Abstract
Objective
To determine the correlation between parental and offspring birthweight (BW) in a developing country like India.
Methods
The study involved two birth cohorts of successive generations. The parental cohort comprised of 472 fathers and 422 mothers from an earlier study. Details of their anthropometry at birth and in adulthood were available. 1525 children born to them comprised the offspring cohort. BW was obtained from hospital records for the offspring cohort. Odds ratios and regression coefficients were calculated to estimate the risks of a low birth weight (LBW) parent producing a LBW baby and quantitate the effects after adjusting for confounders.
Results
A LBW mother had a 2.8 times risk (95%CI 1.2 - 6.4) of delivering a LBW baby (p=0.02) and a LBW father was twice as likely to produce a LBW baby (OR 2.2; 95%CI 1.0 - 4.8; p=0.05). Every 100g increase in maternal BW was associated with an increase in offspring BW of 14g; the equivalent figure for paternal BW was 18.1g (p<0.001 for both). Between the generations, the incidence of LBW decreased from 19.7% to 17.2% (p=0.1). Mean BW increased in males (2846 g v 2861 g; p=0.59) but not in females (2790 g v 2743 g; p=0.08).
Conclusion
Both maternal and paternal BW are strong determinants of offspring BW. The effect of mothers’ BW on offspring BW is weaker than that seen in developed nations. Stronger intrauterine constraint exhibited by Indian women secondary to a higher prevalence of growth restriction in utero may be responsible. Paternal effects may be governed by paternal genes inherited by the offspring.
Keywords: Intergenerational study, low birth weight, birth cohort
INTRODUCTION
LBW is a major health problem in India, affecting 30% of births. Though not a disease by itself, yet its ramifications are vast, with major influence on neonatal and infant survival as well as under-five and long term morbidity. In India, intrauterine growth restriction is considered the main causal factor1 and is attributed to chronic maternal malnutrition and stunting2. In the post-independence era, improvements in obstetric practices, health care, maternal nutrition and socio-economic conditions may have a role in bringing down the incidence of LBW babies. Still, much has to be done to improve BW in India. The first step is to examine the determinants of LBW, so that new factors can be identified and addressed accordingly.
Intergenerational studies, mainly carried out in western populations, have investigated relationships between parental (mainly maternal) BW and that of the offspring.3-21 Only one such study has been published from an Indian population.22 A high correlation was found between maternal and offspring BW to the tune of an increment in birth weight of 15 - 30 g for every 100 g increase in maternal BW. This was explained in terms of heritable maternal genes, similar intrauterine experiences from grandmother to granddaughter through the mother and external environmental factors affecting general health and nutrition. Paternal BW as a determinant of offspring BW has been less well studied.6-11 Paternal and fetal genes regulating intrauterine growth have been described.23-27 Therefore a father could also regulate fetal growth, depending on the regulation of genetic expression by intrauterine environment. This study was undertaken to assess the influence of maternal and paternal BW on offspring BW in India.
MATERIAL AND METHODS
Study design
Intergenerational study involving two birth cohorts.
Period of study
2002 - 2004.
Subjects
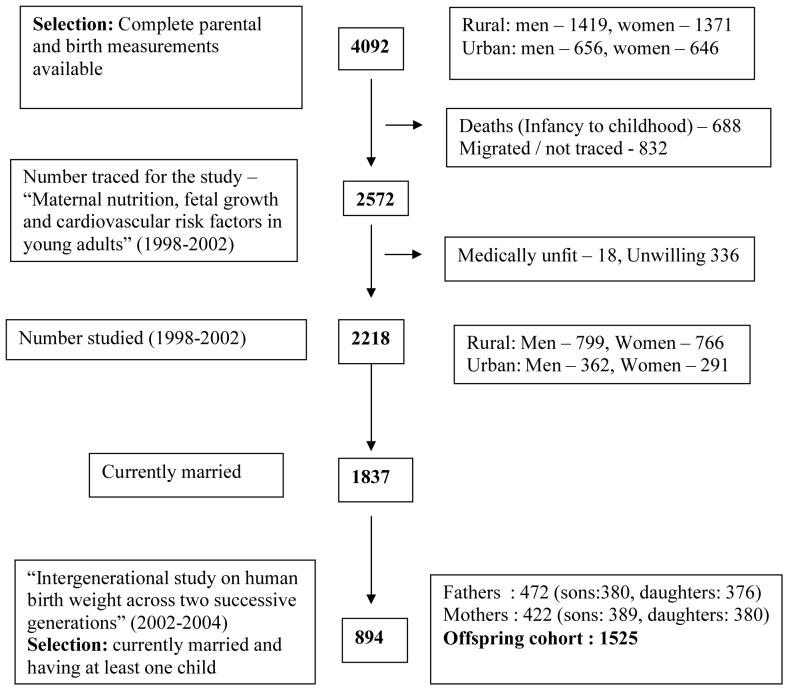
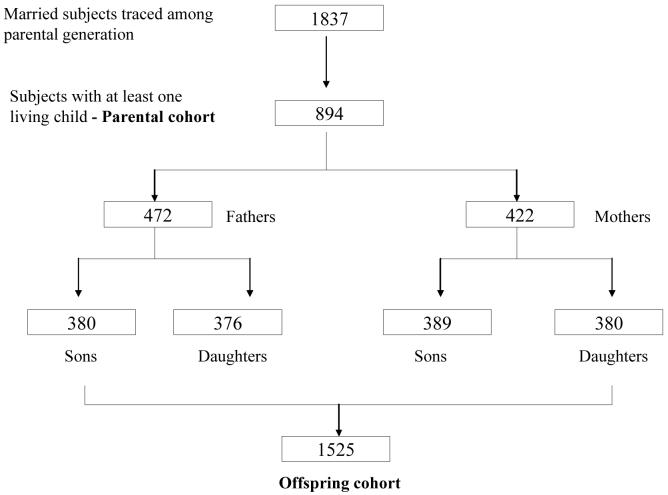
The present study involved two cohorts of successive generations - a parental cohort and an offspring cohort. The parents were drawn from the original cohort which formed the core population for ‘longitudinal studies in human reproduction’, reported in earlier papers.28-30 A sample of 5 localities from a total of 15 in Vellore town representing low, middle and high socio-economic strata were randomly chosen. 25 geographically contiguous villages were selected from a total of 41 villages representing the rural area in the K.V. Kuppam development block in the North Arcot (now Vellore) district of Tamilnadu. All the women in the reproductive age group were recruited and all the babies born to these women during 1969-73 were included for the earlier research studies. Figure 1 provides the details of the parental cohort. 4,092 subjects from this original birth cohort who had complete parental measurements, anthropometry at birth, and longitudinal growth assessment till adolescence recorded in their homes by trained health workers were traced for another research study undertaken during 1998-2002 (“Maternal nutrition, fetal growth and cardiovascular risk factors in young adults” - submitted for publication”). 1,837 were currently married. None had married within the cohort. Hence early life data for both members of a married couple were unavailable and either fathers or mothers with birth data, now having children were studied. Current height and weight of the spouses were recorded prospectively. There were two groups of children, one in whom only maternal data was available and one with paternal data alone (Figure 2).
Figure 1. Flow chart showing the parental cohort derived from earlier studies.
Fig.2. Flow chart showing recruitment of subjects.
Multifetal pregnancy, stillbirth, early infant death, adoptions were excluded
Detailed information was available for the parental cohort from the previous study with regards to anthropometry at birth as well as current anthropometry, SES and parity. SES score was calculated based on 11 factors (education, occupation, type of housing, number of houses owned, number of rooms, number of people per room, water and sanitation, material possessions, farm, land and animals owned, agricultural land ownership, farm equipment ownership).
During the present study, for the offspring data, details of the children such as date of birth, antenatal medical problems, birth order, gestational age and birth weight were obtained from the medical records of hospitals where the equipments are precise, staff are trained and supervised, good records are maintained and available and where a reasonable standard of care is practised. A reliable gestational age was available for only 948 offspring (62%). Length at birth and head circumference were scarcely available for the offspring cohort, and therefore these data could not be used for analysis. Current anthropometry of the children was collected prospectively.
Definitions
LBW was defined as a BW less than 2500 g.
Prematurity was defined as a gestation of less than 37 completed weeks.
Statistical methods
Statistical analysis was carried out using statistical package for social sciences (SPSS) - Version 11.5 and STATA version 8.0. All birth and adult measurements were normally distributed and were used after categorization and also as continuous variables where indicated. Parental BW groups were related to offspring birth size groups using chi square tests. Associations between parent and offspring BWs were examined using linear and logistic regression with and without adjustment for parental adult height, body mass index (BMI), SES score, parity, sex of offspring and age. Random effects generalized least square (GLS) model was used to address the situation where a family had more than one child. This allowed us to include all the offspring born to a parent in the analysis (41.4% of the families had more than one offspring).
RESULTS
Birth weights of parents who were studied (894) was not significantly different from those of the parents who were not studied (3198) - mean birth weights of mothers studied (n=422) and not studied (n=1592) were 2790 (SD 444)g and 2749 (SD 528)g respectively (p=0.14); mean birth weights of fathers studied (n=472) and not studied (1606) were 2846 (SD 489)g and 2824 (SD 565)g respectively (p=0.44).
Two thirds of the families studied hailed from rural areas. In both generations, girls weighed less than boys at birth (Table 1). The mean BW remained unchanged between the two generations (parental cohort 2820 g, offspring cohort 2803 g). The incidence of LBW babies had decreased from 19.7% to 17.2% (p=0.1) between the two generations and the trend was more evident among boys (Table 1). As compared to their fathers or mothers, the sons had a higher mean BW and were less likely to be of LBW. On the other hand daughters were likely to be lighter than their mothers at birth though they were less likely to be of LBW. Parents of higher birth weight were taller as adults. For every Kg increase in birth weight, the adult height of the father increased by 2.45 cm [95% CI: 1.5-3.4] and a mother’s height by 2.04 cm [95%CI: 1.2-2.9] (p <0.001 for both).
Table 1. Baseline characteristics of parents and offspring.
| Mothers |
Daughters |
Sons |
||||
|---|---|---|---|---|---|---|
| n | mean(SD) | n | mean (SD) | n | mean (SD) | |
| Gestational age(wks) | --- | --- | 230 | 39.1(2.24) | 232 | 39.3(1.65) |
| Birth weight (g) | 422 | 2790(444) | 380 | 2716(438) | 389 | 2865(438) |
| Adult height (cm) | 422 | 153.9(5.47) | ||||
| Adult weight (kg) | 422 | 50.3(10.49) | ||||
| BMI (kg/m2) | 422 | 21.2(4.26) | ||||
| Low birth weight [%] | 89 [21.1%] | 73 [19.2%] | 58 [14.9%] | |||
| Fathers |
Daughters |
Sons |
||||
|---|---|---|---|---|---|---|
| n | mean(SD) | n | mean (SD) | n | mean (SD) | |
| Gestational age(wks) | --- | --- | 238 | 39.4(2.17) | 248 | 38.8(2.18) |
| Birth weight (g) | 472 | 2846(489) | 376 | 2771(479) | 380 | 2857(498) |
| Adult height (cm) | 472 | 166.5(6.41) | ||||
| Adult weight (kg) | 472 | 58.5(11.91) | ||||
| BMI (kg/m2) | 472 | 21.0(3.64) | ||||
| Low birth weight [%] | 88 [18.6%] | 76 [20.2%] | 56 [14.7%] | |||
Influence of maternal characteristics on offspring BW
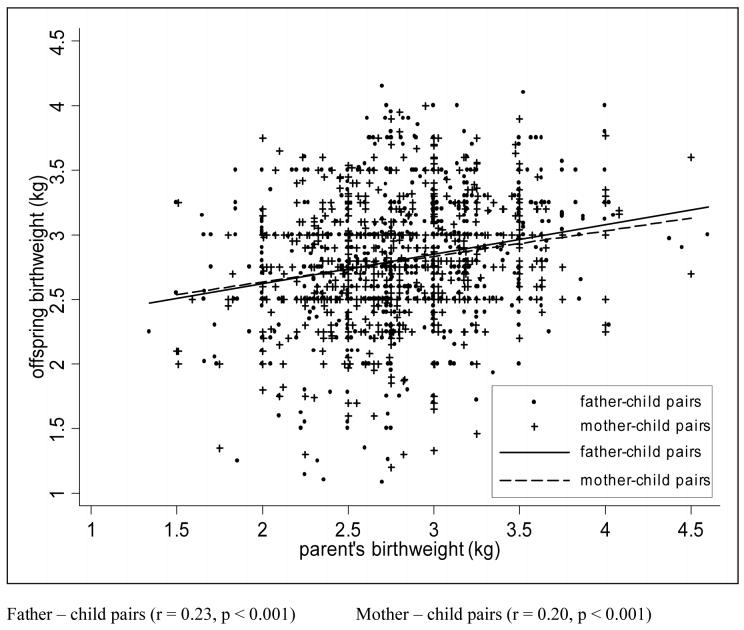
The mean BW of mothers producing LBW offspring was 186 g lower than that of the mothers who produced babies of normal BW (p<0.001). The unadjusted odds ratio revealed that a LBW mother was 3.5 [95%CI: 1.5-8.0] times more likely to produce a LBW baby compared to a woman weighing >3000 g at birth (p <0.001). After adjusting for adult BMI, adult height, SES score, parity and sex of the infant, it was found that a LBW mother had a 2.8 times risk of bearing a LBW baby as compared to a mother who weighed >3000 g at birth (p=0.02; Table 2). In this model, low maternal height was also found to be an independent risk factors for producing a LBW baby (p=0.02) while maternal age, parity, sex of the infant and SES score had no significant association with offspring BW. When birth weight was used as a continuous dependent variable, maternal BW was positively related to offspring BW (20.5g per 100g increment in maternal BW, p<0.001; Fig 2). This effect was slightly blunted after adjusting for maternal adult BMI, height, parity, sex of the infant and SES score where every 100 g increase in maternal BW was associated with an increase in offspring BW of 14.0 g (p<0.001; Table 3). Adult height (p=0.002) and BMI (p<0.001) were also positively related to the BW of the offspring.
Table 2. Random effects logistic regression showing risk of producing a low birth weight offspring.
| Parental * Birth weight (g) | Effects of father (n = 472) |
Effects of mother (n = 422) |
||||
|---|---|---|---|---|---|---|
| Odds ratio | 95%CI | p value | Odds ratio | 95%CI | p value | |
| < 2500 | 2.19 | [1.0-4.8] | 0.05 | 2.76 | [1.2-6.4] | 0.02 |
| 2500 - 3000 | 1.75 | [0.9-3.3] | 0.09 | 2.19 | [1.1-4.6] | 0.03 |
Adjusted for sex of the offspring, parity of the mother, BMI, adult height and SES score of parents
Reference parental birth weight >3000 g
Table 3. Random effects generalized least square regression (regression coefficient β) giving a measure of parental effects on offspring birth weight.
| Parental characteristics | Effects of father (n = 472) |
Effects of mother (n = 422) |
||||
|---|---|---|---|---|---|---|
| Offspring BW (g) | 95%CI | p value | Offspring BW(g) | 95%CI | p value | |
| Birth weight (kg) | 181.4 | [100.7-262.0] | <0.001 | 139.6 | [64.2-214.9] | <0.001 |
| Adult height (cm) | 9.0 | [2.7-15.4] | <0.001 | 9.8 | [3.5-16.0] | 0.002 |
| BMI (kg/m2) | 14.2 | [3.5-24.9] | 0.001 | 16.8 | [8.9-24.5] | <0.001 |
Adjusted for sex of the offspring, parity of the mother and SES
Influence of paternal characteristics on offspring birth weight
A similar relationship was found between the BW of fathers and their offspring. LBW fathers went on to become shorter and lighter as adults (p<0.001). The mean BW of fathers producing LBW babies was 238 g less than that of fathers producing normal BW babies (p=0.001). The unadjusted odds ratio showed that a LBW father had a 2.6 [95%CI: 1.4-5.5] times risk of producing a LBW infant as compared to a father weighing >3000 g at birth (p=0.01). After adjusting for sex of the infant, paternal age, SES, adult height and BMI, the odds ratio was 2.2 (p=0.05; Table 2). Paternal BW was positively related to offspring BW (23.1g per 100g increment in paternal BW, p<0.001; Fig 3). After adjusting for paternal adult BMI, height, age, parity of spouse, sex of the infant and SES score, a 100 g increment in paternal BW was associated with an 18 g increment in the offspring BW (p<0.001; Table 3). Paternal SES score and age were unrelated to offspring BW. In this model paternal height (p=0.01) and BMI (p=0.01) were also positively related to offspring BW.
Fig. 3. Correlation between parental and offspring birthweight.
Accurate gestational age at birth was not available for 38% of the offspring hence the BWs could not be adjusted for this in the analysis. When a sub analysis was done after adjusting for gestational age for the 62% of the offspring for whom gestational age was known, the parental effects on offspring BW were similar.
DISCUSSION
While intergenerational effects on human birth weight have been extensively studied in developed nations3-16,18-21, there is a paucity of data from developing countries17,22. Strengths of our study were a large sample size, birth data were collected prospectively for the parental cohort (for whom birth measurements were made by trained research staff), birth data of the offspring were obtained only from reliable hospital records (rather than recall) and we had good data on potential confounding factors such as parental age, height, weight, sex of infant, birth order and SES. We were able to examine paternal effects while most previous studies did not address the issue. Weaknesses of the study were a lack of gestational age data, and the fact that we did not have birth measurements for both members of the married couple in the parent’s generation. Hence it was not possible to compare both paternal and maternal effects in determining a given offspring’s BW, or to adjust for assortative mating (people tending to marry people of similar size and from the same social background).
The incidence of LBW among the rural population near Vellore lies between 16-18%. In our own study extending over the past 30 years, the prevalence of LBW has decreased from 19.7% to 17.2% between the generations. This is a welcome change though a disappointingly small improvement. It was, however, interesting to note that mean BW did not improve as expected (parental cohort 2820 g and offspring cohort 2803 g). Among girls the mean BW had actually decreased. A similar effect was seen by Ramakrishnan et al in Guatemala where LBW rates increased from 5.1% to 13.5% and mean BW decreased by 90g between generations.17 In contrast a small increment in mean BW of 121 g was observed in the Mysore study.22 Most of the studies from the western world have shown an increment in mean BW between generations.3, 4, 8-10, 12 This phenomenon of decreasing mean BW may be explained by the fact that, a) of late, in developing countries, more LBW babies are surviving and/or b) that birth weights in the parent’s generation were falsely high because only the heavier babies at that time survived to reach reproductive age. A similar analogy can be provided for the static mean BWs.
In our study maternal BW emerged as a significant predictor of offspring BW. After adjusting for confounders, a LBW mother was at a 2.8 times greater risk of giving birth to a LBW baby than a mother who weighed more than 3000g at birth. An increase of 100g in maternal BW in one generation was associated with an increase in offspring BW of 14g in the next generation. Previous western studies and the study in Mysore have shown an increment of 15-30g.3-22 The unimpressive maternal effects on offspring BW in Vellore may be explained by the high prevalence of intrauterine growth restriction in our population. There is an intergenerational cycle of growth failure such that young girls who grow poorly become stunted women and are more likely to give birth to LBW babies.17 The ultimate determinant of intrauterine growth in these settings is probably maternal intrauterine constraint. The effect of a better extra uterine environment and good paternal and fetal genes can only improve the intrauterine growth if the intrauterine constraint is removed.
Until recently it was believed that genetic factors had little influence on BW and that the maternal intrauterine environment had the predominant role in governing offspring BW. This was based on findings that intergenerational effects on BW seem to pass through the maternal and not the paternal line.7, 31-33 A mother can theoretically influence her baby’s BW both through the genes she passes to her fetus and the intrauterine environment that she provides. The effect of maternal genetic influence was questioned by observations that babies born after ovum donation correlated strongly with the ovum recipients rather than the donors with respect to BW.34 Experiments on horses by Walton and Hammond35 also had similar observations and so did others who experimented on domestic animals.35, 36 They opined that somatic growth was regulated chiefly by uterine size and the intrauterine milieu.
Our study showed that paternal BW is a strong predictor of offspring BW. The father’s contribution is mainly genetic6-11,22 ,although it must be remembered that the father also contributes to the environment of the mother, economically and materially, in ways that may not be captured by relatively crude measures of SES. After adjusting for confounding factors, it was found that a LBW father was at a 2.2 times greater risk of begetting a LBW offspring than a father weighing >3000 g at birth. Every 100g increase in the BW of a father was associated with an increase in the BW of his progeny by 18 g. This effect is on a par with western populations despite the higher maternal intrauterine constraint prevalent in India. In the Mysore study this increment was found to be 25 g.
Thus, both parental and fetal genes as well as the intrauterine environment probably influence BW, but the proportion of influence that each factor exerts is debatable. The non-genetic effect probably works in one of the following three ways:
Fetal undernutrition among female fetuses may lead to poor growth and vascularity of reproductive organs in utero and impaired placentation subsequently when they reproduce as adults. This in turn leads to another generation of growth failure. 26
Mother who has a good environment in utero is well nourished and grows taller and heavier as adults and this improves the BW of her own offspring.
A grandmother may have belief systems (e.g. restricting weight during pregnancy), which she passes on to her daughter thus causing generations of growth failure.
Any genetic influence on BW is probably not amenable to public health intervention but improving maternal nutrition, socioeconomic status and education may positively influence the ‘non genetic’ heritable factors (intrauterine nutrition and environment) and this may improve BWs over subsequent generations.
Acknowledgements
We wish to thank the subjects of the study, the field workers and British Heart Foundation for partial funding of this study.
Abbreviations
- LBW
low birth weight
- BW
birth weight
- SES
socio economic status
REFERENCES
- 1.Villar J, Belizan JM. The timing factor in the pathophysiology of the IUGR syndrome. Obs Gyn Sur. 1982;37:499–506. doi: 10.1097/00006254-198208000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Gopalan C. LBWs: Significance and implications. In: Sachdev HPS, Choudhary P, editors. Nutrition in children: developing country concerns. Imprint; New Delhi: 1994. pp. 1–33. [Google Scholar]
- 3.Hackman E, Emanuel I, Van Belle G, Daling J. Maternal BW and subsequent pregnancy outcome. JAMA. 1983;250:2016–2019. [PubMed] [Google Scholar]
- 4.Klebanoff MA, Graubard BI, Kessel SS, Berendes HW. LBW across generations. JAMA. 1984;252:2423–2427. [PubMed] [Google Scholar]
- 5.Magus P, Berg K, Bjerkedal T. No significant difference in BW for offspring of discordant monozygotic female twins. Early Hum Devp. 1985;12:55–59. doi: 10.1016/0378-3782(85)90137-9. [DOI] [PubMed] [Google Scholar]
- 6.Little RE. Mother’s and Father’s BW as predictors of infant BW. Pediatr Perinat Epidemiol. 1987;1:19–31. doi: 10.1111/j.1365-3016.1987.tb00084.x. [DOI] [PubMed] [Google Scholar]
- 7.Emanuel I, Filakti H, Alberman E, Evans SJW. Intergenerational studies of human BW from 1958 birth cohort. I. Evidence for a multigenerational effect. Br J Obs Gyn. 1992;99:67–74. doi: 10.1111/j.1471-0528.1992.tb14396.x. [DOI] [PubMed] [Google Scholar]
- 8.Alberman E, Emanuel I, Filakti H, Evans SWJ. The contrasting effects of parental BW and gestational age on the BW of offspring. Pediatr Perinat Epidemiol. 1992;6:134–144. doi: 10.1111/j.1365-3016.1992.tb00755.x. [DOI] [PubMed] [Google Scholar]
- 9.Godfrey KM, Barker DJP, Robinson S, Osmond C. Maternal BW and diet in pregnancy in relation to infant’s thinness at birth. Br J Obs Gyn. 1997;104:663–667. doi: 10.1111/j.1471-0528.1997.tb11975.x. [DOI] [PubMed] [Google Scholar]
- 10.Klebanoff MA, Mednick BR, Schulsinger C, Sicher NJ, Shiono PH. Father’s effect on infant BW. Am J Obs Gyn. 1998;178:1022–1026. doi: 10.1016/s0002-9378(98)70542-3. [DOI] [PubMed] [Google Scholar]
- 11.Magnus P, Gjessing HK, Skrondal A, Skjaerven R. Paternal contribution to BW. J Epidemiol Com Health. 2001;55:873–877. doi: 10.1136/jech.55.12.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carr-Hill R, Campbell EM, Hall MH, Meredith A. Is BW determined genetically? BMJ. 1987;295:687–689. doi: 10.1136/bmj.295.6600.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klebanoff MA, Meirik O, Berendes HW. Second generation consequences of small-for-dates birth. Pediatrics. 1989;84:343–347. [PubMed] [Google Scholar]
- 14.Magnus P, Bakketeig LS, Skjaerven R. Correlation of BW and gestational age across generations. Ann Hum Bio. 1993;20:231–238. doi: 10.1080/03014469300002662. [DOI] [PubMed] [Google Scholar]
- 15.Sanderson M, Emanuel I, Holt VL. The intergenerational relationship between mother’s BW, infant BW and infant mortality in black and white mothers. Pediatr Perinat Epidemiol. 1995;9:391–405. doi: 10.1111/j.1365-3016.1995.tb00162.x. [DOI] [PubMed] [Google Scholar]
- 16.Skjaerven R, Wilcox AJ, Oyen N, Magnus P. Mother’s BW and survival of their offspring: Population based study. BMJ. 1997;314:1376–1380. doi: 10.1136/bmj.314.7091.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramakrishnan V, Martorell R, Schroeder DG, Flores R. Role of intergenerational effects on linear growth. J Nutr Suppl. 1999;129:5445–5495. doi: 10.1093/jn/129.2.544S. [DOI] [PubMed] [Google Scholar]
- 18.Langhoff-Roos J, Lindmark G, Gustavson KH, Gebre-Medhin M, Merik O. Relative effect of parental BW on infant BW at term. Clin Genet. 1987;32:240–248. doi: 10.1111/j.1399-0004.1987.tb03307.x. [DOI] [PubMed] [Google Scholar]
- 19.Coutinho R, David RJ, Lokins JW. Relation of parental BW to infant BW among African Americans and Whites in Illinois, A transgenerational study. Am J Epidemiol. 1997;146:804–809. doi: 10.1093/oxfordjournals.aje.a009197. [DOI] [PubMed] [Google Scholar]
- 20.Tavares M, Rodrigues T, Cardoso F, Barros H, Leite LP. Independent effect of maternal BW on infant BW. J Perinat Med. 1996;24:391–396. doi: 10.1515/jpme.1996.24.4.391. [DOI] [PubMed] [Google Scholar]
- 21.Klebanoff MA, Yip R. Influence of maternal BW on rate of fetal growth and duration of gestation. J Pediatr. 1987;111:2287–2292. doi: 10.1016/s0022-3476(87)80089-6. [DOI] [PubMed] [Google Scholar]
- 22.Veena SR, Kumaran K, Swarnagowri MN, Jayakumar MN, Leary SD, Fall CHD, et al. Intergenerational effects on size at birth in South India. Pediatr Perinat Epidemiol. 2004;18:361–370. doi: 10.1111/j.1365-3016.2004.00579.x. [DOI] [PubMed] [Google Scholar]
- 23.Dunger DB, Ong KKL, Huxtable SJ, Sherif A, Woods KA, et al. Association of the INS VNTR with size at birth. Nat Genet. 1998;19:98–100. doi: 10.1038/ng0598-98. [DOI] [PubMed] [Google Scholar]
- 24.Frayling TM, Hattersley AT. The role of genetic susceptibility in the association of LBW with type 2 diabetes. Br Med Bull. 2001;60:89–101. doi: 10.1093/bmb/60.1.89. [DOI] [PubMed] [Google Scholar]
- 25.Vaessen N, Janssen JA, Heutink P, Hofman A, Lawberts SWJ, et al. Association between genetic variation in the gene for insulin-like growth factor-I and LBW. Lancet. 2002;359:1036–1037. doi: 10.1016/s0140-6736(02)08067-4. [DOI] [PubMed] [Google Scholar]
- 26.Hau JG. Genomic imprinting: review and relevance to human disease. Am J Hum Genet. 1990;46:857–873. [PMC free article] [PubMed] [Google Scholar]
- 27.De Chiara TM, Robertson EJ, Efstratiadis A. Parental imprinting of the mouse insulin-like growth factor II gene. Cell. 1991;64:849–859. doi: 10.1016/0092-8674(91)90513-x. [DOI] [PubMed] [Google Scholar]
- 28.Rao PSS, Inbaraj SG. Inbreeding effects in human reproduction in Tamilnadu of south India. Ann Hum Genet. 1977;41:87–98. doi: 10.1111/j.1469-1809.1977.tb01964.x. [DOI] [PubMed] [Google Scholar]
- 29.Rao PSS, Inbaraj SG. Inbreeding effects of fertility and sterility in southern India. J Med Genet. 1979;16:24–31. doi: 10.1136/jmg.16.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rao PSS, Inbaraj SG. Inbreeding effects on fetal growth and development. J Med Genet. 1980;17:27–33. doi: 10.1136/jmg.17.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fisher RA. The correlation between relations on the supposition of mendelian inheritance. Trans R Soc Edin. 1918;52:399–404. [Google Scholar]
- 32.Morton NE. The inheritance of human BW. Ann Hum Genet. 1955;20:123–134. doi: 10.1111/j.1469-1809.1955.tb01362.x. [DOI] [PubMed] [Google Scholar]
- 33.Qunsted M, Scott A, Ounsted C. Transmission through the female line of a mechanism constraining human fetal growth. Ann Hum Biol. 1986;13:143–157. doi: 10.1080/03014468600008281. [DOI] [PubMed] [Google Scholar]
- 34.Books AA, Johnson MR, Steer PJ, Pawson ME, Abdalla HI. BW nature or nurture? Early Hum Dev. 1995;42:29–35. doi: 10.1016/0378-3782(95)01637-i. [DOI] [PubMed] [Google Scholar]
- 35.Walton A, Hammond J. The maternal effects on growth and conformation in Shire horse - Shetland pony crosses. Proc R Soc Lond B Biol Sci. 1938;124:311–335. [Google Scholar]
- 36.Lush JL, Hetzer HO, Cellbertson CC. Factors affecting BW of swine. Genetics. 1934;19:329–343. doi: 10.1093/genetics/19.4.329. [DOI] [PMC free article] [PubMed] [Google Scholar]