcinoma: Patient selection and postoperative outcome. Liver Transpl. 2004;10:S39–45. doi: 10.1002/lt.20040. [DOI] [PubMed] [Google Scholar]
- 67.Majno PE, Sarasin FP, Mentha G, Hadengue A. Primary liver resection and salvage transplantation or primary liver transplantation in patients with single, small hepatocellular carcinoma and preserved liver function: An outcome-oriented decision analysis. Hepatology. 2000;31:899–906. doi: 10.1053/he.2000.5763. [DOI] [PubMed] [Google Scholar]
- 68.Marin-Hargreaves G, Azoulay D, Bismuth H. Hepatocellular carcinoma: Surgical indications and results. Crit Rev Oncol Hematol. 2003;47:13–27. doi: 10.1016/s1040-8428(02)00213-5. [DOI] [PubMed] [Google Scholar]
- 69.Lo CM, Fan ST. Liver transplantation for hepatocellular carcinoma. Br J Surg. 2004;91:131–3. doi: 10.1002/bjs.4503. [DOI] [PubMed] [Google Scholar]
- 70.Llovet JM, Fuster J, Bruix J, Barcelona-Clinic Liver Cancer Group The Barcelona approach: Diagnosis, staging, and treatment of hepatocellular carcinoma. Liver Transpl. 2004;10:S115–20. doi: 10.1002/lt.20034. [DOI] [PubMed] [Google Scholar]
- 71.The Organ Procurement and Transplantation Network Waitlist removals: Organ by removal reason: January 1995 – February 29, 2004<www.optn.org> (Version current at June 25, 2007).
- 72.Krahn M, Wong JB, Heathcote J, Scully L, Seeff L. Estimating the prognosis of hepatitis C patients infected by transfusion in Canada between 1986 and 1990. Med Decis Making. 2004;24:20–9. doi: 10.1177/0272989X03261568. [DOI] [PubMed] [Google Scholar]
- 73.Chong CA, Gulamhussein A, Heathcote EJ, et al. Health-state utilities and quality of life in hepatitis C patients. Am J Gastroenterol. 2003;98:630–8. doi: 10.1111/j.1572-0241.2003.07332.x. [DOI] [PubMed] [Google Scholar]
- 74.Dusheiko GM, Roberts JA. Treatment of chronic type B and C hepatitis with interferon alfa: An economic appraisal. Hepatology. 1995;22:1863–73. [PubMed] [Google Scholar]
- 75.Tengs TO, Wallace A. One thousand health-related quality of life estimates. Med Care. 2000;38:583–637. doi: 10.1097/00005650-200006000-00004. [DOI] [PubMed] [Google Scholar]
- 76.Wells CD, Murrill WB, Arguedas MR. Comparison of health-related quality of life preferences between physicians and cirrhotic patients: Implications for cost-utility analyses in chronic liver disease. Dig Dis Sci. 2004;49:453–8. doi: 10.1023/b:ddas.0000020502.46886.c1. [DOI] [PubMed] [Google Scholar]
- 77.Bennett WG, Inoue Y, Beck JR, Wong JB, Pauker SG, Davis GL. Estimates of the cost-effectiveness of a single course of interferon-alpha 2b in patients with histologically mild chronic hepatitis C. Ann Intern Med. 1997;127:855–65. doi: 10.7326/0003-4819-127-10-199711150-00001. [DOI] [PubMed] [Google Scholar]
- 78.Kim WR, Poterucha JJ, Hermans JE, et al. Cost-effectiveness of 6 and 12 months of interferon-alpha therapy for chronic hepatitis C. Ann Intern Med. 1997;127:866–74. doi: 10.7326/0003-4819-127-10-199711150-00002. [DOI] [PubMed] [Google Scholar]
- 79.Thein HH, Krahn M, Kaldor JM, Dore GJ. Estimation of utilities for chronic hepatitis C from SF-36 scores. Am J Gastroenterol. 2005;100:643–51. doi: 10.1111/j.1572-0241.2005.40976.x. [DOI] [PubMed] [Google Scholar]
- 80.Younossi ZM, Boparai N, McCormick M, Price LL, Guyatt G. Assessment of utilities and health-related quality of life in patients with chronic liver disease. Am J Gastroenterol. 2001;96:579–83. doi: 10.1111/j.1572-0241.2001.03537.x. [DOI] [PubMed] [Google Scholar]
- 81.Wong JB, Koff RS. Watchful waiting with periodic liver biopsy versus immediate empirical therapy for histologically mild chronic hepatitis C. A cost-effectiveness analysis. Ann Intern Med. 2000;133:665–75. doi: 10.7326/0003-4819-133-9-200011070-00008. [DOI] [PubMed] [Google Scholar]
- 82.Salomon JA, Weinstein MC, Hammitt JK, Goldie SJ. Cost-effectiveness of treatment for chronic hepatitis C infection in an evolving patient population. JAMA. 2003;290:228–37. doi: 10.1001/jama.290.2.228. [DOI] [PubMed] [Google Scholar]
- 83.Sarasin FP, Majno PE, Llovet JM, Bruix J, Mentha G, Hadengue A. Living donor liver transplantation for early hepatocellular carcinoma: A life-expectancy and cost-effectiveness perspective. Hepatology. 2001;33:1073–9. doi: 10.1053/jhep.2001.23311. [DOI] [PubMed] [Google Scholar]
- 84.Krahn MD, Naglie G, Naimark D, Redelmeier DA, Detsky A. Primer on medical decision analysis: Part 4 – analyzing the model and interpreting the results. Med Decis Making. 1997;17:142–51. doi: 10.1177/0272989X9701700204. [DOI] [PubMed] [Google Scholar]
- 85.Doubilet P, Begg CB, Weinstein MC, Braun P, McNeil BJ. Probabilistic sensitivity analysis using Monte Carlo simulation. A practical approach. Med Decis Making. 1985;5:157–77. doi: 10.1177/0272989X8500500205. [DOI] [PubMed] [Google Scholar]
- 86.Vauthey JN, Lauwers GY, Esnaola NF, et al. Simplified staging for hepatocellular carcinoma. J Clin Oncol. 2002;20:1527–36. doi: 10.1200/JCO.2002.20.6.1527. [DOI] [PubMed] [Google Scholar]
- 87.Vauthey JN, Lauwers GY. Prognostic factors after resection of hepatocellular carcinoma: Are there landmarks in the wild forest? J Hepatol. 2003;38:237–9. doi: 10.1016/s0168-8278(02)00407-5. [DOI] [PubMed] [Google Scholar]
- 88.Llovet JM, Mas X, Aponte JJ, et al. Cost effectiveness of adjuvant therapy for hepatocellular carcinoma during the waiting list for liver transplantation. Gut. 2002;50:123–8. doi: 10.1136/gut.50.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jaeck D, Bachellier P, Oussoultzoglou E, Weber JC, Wolf P. Surgical resection of hepatocellular carcinoma. Post-operative outcome and long-term results in Europe: An overview. Liver Transpl. 2004;10:S58–63. doi: 10.1002/lt.20041. [DOI] [PubMed] [Google Scholar]
- 90.Barbara L, Benzi G, Gaiani S, et al. Natural history of small untreated hepatocellular carcinoma in cirrhosis: A multivariate analysis of prognostic factors of tumor growth and patient survival. Hepatology. 1992;16:132–7. doi: 10.1002/hep.1840160122. [DOI] [PubMed] [Google Scholar]
- 91.Cottone M, Virdone R, Fusco G, et al. Asymptomatic hepatocellular carcinoma in Child’s A cirrhosis. A comparison of natural history and surgical treatment. Gastroenterology. 1989;96:1566–71. doi: 10.1016/0016-5085(89)90528-3. [DOI] [PubMed] [Google Scholar]
- 92.Ebara M, Ohto M, Shinagawa T, et al. Natural history of minute hepatocellular carcinoma smaller than three centimeters complicating cirrhosis. A study in 22 patients. Gastroenterology. 1986;90:289–98. doi: 10.1016/0016-5085(86)90923-6. [DOI] [PubMed] [Google Scholar]
- 93.Richardson WS, Detsky AS. Users’ guides to the medical literature. VII. How to use a clinical decision analysis. B. What are the results and will they help me in caring for my patients? Evidence Based Medicine Working Group. JAMA. 1995;273:1610–3. doi: 10.1001/jama.273.20.1610. [DOI] [PubMed] [Google Scholar]
- 94.Chen MS, Li JQ, Zheng Y, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243:321–8. doi: 10.1097/01.sla.0000201480.65519.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cheng SJ, Pratt DS, Freeman RB, Jr, Kaplan MM, Wong JB. Living-donor versus cadaveric liver transplantation for non-resectable small hepatocellular carcinoma and compensated cirrhosis: A decision analysis. Transplantation. 2001;72:861–8. doi: 10.1097/00007890-200109150-00021. [DOI] [PubMed] [Google Scholar]
- 96.El-Serag HB, Mallat DB, Rabenack L. Management of the single liver nodule in a cirrhotic patient: A decision analysis model. J Clin Gastroenterol. 2005;39:152–9. [PubMed] [Google Scholar]
- 97.Bremner K, Bayoumi A, Sherman M, Krahn M. Biopsy or resection for single small liver nodules in patients with compensated cirrhosis – A decision analysis; 26th Annual Meeting of the Society for Medical Decision Making; Atlanta. October 17 to 20, 2004. [Google Scholar]
- 98.Llovet JM, Bustamante J, Castells A, et al. Natural history of untreated nonsurgical hepatocellular carcinoma: Rationale for the design and evaluation of therapeutic trials. Hepatology. 1999;29:62–7. doi: 10.1002/hep.510290145. [DOI] [PubMed] [Google Scholar]
- 99.Kubota K, Ina H, Okada Y, Irie T. Growth rate of primary single hepatocellular carcinoma: Determining optimal screening interval with contrast enhanced computed tomography. Dig Dis Sci. 2003;48:581–6. doi: 10.1023/a:1022505203786. [DOI] [PubMed] [Google Scholar]
- 100.Okazaki N, Yoshino M, Yoshida T, et al. Evaluation of the prognosis for small hepatocellular carcinoma based on tumor volume doubling time. A preliminary report. Cancer. 1989;63:2207–10. doi: 10.1002/1097-0142(19890601)63:11<2207::aid-cncr2820631124>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 101.Sheu JC, Sung JL, Chen DS, et al. Growth rate of asymptomatic hepatocellular carcinoma and its clinical implications. Gastroenterology. 1985;89:259–66. doi: 10.1016/0016-5085(85)90324-5. [DOI] [PubMed] [Google Scholar]
- 102.Lin OS, Keeffe EB, Sanders GD, Owens DK. Cost-effectiveness of screening for hepatocellular carcinoma in patients with cirrhosis due to chronic hepatitis C. Aliment Pharmacol Ther. 2004;19:1159–72. doi: 10.1111/j.1365-2036.2004.01963.x. [DOI] [PubMed] [Google Scholar]
- 103.Wong JB, Bennett WG, Koff RS, Pauker SG. Pretreatment evaluation of chronic hepatitis C. Risks, benefits, and costs. JAMA. 1998;280:2088–93. doi: 10.1001/jama.280.24.2088. [DOI] [PubMed] [Google Scholar]