Abstract
Dexmedetomidine is a highly specific, potent and selective α2-adrenoceptor agonist. Although intrathecal and epidural administration of dexmedetomidine has been found to produce analgesia, whether this analgesia results from an effect on spinal cord substantia gelatinosa (SG) neurons remains unclear. Here, we investigated the effects of dexmedetomidine on postsynaptic transmission in SG neurons of rat spinal cord slices using the whole-cell patch-clamp technique. In 92% of the SG neurons examined (n= 84), bath-applied dexmedetomidine induced outward currents at −70 mV in a concentration-dependent manner, with the value of effective concentration producing a half-maximal response (0.62 μm). The outward currents induced by dexmedetomidine were suppressed by the α2-adrenoceptor antagonist yohimbine, but not by prazosin, an α1-, α2B- and α2C-adrenoceptor antagonist. Moreover, the dexmedetomidine-induced currents were partially suppressed by the α2C-adrenoceptor antagonist JP-1302, while simultaneous application of JP-1302 and the α2A-adrenoceptor antagonist BRL44408 abolished the current completely. The action of dexmedetomidine was mimicked by the α2A-adrenoceptor agonist oxymetazoline. Plots of the current–voltage relationship revealed a reversal potential at around −86 mV. Dexmedetomidine-induced currents were blocked by the addition of GDP-β-S [guanosine-5′-O-(2-thiodiphosphate)] or Cs+ to the pipette solution. These findings suggest that dexmedetomidine hyperpolarizes the membrane potentials of SG neurons by G-protein-mediated activation of K+ channels through α2A- and α2C-adrenoceptors. This action of dexmedetomidine might contribute, at least in part, to its antinociceptive action in the spinal cord.
Keywords: α2-adrenoceptor, analgesia, dexmedetomidine, dorsal horn, substantia gelatinosa, whole-cell patch-clamp
Introduction
α2-Adrenoceptor agonists mediate a number of physiological phenomena such as antinociception (Yaksh, 1985). They produce an antinociceptive effect by action on the locus ceruleus (Guo et al., 1996) and spinal cord (Yaksh, 1985; Takano & Yaksh, 1991). Clonidine was the first α2-adrenoceptor agonist to be introduced clinically. It has analgesic properties when given epidurally or intrathecally in humans. Epidural clonidine was found to be effective in reducing both postoperative pain (Anzai & Nishikawa, 1995) and intractable neuropathic cancer pain (Eisenach et al., 1995). Intrathecal clonidine also decreased postoperative pain (Filos et al., 1994). Dexmedetomidine is another α2-adrenoceptor agonist. Intravenous administration of dexmedetomidine has been used clinically for sedation in the intensive care unit (Venn & Grounds, 2001). Meanwhile, several lines of evidence indicate that administration of dexmedetomidine produces spinal analgesia as efficiently as clonidine. Behavioral studies in rats have demonstrated an inhibition of nociceptive responses by intrathecally (Fisher et al., 1991) and epidurally (Asano et al., 2000) administered dexmedetomidine. α2-Adrenoceptor agonists are also used in as adjuncts to general anesthesia. The effect of oral clonidine premedication can reduce volatile anesthetic (Ghignone et al., 1987) and opioid (Ghignone et al., 1986) requirements in the perioperative period. Intravenous administration of dexmedetomidine similarly diminishes the volatile anesthetic (Aho et al., 1992) and opioid (But et al., 2006) requirements.
Although both clonidine and dexmedetomidine are α2-adrenoceptor agonists, several differences exist between the two. First, the potency of dexmedetomidine is much greater than that of clonidine at maximum efficacy. Intraperitoneal administration of clonidine decreased minimum alveolar concentration, which produces immobility in 50% of subjects exposed to a noxious stimulus, for halothane about 40% in rats, whereas minimum alveolar concentration was decreased about 90% by dexmedetomidine (Maze et al., 1987; Segal et al., 1988). A second difference lies in the relative α2/α1 selectivity ratio. The α2/α1-adrenoceptor selectivity ratio of clonidine is 220 : 1 (Virtanen et al., 1988). Accordingly, high doses of clonidine may induce cardiovascular side-effects via the α1-adrenoceptor (Virtanen et al., 1988). The α2-/α1-adrenoceptor selectivity ratio of dexmedetomidine is 1620 : 1 (Virtanen et al., 1988). As dexmedetomidine is a highly selective α2-adrenoceptor agonist (Savola & Virtanen, 1991), dexmedetomidine causes fewer side-effects mediated by α1-adrenoceptor activation than clonidine.
Neurons of the superficial dorsal horn, especially lamina II (substantia gelatinosa: SG), are thought to play an important role in modulating nociceptive transmission, because they preferentially receive thin myelinated Aδ- and unmyelinated C-primary afferent fibers, both of which carry nociceptive information from the periphery (Kumazawa & Perl, 1978; Yoshimura & Jessell, 1989). Moreover, binding and immunohistochemical studies show that the highest density of α2-adrenoceptors is in the superficial layers of the spinal dorsal horn (Roudet et al., 1994; Stone et al., 1998). However, whether dexmedetomidine exerts its effect on SG neurons remains unclear. In the present study, we used whole-cell patch-clamp recording to clarify whether dexmedetomidine has postsynaptic effects on SG neurons and to confirm the involvement of α2-adrenoceptors.
Materials and methods
This study was approved by the Animal Research Committee of Niigata University Graduate School of Medical and Dental Sciences in Niigata, Japan. All efforts were made to minimize the number of animals used.
Spinal cord slice preparation
Male Wistar rats (4–8 weeks old) were anesthetized with urethane (1.5 g/kg, i.p.). The lumbosacral segment of the spinal cord was removed as described previously (Yoshimura & Nishi, 1993; Kohno et al., 2003) and placed in a preoxygenated ice-cold (1–3°C) sucrose-substituted Krebs solution containing (in mm): KCl 6.4, MgSO4 4.1, NaHCO3 26, NaH2PO4 1.3, glucose 10 and sucrose 252, bubbled with 95% O2 and 5% CO2 (Wang et al., 1999). Transverse spinal cord slices (500 μm) were cut on a vibrating microslicer. The slices were placed on a nylon mesh in a recording chamber, and then perfused at a rate of 10 ml/min with normal Krebs solution containing (in mM): NaCl 117, KCl 3.6, CaCl2 2.5, MgCl2 1.2, Na2HPO4 1.2, NaHCO3 25, glucose 11, at 36 ± 1°C for at least 1 h prior to recordings.
Electrophysiological recordings
The lamina II was clearly discernible as a relatively translucent band across the dorsal horn under a dissecting microscope with transmitted illumination. Blind whole-cell patch-clamp recordings were made from SG neurons in voltage clamp mode using patch-pipette electrodes with a resistance of 5–10 MΩ (Yoshimura & Nishi, 1993; Kohno et al., 2003). Two pipette solutions were used. The first solution contained (in mm): potassium gluconate 135, KCl 5, CaCl2 0.5, MgCl2 2, EGTA 5, HEPES 5 and ATP-Mg 5 (pH 7.2). Guanosine-5′-O-(2-thiodiphosphate) (GDP-β-S, 2 mm) was used as a GTP-binding protein blocker when necessary. The second solution contained (in mm): Cs2SO4 110, tetraethylammonium (TEA) 5, CaCl2 0.5, MgCl2 2, EGTA 5, HEPES 5 and ATP-Mg 5 (pH 7.2). Cs and TEA were used as K+ channel blockers. Membrane currents were amplified using an Axopatch 200B amplifier (Molecular Devices). Signals were filtered at 2 kHz and digitized at 5 kHz. Data were collected and analysed using pClamp9.0 software (Molecular Devices).
Application of drugs
Drugs were applied by superfusion without alteration of the perfusion rate or temperature. The drugs used were dexmedetomidine (provided by Abbott Japan), prazosin, yohimbine, tetrodotoxin (TTX) (Wako, Japan), oxymetazoline, GDP-β-S, acridin-9-yl-[4-(4-methylpiperazin-1-yl)-phenyl]amine (JP-1302) (Sigma-Aldrich, USA), 2-[(4,5-dihydro-1H-imidazol-2-yl)methyl]-2,3-dihydro-1-methyl-1H-isoindole maleate (BRL44408) (Tocris Bioscience, USA).
Data analysis
Numerical data are expressed as means ± SEM. Statistical significance was assessed as P< 0.05 using the paired Student's t-test. In all cases, n refers to the number of neurons studied. Relative peak amplitude was calculated as an amplitude of dexmedetomidine-induced current in the presence of the drugs, divided by that of currents produced by dexmedetomidine alone. The continuous curve for the concentration–response relationship of dexmedetomidine was drawn according to the following Hill equation: y= 1.3/[1 + (EC50/x)b], where x is the dexmedetomidine concentration and b is the Hill coefficient.
Results
Dexmedetomidine induces currents in dorsal horn neurons
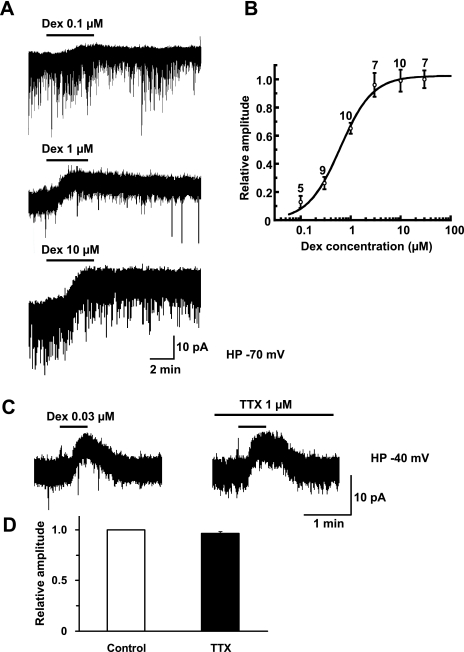
The resting membrane potentials in the SG neurons were −65.8 ± 0.8 mV (n= 17). In 92% of the SG neurons examined (n= 84), bath-applied dexmedetomidine induced outward currents at −70 mV. As shown in Fig. 1A, outward currents exhibited a clear dose-dependency on dexmedetomidine perfused at the surface of the spinal cord. The onset of the responses became slow and recovery was delayed with increasing concentrations of dexmedetomidine. Fig. 1B shows a concentration–response curve of the dexmedetomidine-induced outward currents. Analysis of the curve gave 0.62 μm as the effective concentration producing a half-maximal response (EC50) with a Hill coefficient of 1.34. A higher concentration of dexmedetomidine produced a long duration at a holding potential of −70 mV (Fig. 1A) and it was difficult to be applied repeatedly. Consequently, we used a low concentration of dexmedetomidine (0.03 μm) in order to reproduce responses when it was repeatedly applied. In addition, the dexmedetomidine (0.03 μm)-induced outward currents could not be observed at a holding potential of −70 mV, as shown in Fig. 1B. Therefore, we recorded the currents at a holding potential of −40 mV (Fig. 1C and D). To confirm that the dexmedetomidine-induced currents were postsynaptic effects, we examined the currents in the presence of TTX to remove any possible influence of α2-adrenoceptors on presynaptic terminals. We compared peak amplitude elicited by dexmedetomidine (0.03 μm) in the absence and presence of TTX (1 μm). TTX had no significant effect on the amplitude of dexmedetomidine (Fig. 1Cand D, 96.2 ± 1.3% of control, P= 0.91, n= 5).
Fig. 1.
Dexmedetomidine induces an outward current in a concentration-dependent manner in SG neurons. (A) Outward currents induced by dexmedetomidine (0.1, 1 and 10 μm). Duration of drug superfusion is shown by horizontal bars above chart recordings. Holding potential = −70 mV. (B) Relative peak amplitude was calculated as an amplitude of the dexmedetomidine (0.1–30 μm)-induced current divided by that of the current produced by dexmedetomidine (30 μm). The continuous curve was drawn according to the Hill plot with an EC50 value of 0.62 μm (95% confidence interval, 0.51–0.77 μm) and a Hill coefficient of 1.34. The number next to each point denotes the number of neurons examined. Holding potential = −70 mV. (C) Outward currents elicited by dexmedetomidine (0.03 μm) in the absence and presence of TTX (1 μm). These currents were obtained from the same neuron (n= 5). Holding potential = −40 mV. (D) Relative peak amplitude was calculated as an amplitude of dexmedetomidine-induced current in the presence of TTX (5.3 ± 1.3 pA) divided by that of currents produced by dexmedetomidine (5.6 ± 1.4 pA) without TTX.
Pharmacological analysis of dexmedetomidine-induced responses
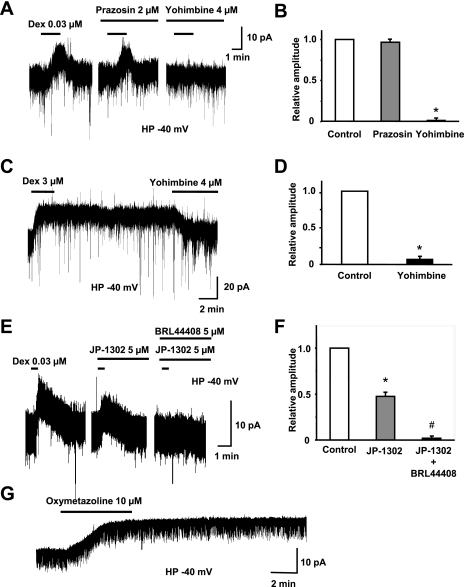
We examined the effects on dexmedetomidine-induced responses of the antagonists prazosin (2 μm) and yohimbine (4 μm), as compared with those of previous studies (prazosin 0.5–2 μm, yohimbine 1–4 μm) (Baba et al., 2000; Kawasaki et al., 2003; Sonohata et al., 2004). The dexmedetomidine-induced outward currents were attenuated by the application of the α2-adrenoceptor antagonist yohimbine (4 μm), while the α1-adrenoceptor antagonist prazosin (2 μm) had no significant effect (Fig. 2A). Figure 2B shows a summary of the suppressive effects of each adrenergic antagonist. We next examined the effects on dexmedetomidine-induced responses using a lower concentration of prazosin (0.2 μm) and yohimbine (0.4 μm). However, these antagonists did not have any effect on the dexmedetomidine-induced outward currents (prazosin, 93.3 ± 4.1% of control, P= 0.71; yohimbine, 91.0 ± 7.4% of control, P= 0.88, n= 5). These results suggested that the dexmedetomidine-induced outward current was mediated by α2-adrenoceptors.
Fig. 2.
Dexmedetomidine-induced currents are mediated by α2- (α2A- and α2C-) but not by α1-adrenoceptors. (A) Prazosin (2 μm), an α1-adrenoceptor antagonist, did not affect the amplitude of the outward current induced by dexmedetomidine (0.03 μm), whereas the currents were suppressed by application of yohimbine (4 μm), an α2-adrenoceptor antagonist. These currents were obtained from the same neuron (n= 6). Holding potential = −40 mV. (B) Relative peak amplitude was calculated as an amplitude of dexmedetomidine-induced current in the presence of prazosin (8.7 ± 2.2 pA) or yohimbine (0.1 ± 0.1 pA) divided by that of currents produced by dexmedetomidine (9.3 ± 2.6 pA) alone. *P< 0.05, control vs. yohimbine. Holding potential = −40 mV. (C) Dexmedetomidine (3 μm) produced an outward current of long duration. A persistent outward current after washout of dexmedetomidine was completely reduced in amplitude by yohimbine (4 μm). Holding potential = −40 mV. (D) The dexmedetomidine-induced outward currents in the presence of yohimbine were significantly smaller than control (*P< 0.05, n= 10). (E) Application of JP-1302 (5 μm) partially blocked the dexmedetomidine (0.03 μm)-induced current, and simultaneous application of JP-1302 (5 μm) and BRL44408 (5 μm) abolished the current completely. These currents were obtained from the same neuron (n= 6). Holding potential = −40 mV. (F) Relative peak amplitude was calculated as an amplitude of dexmedetomidine-induced current in the presence of JP-1302 (3.3 ± 0.3 pA) or JP-1302 plus BRL44408 (0.1 ± 0.1 pA) divided by that of currents produced by dexmedetomidine (6.9 ± 0.6 pA) alone. *P< 0.05, control vs. JP-1302. #P< 0.05, control vs. JP-1302 plus BRL44408. (G) Dexmedetomidine-induced outward current was mimicked by the α2A-adrenoceptor agonist oxymetazoline (10 μm) on SG neurons. Holding potential = −40 mV.
Application of a high concentration of dexmedetomidine (3 μm) produced an outward current of long duration. Superfusing yohimbine (4 μm) alone accelerated the recovery of outward currents to baseline level (Fig. 2Cand D), confirming that the dexmedetomidine-induced current was mediated by α2-adrenoceptors. Furthermore, to examine the involvement of α2A- and α2C-adrenoceptors on the dexmedetomidine-induced current, we used α2A- and α2C-adrenoceptor subtype-preferring antagonists (Fig. 2E andF). Application of the α2C-adrenoceptor antagonist JP-1302 (5 μm) partially suppressed the dexmedetomidine (0.03 μm)-induced current. In addition, it was markedly abolished when dexmedetomidine was again applied in the presence of JP-1302 (5 μm) and the α2A-adrenoceptor antagonist BRL44408 (5 μm). Relative peak amplitude was reduced to 3.3 ± 0.3 pA in the presence of JP-1302 and to 0.1 ± 0.1 pA in the presence of JP-1302 plus BRL44408 (dexmedetomidine alone, 6.9 ± 0.6 pA, Fig. 2F). We next used oxymatazoline, an α2A-adrenoceptor agonist (Miyazaki et al., 1998; Kawasaki et al., 2003). In nine of ten neurons, oxymatazoline (10 μm) induced an outward current similar to that of dexmedetomidine (Fig. 2G) with a peak amplitude of 10.2 ± 1.0 pA (n= 9). This result suggested that α2A-adrenoceptors were present and activatable on SG neurons. These findings indicated that both α2A- and α2C-adrenoceptors were involved in the dexmedetomidine-induced current.
Dexmedetomidine activates K+ channels through the activation of G-proteins on SG neurons
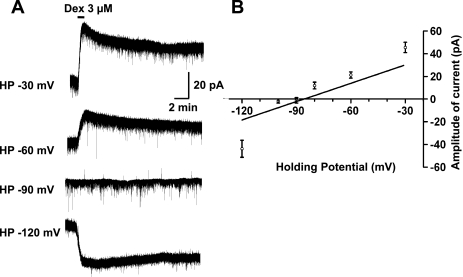
We next investigated what kinds of channels mediate the dexmedetomidine current. Figure 3A shows the dexmedetomidine (3 μm)-induced currents recorded at different holding potentials. Plots of the current–voltage relationship revealed a reversal potential at around −86 mV (Fig. 3B), which is slightly different from the equilibrium potential (−97 mV) of K+, as calculated from the Nernst equation using K+ concentrations ([K+]o, 3.6 mm; [K+]i, 140 mm) of the solutions. This slight difference might be considered to reflect a liquid junction potential (9–10 mV) existing between the Krebs and patch-pipette solutions (Yajiri et al., 1997). Superfusing dexmedetomidine opens K+ channels on postsynaptic SG neurons, generating an outward current at holding potentials below −90 mV.
Fig. 3.
(A) Dexmedetomidine-induced currents show voltage dependency. (B) Relationship between holding potential and current amplitude. The regression equation was y= 0.901x + 77.1 [R2 = 0.92, P= 0.002, y= amplitude of current (pA), x= holding potential (mV)].
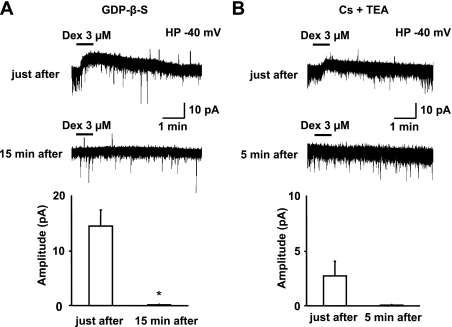
To determine whether G-proteins are responsible for the dexmedetomidine-induced current, GDP-β-S (2 mm), a non-hydrolysable analog of GDP that competitively inhibits G-proteins, was used in the pipette solution to prevent the postsynaptic activation of α2-adrenoceptors. When dexmedetomidine (3 μm) was applied shortly after establishing the whole-cell configuration, an outward current was observed (Fig. 4A). This outward current was completely abolished when dexmedetomidine was again applied 15 min later (Fig. 4A, n= 5). We next used the pipette solution containing Cs+ and TEA to inhibit the postsynaptic effect of K+ channels. Dexmedetomidine (3 μm)-induced outward current was recorded just after establishing the whole-cell configuration (Fig. 4B). However, this current was abolished after the second application of dexmedetomidine, which was performed more than 5 min later (Fig. 4B, n= 7). These findings suggested that the dexmedetomidine-induced outward current was mediated by K+ channels through the activation of G-proteins.
Fig. 4.
Inhibition of dexmedetomidine-induced current by GDP-β-S or Cs+/TEA. (A) The dexmedetomidine (3 μm)-induced outward current was examined with K-gluconate pipette solution containing GDP-β-S (2 mm). Dexmedetomidine produced an outward current just after establishing the whole-cell configuration, but this was markedly abolished when dexmedetomidine was again applied 15 min later (*P< 0.05, n= 5). Holding potential = −40 mV. (B) Dexmedetomidine (3 μm) induced an outward current just after establishing whole-cell recording with Cs+ and TEA-containing pipette. When dexmedetomidine was again applied 5 min later, the current was completely abolished (n= 7). Holding potential = −40 mV.
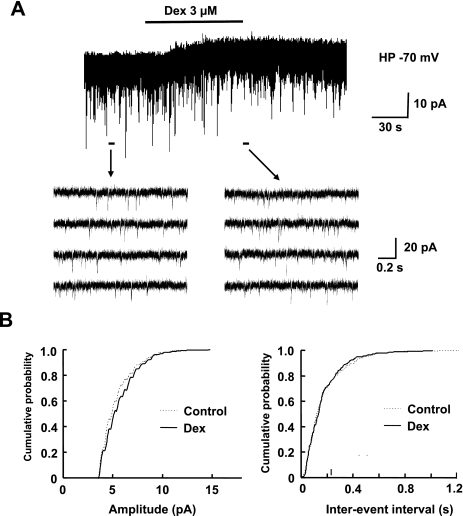
Effects of dexmedetomidine on spontaneous excitatory postsynaptic currents
We next examined the effects of dexmedetomidine on spontaneous excitatory postsynaptic currents (sEPSCs) in SG neurons (Fig. 5A). Amplitude and inter-event interval distributions were not changed by dexmedetomidine (Fig. 5B; amplitude, P> 0.05, Kolmogorov–Smirnov test; inter-event interval, P> 0.05). These data indicate that dexmedetomidine does not affect glutamate release from presynaptic terminals.
Fig. 5.
Dexmedetomidine dose not affect the frequency or amplitude of spontaneous EPSCs in SG neurons. (A) Continuous chart recording of sEPSCs before and during the application of dexmedetomidine (3 μm) (upper trace). Four consecutive traces of sEPSCs for a period indicated by a short bar below the chart recording are shown on an expanded time scale (lower traces). Holding potential = −70 mV. (B) Cumulative distributions of the amplitude (left) and the inter-event interval (right) of sEPSCs before (dashed line) and during the application of dexmedetomidine (continuous line). Dexmedetomidine had no significant effect on the amplitude (P> 0.05; Kolmogorov–Smirnov test) or the inter-event interval distribution (P> 0.05).
Discussion
Intrathecal dexmedetomidine is a highly potent antinociceptive agent in animals (Fisher et al., 1991). Lipophilicity may affect the analgesic potency of intrathecally administered agents (Eisenach et al., 1994). Highly lipophilic agents bind to spinal cord more efficiently than poorly lipophilic drugs. Dexmedetomidine is more lipophilic than clonidine (Savola et al., 1986). Eisenach et al. (1994) showed that intrathecal administration of dexmedetomidine at a dose of 100 μg produced an antinociceptive effect that was first evident at 30 min and lasted for 90 min after the injection. As these authors demonstrated in a separate set of experiments in the same study, intrathecal administration of dexmedetomidine at this dose resulted in cerebrospinal fluid concentrations ranging from 300 to 3000 ng/ml (nearly 1–10 μm) during the period 30–90 min after injection. These results are consistent with the concentrations of dexmedetomidine that induced outward currents in the present study (Fig. 1B). Taken together, these data suggest that the cerebrospinal fluid concentration of dexmedetomidine required for antinociception is 1–10 μm.
Behavioral studies in rats have demonstrated that intrathecal administration of dexmedetomidine produces dose-dependent antinociception (Fisher et al., 1991). Low doses of dexmedetomidine produced a transient period of antinociception. On the other hand, profound antinociception was prolonged by high doses of dexmedetomidine. For example, a transient current was evoked by a low concentration of dexmedetomidine (0.03 μm) in the present study (Fig. 2A). By contrast, a high concentration of dexmedetomidine (3 μm) produced a current with a long duration (Fig. 2C). The durations of dexmedetomidine (3 μm)-induced currents were longer than 30 min in most neurons without desensitization. The higher the concentration of dexmedetomidine, the longer the duration tended to be. The durations of dexmedetomidine-induced outward currents were much longer than those induced by noradrenaline (Sonohata et al., 2004). This long duration may be caused by the high affinity of dexmedetomidine. A radioligand binding study showed that the binding affinity (pKi) value of dexmedetomidine was higher than that of noradrenaline for the α2-adrenoceptor (Jasper et al., 1998). Alternatively, the long-term effect may be due to a slow rate of diffusion of dexmedetomidine out of the spinal cord slice (Koga et al., 2005). This issue remains to be established.
As mentioned above, superficial dorsal horn neurons are important for processing nociceptive information from primary afferent fibers. Binding and immunohistochemical studies have suggested that the highest density of α2-adrenoceptors exist in the superficial layers of the spinal dorsal horn (Roudet et al., 1994; Stone et al., 1998). In addition, the binding sites of the α2A-adrenoceptors in the superficial dorsal horn were dramatically reduced in number after neonatal capsaicin treatment or dorsal rhizotomy, whereas α2C-adrenoceptors were not significantly reduced by either of these treatments. This finding suggests that α2C-adrenoceptors exist in postsynaptic sites (Stone et al., 1998; Olave & Maxwell, 2002), and that α2A-adrenoceptors are located mainly in presynaptic sites (Stone et al., 1998; Kawasaki et al., 2003) of the dorsal horn. By contrast, few α2B-adrenoceptors were seen in the spinal dorsal horn by in situ hybridization studies of the distribution of α2B mRNA (Nicholas et al., 1993; Shi et al., 1999). Furthermore, an α2-adrenoceptor agonist did not change spinal antinociception in α2B-adrenoceptor knockout mice, suggesting that α2B-adrenoceptors do not participate in the antinociceptive effect in the spinal cord (Fairbanks et al., 2002). However, these immunohistochemical studies showed a dramatic, but incomplete, reduction in α2A-adrenoceptor immunoreactivity. This result raised the question of whether α2A-adrenoceptors were also located on postsynaptic SG neurons, in addition to α2C-adrenoceptors. We used prazosin as the α1-adrenoceptor antagonist, but prazosin acts not only as an α1-adrenoceptor, but also has some properties of α2B- and α2C-adrenoceptor antagonists (Bylund, 1988; MacDonald et al., 1997). The binding inhibition coefficients (Ki) of prazosin were 2750, 108 and 98 nm for the α2A-, α2B- and α2C-adrenoceptor, respectively (Marjamaki et al., 1993). Nevertheless, our study showed that dexmedetomidine-induced currents were not blocked by the α2B- and α2C-adrenoceptor antagonistic action of prazosin in SG neurons (Fig. 2A andB). Therefore, highly selective α2A- or α2C-adrenoceptor antagonists were superfused to investigate this question. BRL44408 was noted as a selective α2A-adrenoceptor antagonist having Ki of 4, 174 and 187 nm for the α2A-, α2B- and α2C-adrenoceptor, respectively (Bylund et al., 1994). JP-1302 was recently described as a novel, highly selective α2C-adrenoceptor antagonist (Sallinen et al., 2007). The Ki were 1500, 2200 and 16 nm for the α2A-, α2B- and α2C-adrenoceptor, respectively. Application of JP-1302 partially suppressed the dexmedetomidine-induced current, and simultaneous application of JP-1302 and BRL44408 abolished the amplitude of the current completely (Fig. 2EandF). Our findings clarify the involvement of both α2A- and α2C-adrenoceptors in the dexmedetomidine-induced current. Furthermore, we showed that the dexmedetomidine-induced current seen in postsynaptic SG neurons was mimicked by oxymetazoline, an α2A-adrenoceptor agonist (Fig. 2G). The Ki were 6, 3150 and 180 nm for the α2A-, α2B- and α2C-adrenoceptor, respectively (Marjamaki et al., 1993). Taking these data together, it is likely that dexmedetomidine activates both α2A- and α2C-adrenoceptors on postsynaptic SG neurons and induces currents. As we did not examine the application of an α2C-adrenoceptor agonist, we could not conclude whether there were α2C-adrenoceptors in postsynaptic SG neurons. Further studies are required with highly selective α2A- or α2C-adrenoceptor agonists and antagonists.
We showed that activation of α2-adrenoceptors with dexmedetomidine resulted in outward currents that were mediated by activation of K+ channels through the activation of G-proteins (Figs 3 and4). These findings suggest that dexmedetomidine hyperpolarizes the membrane potentials of SG neurons by opening G-protein-coupled inwardly rectifying potassium (GIRK) channels. The GIRK1 and GIRK2 subunits are concentrated in lamina II of the mouse spinal cord (Marker et al., 2005). As the antinociceptive effect of clonidine was reduced in GIRK2-knockout mice (Mitrovic et al., 2003), it is possible that dexmedetomidine exerts its effects on GIRK channels similarly to clonidine.
We focussed on the postsynaptic effects of dexmedetomidine in SG neurons. However, α2-adrenoceptors are also localized on primary afferent terminals (Stone et al., 1998). Noradrenaline acts on α2-adrenoceptors on postsynaptic (Sonohata et al., 2004) and presynaptic (Kawasaki et al., 2003) SG neurons, and produces an antinociceptive effect. Kawasaki et al. (2003) showed that noradrenaline, clonidine and oxymetazoline inhibited the peak amplitudes of monosynaptically evoked Aδ- and C-fiber EPSCs, while miniature EPSC (mEPSC) amplitude and frequency was unaffected by noradrenaline. Pan et al. (2002) reported that the peak amplitude of evoked EPSCs was attenuated by clonidine in the outer zone of SG. Furthermore, clonidine significantly decreased the frequency of mEPSCs. A discrepancy between this and the current study may be due to the fact that different SG neurons were tested; Pan et al. examined neurons in the outer layer of SG, while we investigated neurons located at the center of SG. The possibility cannot be ruled out that SG neurons exhibiting no effect of noradrenaline on mEPSCs (Kawasaki et al., 2003) or no effect of dexmedetomidine on sEPSCs in the current study (where the blind patch-clamp technique was used) had located in the inner layer of SG because visually identified neurons in the inner layer of SG appeared to be without actions of clonidine on mEPSCs (Pan et al., 2002). Li & Zhuo (2001) showed that clonidine inhibited glutamate-mediated evoked EPSCs. However, clonidine preferentially depressed polysynaptic but not monosynaptic Aδ-fiber-evoked field potentials in superficial spinal dorsal horn (Ruscheweyh & Sandkuhler, 2000). Moreover, α2-adrenoceptor agonists depressed the NMDA receptor-mediated excitatory postsynaptic potential on A- and C-primary afferent fibers (Faber et al., 1998). Our results showed that dexmedetomidine did not affect glutamate release from presynaptic terminals. Whether dexmedetomidine exhibits a similar presynaptic action on these currents needs to be established.
In conclusion, the present study suggests that dexmedetomidine hyperpolarizes the membrane potentials of spinal dorsal horn neurons via G-protein-mediated activation of K+ channels through α2-adrenoceptors. This action of dexmedetomidine might contribute, at least in part, to its antinociceptive action in the spinal cord.
Acknowledgments
This study was supported by a Grant-In-Aid for Scientific Research (No. 16591529) from the Ministry of Education, Science, Sports and Culture of Japan, Tokyo, Japan and Yujin Memorial Grant.
Abbreviations
- BRL44408
2-[(4,5-dihydro-1H-imidazol-2-yl)methyl]-2,3-dihydro-1-methyl-1H-isoindole maleate
- EPSCs
excitatory postsynaptic currents
- GDP-β-S
guanosine-5′-O-(2-thiodiphosphate)
- GIRK
G-protein-coupled inwardly rectifying potassium
- JP-1302
acridin-9-yl-[4-(4-methylpiperazin-1-yl)-phenyl]amine
- Ki
binding inhibition coefficient
- pKi
binding affinity
- SG
substantia gelatinosa
- TEA
tetraethylammonium
- TTX
tetrodotoxin
References
- Aho M, Erkola O, Kallio A, Scheinin H, Korttila K. Dexmedetomidine infusion for maintenance of anesthesia in patients undergoing abdominal hysterectomy. Anesth. Analg. 1992;75:940–946. [PubMed] [Google Scholar]
- Anzai Y, Nishikawa T. Thoracic epidural clonidine and morphine for postoperative pain relief. Can. J. Anaesth. 1995;42:292–297. doi: 10.1007/BF03010705. [DOI] [PubMed] [Google Scholar]
- Asano T, Dohi S, Ohta S, Shimonaka H, Iida H. Antinociception by epidural and systemic alpha(2)-adrenoceptor agonists and their binding affinity in rat spinal cord and brain. Anesth. Analg. 2000;90:400–407. doi: 10.1097/00000539-200002000-00030. [DOI] [PubMed] [Google Scholar]
- Baba H, Shimoji K, Yoshimura M. Norepinephrine facilitates inhibitory transmission in substantia gelatinosa of adult rat spinal cord (part 1): effects on axon terminals of GABAergic and glycinergic neurons. Anesthesiology. 2000;92:473–484. doi: 10.1097/00000542-200002000-00030. [DOI] [PubMed] [Google Scholar]
- But AK, Ozgul U, Erdil F, Gulhas N, Toprak HI, Durmus M, Ersoy MO. The effects of pre-operative dexmedetomidine infusion on hemodynamics in patients with pulmonary hypertension undergoing mitral valve replacement surgery. Acta Anaesthesiol. Scand. 2006;50:1207–1212. doi: 10.1111/j.1399-6576.2006.01136.x. [DOI] [PubMed] [Google Scholar]
- Bylund DB. Subtypes of alpha 2-adrenoceptors: pharmacological and molecular biological evidence converge. Trends Pharmacol. Sci. 1988;9:356–361. doi: 10.1016/0165-6147(88)90254-4. [DOI] [PubMed] [Google Scholar]
- Bylund DB, Eikenberg DC, Hieble JP, Langer SZ, Lefkowitz RJ, Minneman KP, Molinoff PB, Ruffolo RR, Jr, Trendelenburg U. International Union of Pharmacology nomenclature of adrenoceptors. Pharmacol. Rev. 1994;46:121–136. [PubMed] [Google Scholar]
- Eisenach JC, Shafer SL, Bucklin BA, Jackson C, Kallio A. Pharmacokinetics and pharmacodynamics of intraspinal dexmedetomidine in sheep. Anesthesiology. 1994;80:1349–1359. doi: 10.1097/00000542-199406000-00023. [DOI] [PubMed] [Google Scholar]
- Eisenach JC, DuPen S, Dubois M, Miguel R, Allin D. Epidural clonidine analgesia for intractable cancer pain. The Epidural Clonidine Study Group. Pain. 1995;61:391–399. doi: 10.1016/0304-3959(94)00209-W. [DOI] [PubMed] [Google Scholar]
- Faber ES, Chambers JP, Evans RH. Depression of NMDA receptor-mediated synaptic transmission by four alpha2 adrenoceptor agonists on the in vitro rat spinal cord preparation. Br. J. Pharmacol. 1998;124:507–512. doi: 10.1038/sj.bjp.0701873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks CA, Stone LS, Kitto KF, Nguyen HO, Posthumus IJ, Wilcox GL. alpha(2C)-Adrenergic receptors mediate spinal analgesia and adrenergic-opioid synergy. J. Pharmacol. Exp. Ther. 2002;300:282–290. doi: 10.1124/jpet.300.1.282. [DOI] [PubMed] [Google Scholar]
- Filos KS, Goudas LC, Patroni O, Polyzou V. Hemodynamic and analgesic profile after intrathecal clonidine in humans. A dose-response study. Anesthesiology. 1994;81:591–601. doi: 10.1097/00000542-199409000-00011. [DOI] [PubMed] [Google Scholar]
- Fisher B, Zornow MH, Yaksh TL, Peterson BM. Antinociceptive properties of intrathecal dexmedetomidine in rats. Eur. J. Pharmacol. 1991;192:221–225. doi: 10.1016/0014-2999(91)90046-s. [DOI] [PubMed] [Google Scholar]
- Ghignone M, Quintin L, Duke PC, Kehler CH, Calvillo O. Effects of clonidine on narcotic requirements and hemodynamic response during induction of fentanyl anesthesia and endotracheal intubation. Anesthesiology. 1986;64:36–42. doi: 10.1097/00000542-198601000-00007. [DOI] [PubMed] [Google Scholar]
- Ghignone M, Calvillo O, Quintin L. Anesthesia and hypertension: the effect of clonidine on perioperative hemodynamics and isoflurane requirements. Anesthesiology. 1987;67:3–10. [PubMed] [Google Scholar]
- Guo TZ, Jiang JY, Buttermann AE, Maze M. Dexmedetomidine injection into the locus ceruleus produces antinociception. Anesthesiology. 1996;84:873–881. doi: 10.1097/00000542-199604000-00015. [DOI] [PubMed] [Google Scholar]
- Jasper JR, Lesnick JD, Chang LK, Yamanishi SS, Chang TK, Hsu SA, Daunt DA, Bonhaus DW, Eglen RM. Ligand efficacy and potency at recombinant alpha2 adrenergic receptors: agonist-mediated [35S]GTPgammaS binding. Biochem. Pharmacol. 1998;55:1035–1043. doi: 10.1016/s0006-2952(97)00631-x. [DOI] [PubMed] [Google Scholar]
- Kawasaki Y, Kumamoto E, Furue H, Yoshimura M. Alpha 2 adrenoceptor-mediated presynaptic inhibition of primary afferent glutamatergic transmission in rat substantia gelatinosa neurons. Anesthesiology. 2003;98:682–689. doi: 10.1097/00000542-200303000-00016. [DOI] [PubMed] [Google Scholar]
- Koga A, Fujita T, Totoki T, Kumamoto E. Tramadol produces outward currents by activating mu-opioid receptors in adult rat substantia gelatinosa neurones. Br. J. Pharmacol. 2005;145:602–607. doi: 10.1038/sj.bjp.0706225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno T, Moore KA, Baba H, Woolf CJ. Peripheral nerve injury alters excitatory synaptic transmission in lamina II of the rat dorsal horn. J. Physiol. 2003;548:131–138. doi: 10.1113/jphysiol.2002.036186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumazawa T, Perl ER. Excitation of marginal and substantia gelatinosa neurons in the primate spinal cord: indications of their place in dorsal horn functional organization. J. Comp. Neurol. 1978;177:417–434. doi: 10.1002/cne.901770305. [DOI] [PubMed] [Google Scholar]
- Li P, Zhuo M. Cholinergic, noradrenergic, and serotonergic inhibition of fast synaptic transmission in spinal lumbar dorsal horn of rat. Brain Res. Bull. 2001;54:639–647. doi: 10.1016/s0361-9230(01)00470-1. [DOI] [PubMed] [Google Scholar]
- MacDonald E, Kobilka BK, Scheinin M. Gene targeting – homing in on alpha 2-adrenoceptor-subtype function. Trends Pharmacol. Sci. 1997;18:211–219. doi: 10.1016/s0165-6147(97)01063-8. [DOI] [PubMed] [Google Scholar]
- Marjamaki A, Luomala K, Ala-Uotila S, Scheinin M. Use of recombinant human alpha 2-adrenoceptors to characterize subtype selectively of antagonist binding. Eur. J. Pharmacol. 1993;246:219–226. doi: 10.1016/0922-4106(93)90034-7. [DOI] [PubMed] [Google Scholar]
- Marker CL, Lujan R, Loh HH, Wickman K. Spinal G-protein-gated potassium channels contribute in a dose-dependent manner to the analgesic effect of mu- and delta- but not kappa-opioids. J. Neurosci. 2005;25:3551–3559. doi: 10.1523/JNEUROSCI.4899-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maze M, Birch B, Vickery RG. Clonidine reduces halothane MAC in rats. Anesthesiology. 1987;67:868–869. doi: 10.1097/00000542-198711000-00061. [DOI] [PubMed] [Google Scholar]
- Mitrovic I, Margeta-Mitrovic M, Bader S, Stoffel M, Jan LY, Basbaum AI. Contribution of GIRK2-mediated postsynaptic signaling to opiate and alpha 2-adrenergic analgesia and analgesic sex differences. Proc. Natl Acad. Sci. U.S.A. 2003;100:271–276. doi: 10.1073/pnas.0136822100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki T, Kobayashi H, Tosaka T. Presynaptic inhibition by noradrenaline of the EPSC evoked in neonatal rat sympathetic preganglionic neurons. Brain Res. 1998;790:170–177. doi: 10.1016/s0006-8993(97)01549-7. [DOI] [PubMed] [Google Scholar]
- Nicholas AP, Pieribone V, Hokfelt T. Distributions of mRNAs for alpha-2 adrenergic receptor subtypes in rat brain: an in situ hybridization study. J. Comp. Neurol. 1993;328:575–594. doi: 10.1002/cne.903280409. [DOI] [PubMed] [Google Scholar]
- Olave MJ, Maxwell DJ. An investigation of neurones that possess the alpha 2C-adrenergic receptor in the rat dorsal horn. Neuroscience. 2002;115:31–40. doi: 10.1016/s0306-4522(02)00407-4. [DOI] [PubMed] [Google Scholar]
- Pan YZ, Li DP, Pan HL. Inhibition of glutamatergic synaptic input to spinal lamina II(o) neurons by presynaptic alpha(2)-adrenergic receptors. J. Neurophysiol. 2002;87:1938–1947. doi: 10.1152/jn.00575.2001. [DOI] [PubMed] [Google Scholar]
- Roudet C, Mouchet P, Feuerstein C, Savasta M. Normal distribution of alpha 2-adrenoceptors in the rat spinal cord and its modification after noradrenergic denervation: a quantitative autoradiographic study. J. Neurosci. Res. 1994;39:319–329. doi: 10.1002/jnr.490390309. [DOI] [PubMed] [Google Scholar]
- Ruscheweyh R, Sandkuhler J. Differential actions of spinal analgesics on mono-versus polysynaptic Adelta-fibre-evoked field potentials in superficial spinal dorsal horn in vitro. Pain. 2000;88:97–108. doi: 10.1016/S0304-3959(00)00325-0. [DOI] [PubMed] [Google Scholar]
- Sallinen J, Hoglund I, Engstrom M, Lehtimaki J, Virtanen R, Sirvio J, Wurster S, Savola JM, Haapalinna A. Pharmacological characterization and CNS effects of a novel highly selective alpha2C-adrenoceptor antagonist JP-1302. Br. J. Pharmacol. 2007;150:391–402. doi: 10.1038/sj.bjp.0707005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savola JM, Virtanen R. Central alpha 2-adrenoceptors are highly stereoselective for dexmedetomidine, the dextro enantiomer of medetomidine. Eur. J. Pharmacol. 1991;195:193–199. doi: 10.1016/0014-2999(91)90535-x. [DOI] [PubMed] [Google Scholar]
- Savola JM, Ruskoaho H, Puurunen J, Salonen JS, Karki NT. Evidence for medetomidine as a selective and potent agonist at alpha 2-adrenoreceptors. J. Auton. Pharmacol. 1986;6:275–284. doi: 10.1111/j.1474-8673.1986.tb00654.x. [DOI] [PubMed] [Google Scholar]
- Segal IS, Vickery RG, Walton JK, Doze VA, Maze M. Dexmedetomidine diminishes halothane anesthetic requirements in rats through a postsynaptic alpha 2 adrenergic receptor. Anesthesiology. 1988;69:818–823. doi: 10.1097/00000542-198812000-00004. [DOI] [PubMed] [Google Scholar]
- Shi TJ, Winzer-Serhan U, Leslie F, Hokfelt T. Distribution of alpha2-adrenoceptor mRNAs in the rat lumbar spinal cord in normal and axotomized rats. Neuroreport. 1999;10:2835–2839. doi: 10.1097/00001756-199909090-00025. [DOI] [PubMed] [Google Scholar]
- Sonohata M, Furue H, Katafuchi T, Yasaka T, Doi A, Kumamoto E, Yoshimura M. Actions of noradrenaline on substantia gelatinosa neurones in the rat spinal cord revealed by in vivo patch recording. J. Physiol. 2004;555:515–526. doi: 10.1113/jphysiol.2003.054932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone LS, Broberger C, Vulchanova L, Wilcox GL, Hokfelt T, Riedl MS, Elde R. Differential distribution of alpha2A and alpha2C adrenergic receptor immunoreactivity in the rat spinal cord. J. Neurosci. 1998;18:5928–5937. doi: 10.1523/JNEUROSCI.18-15-05928.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano Y, Yaksh TL. Relative efficacy of spinal alpha-2 agonists, dexmedetomidine, clonidine and ST-91, determined in vivo by using N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline, an irreversible antagonist. J. Pharmacol. Exp. Ther. 1991;258:438–446. [PubMed] [Google Scholar]
- Venn RM, Grounds RM. Comparison between dexmedetomidine and propofol for sedation in the intensive care unit: patient and clinician perceptions. Br. J. Anaesth. 2001;87:684–690. doi: 10.1093/bja/87.5.684. [DOI] [PubMed] [Google Scholar]
- Virtanen R, Savola JM, Saano V, Nyman L. Characterization of the selectivity, specificity and potency of medetomidine as an alpha 2-adrenoceptor agonist. Eur. J. Pharmacol. 1988;150:9–14. doi: 10.1016/0014-2999(88)90744-3. [DOI] [PubMed] [Google Scholar]
- Wang MY, Rampil IJ, Kendig JJ. Ethanol directly depresses AMPA and NMDA glutamate currents in spinal cord motor neurons independent of actions on GABAA or glycine receptors. J. Pharmacol. Exp. Ther. 1999;290:362–367. [PubMed] [Google Scholar]
- Yajiri Y, Yoshimura M, Okamoto M, Takahashi H, Higashi H. A novel slow excitatory postsynaptic current in substantia gelatinosa neurons of the rat spinal cord in vitro. Neuroscience. 1997;76:673–688. doi: 10.1016/s0306-4522(96)00291-6. [DOI] [PubMed] [Google Scholar]
- Yaksh TL. Pharmacology of spinal adrenergic systems which modulate spinal nociceptive processing. Pharmacol. Biochem. Behav. 1985;22:845–858. doi: 10.1016/0091-3057(85)90537-4. [DOI] [PubMed] [Google Scholar]
- Yoshimura M, Jessell TM. Primary afferent-evoked synaptic responses and slow potential generation in rat substantia gelatinosa neurons in vitro. J. Neurophysiol. 1989;62:96–108. doi: 10.1152/jn.1989.62.1.96. [DOI] [PubMed] [Google Scholar]
- Yoshimura M, Nishi S. Blind patch-clamp recordings from substantia gelatinosa neurons in adult rat spinal cord slices: pharmacological properties of synaptic currents. Neuroscience. 1993;53:519–526. doi: 10.1016/0306-4522(93)90216-3. [DOI] [PubMed] [Google Scholar]