Abstract
Cells of the yeast Saccharomyces cerevisiae contain three NAD kinases; namely, cytosolic Utr1p, cytosolic Yef1p, and mitochondrial Pos5p. Previously, the NADH kinase reaction catalyzed by Pos5p, rather than the NAD kinase reaction followed by the NADP+-dependent dehydrogenase reaction, had been regarded as a critical source of mitochondrial NADPH, which plays vital roles in various mitochondrial functions. This study demonstrates that the mitochondrial NADH kinase reaction is dispensable as a source of mitochondrial NADPH and emphasizes the importance of the NAD kinase reaction, followed by the mitochondrial NADP+-dependent dehydrogenase reaction. Of the potential dehydrogenases (malic enzyme, Mae1p; isocitrate dehydrogenase, Idp1p; and acetaldehyde dehydrogenases, Ald4/5p), evidence is presented that acetaldehyde dehydrogenases, and in particular Ald4p, play a prominent role in generating mitochondrial NADPH in the absence of the NADH kinase reaction. The physiological significance of the mitochondrial NADH kinase reaction in the absence of Ald4p is also demonstrated. In addition, Pos5p is confirmed to have a considerably higher NADH kinase activity than NAD kinase activity. Taking these results together, it is proposed that there are two sources of mitochondrial NADPH in yeast: one is the mitochondrial Pos5p-NADH kinase reaction and the other is the mitochondrial Pos5p-NAD kinase reaction followed by the mitochondrial NADP+-dependent acetaldehyde dehydrogenase reaction.
NADPH plays vital roles in reactions that protect against oxidative stress as well as participating in a large number of biosynthetic reactions (1). It is generated by the NAD kinase (EC 2.7.1.23) reaction followed by the NADP+-dependent dehydrogenase reaction. NAD kinase catalyzes the phosphorylation of NAD+ to give NADP+, and NADP+-dependent dehydrogenase reduces the NADP+ to yield NADPH. NADPH is also synthesized by the activity of NADH kinase (EC 2.7.1.86) or pyridine nucleotide transhydrogenase (EC 1.6.1.1) (1). NADH kinase catalyzes the phosphorylation of NADH to give NADPH, whereas pyridine nucleotide transhydrogenase transports protons across the membrane in concert with hydride exchange between NADH and NADP+ or NAD+ and NADPH, resulting in the formation of NADPH from NADP+ (1).
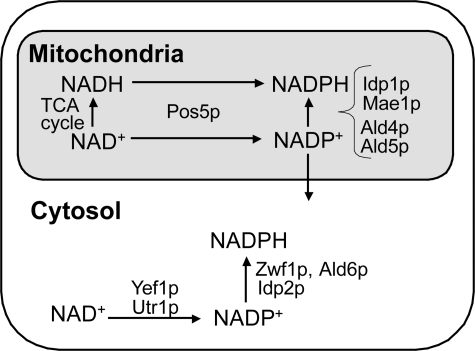
In the cytosol of the yeast Saccharomyces cerevisiae, the major sources of NADPH appear to be cytosolic NADP+-dependent dehydrogenases, including glucose-6-phosphate dehydrogenase (Zwf1p), acetaldehyde dehydrogenase (Ald6p), and isocitrate dehydrogenase (Idp2p) (2, 3) (see Fig. 1). S. cerevisiae has three NAD kinases (cytosolic Utr1p, mitochondrial Pos5p, and Yef1p, which is assumed to reside in the cytosol) (4–6) (see Fig. 1). Although we initially reported that the triple mutant utr1yef1pos5 (MK1219: BY4742 utr1Δ::kanMX4 yef1Δ::HIS3 pos5Δ::CgLEU2) is viable (5), we subsequently found, as described in this report, that the triple mutant utr1yef1pos5 (utr1Δ::kanMX4 yef1Δ::HIS3 pos5Δ::hphMX4) is lethal. The triple mutant in an SEY6210.5 background is also lethal (7). Given the viability of the double mutants (utr1yef1) for the cytosolic NAD kinases in BY4742 and SEY6210.5 backgrounds (5, 7), it is considered that cytosolic NADP(H) can be generated by Pos5p, although how cytosolic NADP+ is produced by mitochondrial Pos5p remains to be determined. The three NAD kinases also exhibit NADH kinase activity, although the physiological contribution of the NADH kinase activity of Utr1p and Yef1p appears to be negligible (5–9).
FIGURE 1.
Scheme illustrating the reactions that produce cytosolic and mitochondrial NADPH in S. cerevisiae. In the cytosol, Zwf1p, Ald6p, and Idp2p are considered to be significant for the generation of NADPH from NADP+ (35, 36). Cytosolic NADP+ is mainly produced by Utr1p, but not by Yef1p (7, 10). Cytosolic NADH kinase reactions are not depicted due to the negligible contribution of the NADH kinase activity of Utr1p or Yef1p to the cytosolic production of NADPH from NADH (5). In mitochondria, the NADH kinase reaction catalyzed by Pos5p has been proposed to be important (6). In this study, we demonstrated that, in addition to the NADH kinase reaction, the NAD kinase reaction catalyzed by Pos5p followed by dehydrogenase reactions catalyzed by Ald4/Ald5p (particularly Ald4p), but not Idp1p and Mae1p, is also critical to the supply of mitochondrial NADPH. The viability of the utr1yef1 double mutant implies that cytosolic NADP+ can be supplied by Pos5p (5, 7).
In contrast to the cytosol, the major source of mitochondrial NADPH in S. cerevisiae is the mitochondrial NAD kinase Pos5p (6) (see Fig. 1). pos5 exhibits several phenotypes, which either directly or indirectly result from decreased mitochondrial NADPH. The phenotypes include Arg– and sensitivity to oxidative stresses (paraquat, hyperoxia, and H2O2), slow growth on non-fermentable carbon sources, defective biosynthesis of enzymes containing the Fe-S cluster, up-regulated transcription of the genes for iron uptake, abnormal accumulation of iron in the mitochondria, and accumulation of mutations in mitochondrial DNA (6, 9, 10).
In S. cerevisiae, mitochondrial NADP+-dependent dehydrogenase appears to be nonessential as a source of mitochondrial NADPH. In mammals, mitochondrial NADP+-dependent isocitrate dehydrogenase is a source of mitochondrial NADPH (11). Decreased expression of isocitrate dehydrogenase causes severe phenotypes, including elevation of reactive oxygen species (ROS)2 production, lipid peroxidation, and mitochondrial damage (11). In the case of S. cerevisiae, however, the lack of a mitochondrial NADP+-dependent isocitrate dehydrogenase gene (IDP1) results in no detectable phenotype in the presence of 0.5 mm H2O2 (12). The higher NADH kinase activity of Pos5p compared with its NAD kinase activity led Otten and Culotta (6, 13) to speculate that the NADH kinase reaction catalyzed by Pos5p, rather than the NAD kinase reaction, is critical to the supply of mitochondrial NADPH. However, these authors presented no evidence to corroborate this speculation, and neither were the kinetic values of Pos5p determined. Only two studies have demonstrated that Pos5p uses NADH in preference to NAD+ (6, 9). Moreover, in S. cerevisiae, there are other known mitochondrial NADP+-dependent dehydrogenases such as malic enzyme (Mae1p) and two acetaldehyde dehydrogenases (Ald4/Ald5p) (Fig. 1), although there is no pyridine nucleotide transhydrogenase in the mitochondria (14–16). The possibility remains that, in addition to the NAD kinase reaction catalyzed by Pos5p, any one of these other dehydrogenases may contribute physiologically to the supply of mitochondrial NADPH.
This study focuses on the reactions responsible for the generation of mitochondrial NADPH in S. cerevisiae (BY4742 background). Initially, we demonstrate that the NAD kinase triple mutant (utr1yef1pos5) is lethal, and using this triple mutant we reveal the dispensability of the mitochondrial NADH kinase reaction, thus emphasizing that the NAD kinase reaction followed by the NADP+-dependent dehydrogenase reaction is able to produce mitochondrial NADPH. We also demonstrate that mitochondrial NADP+-dependent acetaldehyde dehydrogenases (Ald4/Ald5p), and in particular Ald4p, are critical as a source of mitochondrial NADPH. The physiological significance of the NADH kinase reaction in the absence of Ald4p is also demonstrated.
EXPERIMENTAL PROCEDURES
Plasmids—The plasmids and primers used in this study are listed in Table 1 and supplemental Table S1, respectively. POS5 and its 406-bp upstream region (PPOS5) were amplified by PCR using the genomic DNA from S. cerevisiae BY4742 and then inserted into pRS415, yielding pMK1643 (PPOS5+POS5). Similarly, a BamHI fragment consisting of UTR1 plus its 503-bp upstream region (PUTR1) from YCp-UTR1 was inserted into pRS415, yielding pMK1702 (PUTR1+UTR1). Following disruption of the endogenous NcoI site in the POS5 insert of pMK1643 to give pMK1645, NcoI sites were again introduced at positions +1 and +185 of the POS5 insert of pMK1645 to yield pMK1646 and pMK1647, respectively. UTR1 from pSK49 and yfjB from pSK65 were then inserted into the NcoI/BamHI sites of pMK1646 and pMK1647, resulting in pMK1700 (PPOS5+UTR1), pMK1701 (PPOS5+yfjB), pMK1722 (PPOS5+ 62MTS+UTR1), and pMK1723 (PPOS5+62MTS+yfjB). 62MTS encodes a Pos5p-specific N-terminal additional sequence (62 amino acid residues) containing a putative mitochondrial targeting sequence (MTS) (Fig. 2) (9). Although a deficiency in nucleotide A at the +957 position of POS5 in pMK1643 was later found, PPOS5 plus the correct POS5 was again inserted into pRS415, yielding pMK2127 (PPOS5+POS5). An NcoI site was again introduced at the +1 position of POS5 in pMK2127, giving pMK2147 (PPOS5+POS5 (NcoI at +1)). MTS was removed from POS5 in pMK2127, giving pMK2145 (PPOS5+POS5ΔMTS) encoding Pos5ΔMTSp (Fig. 2), using the primers pos5f-17 and pos5r-17p normal (supplemental Table S1) and pMK2127 as a template. An NdeI site was then introduced at the +1 position of POS5ΔMTS using the primers pos5f-17 and pos5rNdeI-17p (supplemental Table S1) and pMK2127 as a template, giving pMK2148. The NdeI/BamHI fragment (POS5ΔMTS) from pMK2148 was then inserted into pET-28b (Novagen, Darmstadt, Germany), yielding pMK2159. Accurate synthesis of all the constructed plasmids was confirmed by DNA sequencing.
TABLE 1.
Plasmids used in this study
| Plasmids | Description | Resource |
|---|---|---|
| pRS415 | LEU2, CEN, Apr | (37) |
| YCp-UTR1 | 5′-503-bp (PUTR1)+UTR1 in SmaI of YCplac33 | (5) |
| pSK49 | UTR1 in NcoI/BamHI of pET-14b | (4) |
| pSK65 | yfjB in NcoI/BamHI of pET-14b | (25) |
| pET-28b | For an expression in E. coli, Kanr | Novagen |
| pMK1643a | 5′-406-bp (PPOS5)+POS5, amplified by PCR, in SacI/BamHI of pRS415 | This study |
| pMK1702 | PUTR1+UTR1 in BamHI of pRS415, from YCp-UTR1 | This study |
| pMK1645b | PPOS5+POS5 (ΔNcoI) in pRS415, from pMK1643 | This study |
| pMK1646c | PPOS5+POS5 (NcoI at +1) in pRS415, from pMK1645 | This study |
| pMK1647d | PPOS5+POS5 (NcoI at +185) in pRS415, from pMK1645 | This study |
| pMK1700 | PPOS5+UTR1 in pRS415, from NcoI/BamHI fragments of pMK1646 and pSK49 | This study |
| pMK1701 | PPOS5+yfjB in pRS415, from NcoI/BamHI fragments of pMK1646 and pSK65 | This study |
| pMK1722 | PPOS5+62MTS+UTR1 in pRS415, from NcoI/BamHI fragments of pMK1647 and pSK49 | This study |
| pMK1723 | PPOS5+62MTS+yfjB in pRS415, from NcoI/BamHI fragments of pMK1647 and pSK65 | This study |
| pMK2127 | PPOS5+POS5 in SacI/BamHI of pRS415 | This study |
| pMK2147c | PPOS5+POS5 (NcoI at +1) in pRS415, from pMK2127 | This study |
| pMK2145e | PPOS5+POS5ΔMTS in pRS415, from pMK2127 | This study |
| pMK2148f | PPOS5+POS5ΔMTS (NdeI at +1) in pRS415, from pMK2127 | This study |
| pMK2159 | POS5ΔMTS in pET-28b, from NcoI/BamHI fragments of pMK2148 and pET-28b | This study |
POS5 in pMK1643 lacks nucleotide A at +957.
NcoI in POS5 was disrupted by changing +226 CCATGG +231 to +226 CCTTGG +231 but had no effect on the encoding of amino acids.
NcoI was introduced into +1 of POS5 in pMK1645 by changing -2 AAATGT +4 to -2 CCATGG +4, resulting in a change of encoded residues from 1MF2 to 1MV2.
NcoI was introduced into a site at +185 of POS5 in pMK1645 by changing +185 TCTGGC +190 to +185 CCATGG +190, resulting in a change in the encoded residues from 62IWQ64 to 62TME64.
MTS (48 bp: +4 to +51), encoding 16 amino acid residues, was removed from POS5.
NdeI was introduced into +1 of POS5ΔMTS by changing -3 AAAATG +3 to -3 CATATG +3, giving no change of residues.
FIGURE 2.
Alignment of the primary structures of Pos5p and YfjB. Alignment was conducted using ClustalW (28). The Pos5p-specific additional N-terminal amino acid sequence consisting of 62 amino acid residues (62MTS) is underlined. The putative MTS is double-underlined, and the putative cleavage site is denoted by an arrow (9). Pos5ΔMTSp has an N-terminal sequence, MSTLDSHS, lacking 16 residues (2nd to 17th residue) from Pos5p. Identical residues are denoted by an asterisk (*), strongly conserved residues by a colon (:) and weakly conserved residues by a period (.).
Strains and Cultivations—For the cultivation of yeast, standard yeast media were used (17). If required, arginine (Arg) was added to the medium to a final concentration of 20 μg/ml. The yeast strains used in this study were derived from the parental strains BY4742 (MATα leu2Δ0 lys2Δ0 ura3Δ0 his3Δ1) and BY4741 (MATa leu2Δ0 met15Δ0 ura3Δ0 his3Δ1) (EUROSCARF). BY4742 deletion strains (pos5, idp1, mae1, ald4, and ald5), constructed using the kanMX4 cassette, were purchased from EUROSCARF. MK933 (BY4742 utr1Δ::kanMX4 yef1Δ::HIS3 YCp-UTR1), MK1219 (BY4742 utr1Δ::kanMX4 yef1Δ::HIS3 pos5Δ::CgLEU2), and MK1224 (BY4742 pos5Δ::CgLEU2) have been described previously (5). MK1598 (BY4742 utr1Δ::kanMX4 yef1Δ::HIS3 pos5Δ::hphMX4 YCp-UTR1) was constructed by replacing POS5 in MK933 with hphMX4 (18). MK805 (BY4741 pos5Δ::HIS3) was constructed by replacing POS5 in BY4741 with HIS3MX6 (19). IDP1, MAE1, ALD4, and ALD5 in MK805 were each replaced by kanMX4, resulting in MK2190 (pos5idp1), MK2191 (pos5mae1), MK2164 (pos5ald4), and MK2192 (pos5ald5), respectively. Correct replacement was confirmed by genomic PCR as described previously (19). Following introduction of the pRS415-derived plasmids containing POS5, UTR1, and yfjB, or their chimeric DNA into MK1598 (utr1yef1pos5 carrying YCp-UTR1), YCp-UTR1 (URA3) was removed by plasmid shuffling on SD medium containing 0.1% 5-fluoroorotic acid (20). The phenotype of the resultant viable cells lacking YCp-UTR1 was further examined after confirmation of the cell as uracil (Ura)–. To check growth phenotypes, cells were grown in SD media, suspended in sterilized water, normalized to an A600 of 2.0, and then serially diluted 10-fold. Each dilution (5.0 μl) was spotted onto each solid medium and incubated at 30 °C for 3 days.
Subcellular Fractionation—Subcellular fractionation into a mitochondrial fraction and a post-mitochondrial supernatant (PMS) fraction was conducted as described previously (21). The solution containing mitochondria was sonicated to disrupt the mitochondria and centrifuged at 4 °C and 20,000 × g for 10 min, and the supernatant was used as the mitochondrial fraction.
Assays—Protein concentrations were determined as described previously using bovine serum albumin as a standard (22). All activities were assayed at 30 °C. NAD kinase activity was assayed in a 1.0-ml reaction mixture containing 5.0 mm NAD+, 5.0 mm ATP, 5.0 mm MgCl2, and 100 mm Tris-HCl, pH 8.0. The reaction was terminated by heating the mixture in boiling water for 5 min. To assay the NAD kinase activity of the mitochondrial or PMS fraction from yeast, the NADP+ formed was determined by a cycling assay in a 1.0-ml reaction mixture containing 5.0 mm glucose 6-phosphate, 0.5 unit of glucose-6-phosphate dehydrogenase, 0.2 mg/ml thiazolyl blue tetrazolium bromide, and 0.03 mg/ml 1-methoxy-5-methylphenazinium methyl sulfate (23). To assay the NAD kinase activity of purified Pos5ΔMTSp (to determine the optimum pH), the NADP+ formed was determined without either thiazolyl blue tetrazolium bromide or 1-methoxy-5-methylphenazinium methyl sulfate (5), and the assay was conducted in the presence of 2.0 mm NAD+. The NAD kinase activity of purified Pos5ΔMTSp or purified Escherichia coli NAD kinase (YfjB) was also continuously assayed in a 1.0-ml reaction mixture containing 5.0 mm NAD+, 5.0 mm ATP, 5.0 mm MgCl2, 5.0 mm glucose 6-phosphate, 0.5 unit of glucose-6-phosphate dehydrogenase, and 100 mm Tris-HCl, pH 8.0 (5). The NADH kinase activity was assayed as described previously (5) in a 1.0-ml reaction mixture containing 2.0 mm NADH, 5.0 mm ATP, 5.0 mm MgCl2, and 100 mm Tris-HCl, pH 8.0. The reaction was terminated by the addition of 0.1 ml of 1.0 m NaOH followed by immediate immersion of the test tube in boiling water for 1.5 min. We confirmed that this boiling treatment under alkaline conditions completely degrades at least 0.1 mm NADP+ (data not shown). The mixture was neutralized by the addition of 0.3 ml of neutralization solution (0.5 m triethanolamine-HCl, 0.4 m Tris-HCl, 25 mm NH4Cl, 25 mm α-ketoglutarate, pH 7.8). The NADH and NADPH thus formed were enzymatically oxidized to NAD+ and NADP+, respectively, by the addition of 12.5 units of glutamate dehydrogenase followed by incubation at 30 °C for 10 min. Oxidation was monitored by observing the decrease in A340. After the oxidation reaction was terminated by immersing the test tube in boiling water for 5 min, the amount of NADP+ was determined. For the assay of the NADH kinase activity of purified Pos5ΔMTSp or purified YfjB, the amount of NADP+ was determined as described above without either thiazolyl blue tetrazolium bromide or 1-methoxy-5-methylphenazinium methyl sulfate. For the assay of the NADH kinase activity of the mitochondrial or PMS fraction from yeast, the amount of NADP+ was determined by the cycling assay as described above. Glucose-6-phosphate dehydrogenase activity itself was continuously assayed in a 1.0-ml reaction mixture containing 1.0 mm glucose 6-phosphate, 0.5 mm NADP+, 10 mm MgCl2, and 100 mm Tris-HCl, pH 8.0 (24). Cytochrome c oxidase activity was assayed using a cytochrome c oxidase assay kit (CYTOCOX1, Sigma). One unit of enzyme activity was defined as 1.0 μmol of product formed in 1 min at 30 °C, and specific activity was expressed in units/mg of protein.
Expression and Purification—Pos5ΔMTSp was expressed in E. coli RosettaBlue (Novagen) carrying pMK2159 (MK2162) as described previously (9), except that expression was induced at 16 °C for 3 days after the addition of isopropyl thio-β-galactoside to a final concentration of 0.2 mm. Purification was conducted as described previously (9). Purified Pos5ΔMTSp (7.6 mg) was obtained from an approximate 350-ml culture. The Pos5ΔMTSp expressed from MK2162 contains a tag (MGSSHHHHHHSSGLVPRGSH) at its N terminus, but no tag at its C terminus. YfjB was expressed and purified as described previously (25) and was stored at –30 °C.
Phylogenic Tree—A BLAST search (26) was conducted using the primary structure of Pos5p as the query and the Kyoto Encyclopedia of Genes and Genomes (KEGG) as the data base. Using the primary structure of the 50 proteins that show the highest homology with Pos5p, as well as that of Arabidopsis thaliana NADK3 (27) exhibiting only about a 750th homology with Pos5p, a ClustalW search (28) was performed. An unrooted NJ tree was then constructed. These searches were performed using the KEGG web site. Localization was predicted using TargetP (29).
RESULTS
Lethality of Utr1yef1pos5—We previously reported that the S. cerevisiae NAD kinase triple mutant utr1yef1pos5 (MK1219: BY4742 utr1Δ::kanMX4 yef1Δ::HIS3 pos5Δ::CgLEU2) was viable (5). However, during further analysis of MK1219, we found that, although POS5 was certainly replaced by CgLEU2, native POS5 was still unexpectedly detected by PCR in the genomic DNA of MK1219. Therefore, another triple mutant was constructed carrying YCp-UTR1 (MK1598 (utr1Δ::kanMX4 yef1Δ::HIS3 pos5Δ::hphMX4 YCp-UTR1)). It was confirmed that POS5 was replaced by hphMX4, because no native POS5 was detectable by PCR in the genomic DNA of MK1598. MK1598 was Arg– and was unable to grow on medium containing 5-fluoroorotic acid (data not shown), indicating that the triple mutant is lethal. Furthermore, it was subsequently reported that the utr1pos5 double mutant in S288C and SEY6210.5 backgrounds is lethal, and hence the utr1yef1pos5 triple mutant in a SEY6210.5 background is also lethal (7, 10). In addition, re-examination of the genotype of the double mutant utr1pos5 (MK803: BY4742 utr1Δ::kanMX4 pos5Δ::HIS3) (5) revealed that UTR1 was not correctly replaced by kanMX4 in MK803. Thus, we concluded that the NAD kinase triple mutant is lethal and realized that MK1219 and MK803 were not the true triple and utr1pos5 double mutants, respectively.
Dispensability of the Mitochondrial NADH Kinase Reaction for the Generation of Mitochondrial NADPH—YfjB has previously been demonstrated to exhibit no NADH kinase activity (8, 25). In the present study, we re-confirmed that purified YfjB (0.26 μg), which has detectable NAD kinase activity (ΔA340 of 0.56 for a 10-min reaction), exhibits no NADH kinase activity after a 10-min reaction. We further confirmed that purified excess YfjB (5.2 μg and 130 μg) exhibits no NADH kinase activity after a 10-min reaction, which is consistent with our previous results (8).
In Pos5p, the N-terminal amino acid sequence consisting of 17 residues is predicted to be the MTS required for transfer of Pos5p to the mitochondria (Fig. 2) (9). Alignment of the primary structures of Pos5p and YfjB shows that, although they are very similar to each other, Pos5p has an additional sequence (62MTS) consisting of 62 amino acid residues (Fig. 2). This suggested that Utr1p and YfjB might be delivered to the mitochondria if they are fused to the 62MTS.
MK1598 (utr1yef1pos5 carrying YCp-UTR1) cells were transformed with pRS415 (LEU2) or pRS415-based plasmids as shown in Fig. 3. The viability of each transformant was examined on solid medium containing 5-fluoroorotic acid. Although the transformant with pRS415 alone was not viable, the other transformants were at least viable in the presence of Arg and were Ura– (data not shown), indicating that utr1yef1pos5 survives due to the help of the NAD kinase gene supplied from the pRS415-based plasmid and that the NADH kinase reaction is dispensable for cellular viability.
FIGURE 3.
Growth phenotypes of the NAD kinase triple mutants (utr1yef1pos5) having POS5, UTR1, and yfjB with or without 62MTS. The triple mutants carrying the indicated plasmids containing a denoted insert were spotted onto SD solid media with Arg (+Arg) or without Arg (–Arg), and SD solid medium containing both Arg and 2 mm H2O2 (+Arg +H2O2). As a control, wild-type (BY4742) cells carrying pRS415 alone (marked by asterisk) were also spotted. The triple mutant carrying POS5 (NcoI site at +1) (pMK2147) was Arg+ and H2O2r, indicating that Arg– and H2O2s in the absence of 62MTS were not attributed to the NcoI site that was introduced at +1of UTR1 and yfjB in pMK1700 and pMK1701, respectively. The mutant carrying PUTR1+UTR1 (pMK1702) was also Arg– and H2O2s and grew at approximately the same rate as that carrying PPOS5+UTR1 (pMK1700) (data not shown), indicating that replacement of PUTR1 by PPOS5 had no effect.
The mutants were Arg+ and exhibited resistance to H2O2 (H2O2r) in the presence of 62MTS, whereas they were Arg– and exhibited sensitivity to H2O2 (H2O2s) in the absence of 62MTS or MTS (Fig. 3). This result also implied that 62MTS-tagged enzymes (Utr1p and YfjB) are delivered to the mitochondria, whereas untagged-enzymes or Pos5ΔMTSp fail to enter the mitochondria. If this is the case, it can be concluded that the mitochondrial NADH kinase reaction is dispensable for the supply of mitochondrial NADPH.
Delivery of 62MTS-tagged Enzymes to the Mitochondria—To demonstrate possible delivery of 62MTS-tagged enzymes to the mitochondria, triple mutants carrying POS5 as well as UTR1 and yfjB with or without 62MTS were biochemically fractionated into mitochondrial and PMS fractions (Fig. 4). The NAD kinase activity in each fraction was then assayed. The activities of cytochrome c oxidase and glucose-6-phosphate dehydrogenase, which are markers for the mitochondrial and PMS fractions (30), respectively, ensured correct fractionation (supplemental Table S2).
FIGURE 4.
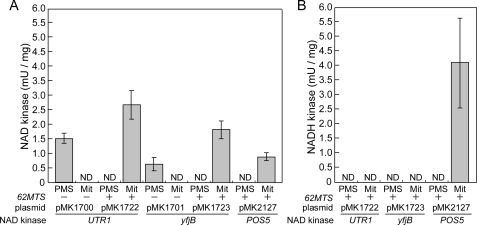
NAD kinase (A) and NADH kinase (B) activities in mitochondrial and PMS fractions from NAD kinase triple mutants (utr1yef1pos5) containing POS5 as well as UTR1 and yfjB with (+) or without (–) 62MTS. The mutants carrying POS5 (MK2127), UTR1 (pMK1700), 62MTS+UTR1 (pMK1722), yfjB (pMK1701), or 62MTS+yfjB (pMK1723) were fractionated into mitochondrial and PMS fractions (supplemental Table S2). The NAD kinase (A) and NADH kinase (B) activities in each fraction were assayed. NADH kinase activity in the fractions from mutants without 62MTS was not determined. NAD kinase activity was assayed in the presence of mitochondrial fractions from mutants carrying pMK1700 (36 and 72 μg), pMK1722 (16, 32, 34, 63, 68, and 126 μg), pMK1701 (28 and 56 μg), pMK1723 (20, 22, 34, 50, 68, and 100 μg), or pMK2127 (20 and 40 μg), and in the presence of PMS fractions from mutants carrying pMK1700 (17 and 34 μg), pMK1722 (39, 42, 60, and 120 μg), pMK1701 (12 and 24 μg), pMK1723 (27, 42, 49, and 98 μg), or pMK2127 (28 and 43 μg) for 30- and 60-min reactions. NADH kinase activity was assayed in the presence of mitochondrial fractions from mutants carrying pMK1722 (16, 32, 52, and 208 μg), pMK1723 (18, 36, 52, and 208 μg), or pMK2127 (17, 20, 34, and 40 μg), and in the presence of PMS fractions from mutants carrying pMK1722 (39 and 128 μg), pMK1723 (45 and 128 μg), or pMK2127 (47 and 55 μg) for 10- and 20-min reactions. NADH kinase activity of mitochondrial fractions from mutants carrying pMK1722 (16, 32, and 52 μg) or pMK1723 (52 μg) was not detected even after 60-, 120-, 240-, and 600-min reactions. Means ± S.D. values of NAD kinase and NADH kinase activities are presented (details are described in the legend of supplemental Table S2). ND, not detected; Mit, mitochondrial.
NAD kinase activity was detected in the mitochondrial fraction from the mutants carrying POS5 or UTR1 and yfjB with 62MTS, but not in the PMS fraction. Conversely, activity was detected in the PMS fraction, but not in the mitochondrial fraction, in the absence of 62MTS (Fig. 4A), demonstrating that the 62MTS-tagged enzymes (Utr1p and YfjB) as well as Pos5p are delivered to the mitochondria, whereas untagged enzymes are not. Furthermore, NADH kinase activity was detected in the mitochondrial fraction from the mutants carrying POS5, but not in the PMS fraction (Fig. 4B). In the mitochondrial fraction from the mutants carrying UTR1 and yfjB with 62MTS, no NADH kinase activity was detected (Fig. 4B). We attribute this lack of NADH kinase activity, at least that of the 62MTS-tagged Utr1p, to the sensitivity of the assay system, because purified Utr1p shows lower NADH kinase activity than NAD kinase activity (Table 2) (7). Collectively, these results suggest that the mitochondrial NADH kinase reaction is dispensable for the supply of mitochondrial NADPH.
TABLE 2.
Kinetic values for the NAD+ and NADH of Pos5p, Utr1p, Yef1p, and NADK3
Values were determined by varying the concentrations of NAD+ or NADH in the presence of ATP at 5.0 mm (Pos5p, Utr1p, and Yef1p) and 4.0 mm (NADK3), except for the Vmax of Utr1p and Yef1p, which was the specific activity assayed in the presence of 1.0 mm ATP and 1.0 mm NAD+ or 1.0 mm NADH (7).
The Mitochondrial NADP+-dependent Dehydrogenase Reaction as a Source of Mitochondrial NADPH—The dispensability of the NADH kinase reaction emphasizes the significance of the NAD kinase reaction as well as the mitochondrial NADP+-dependent dehydrogenase for the generation of mitochondrial NADPH. Pyridine nucleotide transhydrogenase activity is not detected in the mitochondria of S. cerevisiae (16). The known mitochondrial NADP+-dependent dehydrogenases of S. cerevisiae are isocitrate dehydrogenase (Idp1p) (31), malic enzyme (Mae1p) (14), and acetaldehyde dehydrogenases (Ald4p and Ald5p) (15).
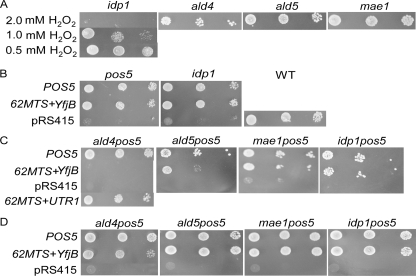
To evaluate the contribution of each dehydrogenase, the phenotypes of pos5, idp1, mae1, ald4, and ald5 carrying only pRS415 were examined. idp1 exhibited H2O2s at 1.0 mm and 2.0 mm H2O2, but not at 0.5 mm H2O2, whereas the other single mutants were H2O2r at 2.0 mm H2O2 (Fig. 5A). However, idp1 mutants carrying POS5 (pMK2127) or 62MTS+yfjB (pMK1723) were also H2O2r (Fig. 5B). Moreover, pos5idp1 cells carrying 62MTS+yfjB were resistant to 2.0 mm H2O2 (Fig. 5C), indicating that Idp1p is not required for the generation of mitochondrial NADPH, even in the absence of the NADH kinase reaction.
FIGURE 5.
Phenotypes of the mutants lacking mitochondrial NADP+-dependent dehydrogenase genes. A, growth of the single mutant carrying pRS415 on SD solid media containing Arg and 0.5, 1.0, or 2.0 mm H2O2. B–D, growth of the single (B) or double (C and D) mutants carrying POS5 (pMK2127), 62MTS+yfjB (pMK1723), pRS415, or 62MTS+UTR1 (pMK1722) on SD solid media containing both Arg and 2.0 mm H2O2 (B and C), and with neither Arg nor H2O2 (D). Mutants carrying pRS415 grew as well as the WT carrying pRS415 on SD solid media containing Arg (data not shown). In the single mutants, each gene is replaced by kanMX4. In the double mutants, POS5 is replaced by HIS3MX6 and each dehydrogenase gene by kanMX4.
pos5mae1 cells carrying 62MTS+yfjB were also H2O2r (Fig. 5C), again demonstrating the dispensability of Mae1p. However, pos5ald4 carrying 62MTS+yfjB was H2O2s at 2.0 mm H2O2, whereas the double mutants having POS5 or 62MTS+UTR1 (pMK1722) were H2O2r (Fig. 5C). pos5ald5 carrying 62MTS+yfjB was slightly H2O2s (Fig. 5C). The possibility that 62MTS+yfjB was not functionally expressed in pos5ald4 and pos5ald5 can be excluded, because both pos5ald4 and pos5ald5 carrying 62MTS+yfjB were Arg+ (Fig. 5D), whereas both pos5ald4 and pos5ald5 carrying pRS415 alone were Arg– (data not shown). This indicates that the NAD kinase reaction catalyzed by the expressed 62MTS+yfjB followed by the dehydrogenase reaction can supply the NADPH needed for Arg biosynthesis, but cannot supply that required to protect cells against 2.0 mm H2O2.
The H2O2s phenotype of pos5ald4 carrying 62MTS+yfjB indicated the essentiality of the mitochondrial acetaldehyde dehydrogenase reaction catalyzed by Ald4p in the absence of the mitochondrial NADH kinase reaction. The slight H2O2s phenotype of pos5ald5 carrying 62MTS+yfjB also demonstrated that Ald5p partially contributes to the supply of mitochondrial NADPH. Simultaneously, the H2O2r phenotypes of pos5ald4 carrying POS5 and 62MTS+UTR1 demonstrated that the mitochondrial NADH kinase reaction is indispensable in the absence of Ald4p, i.e. that the mitochondrial NADH kinase reaction is physiologically significant.
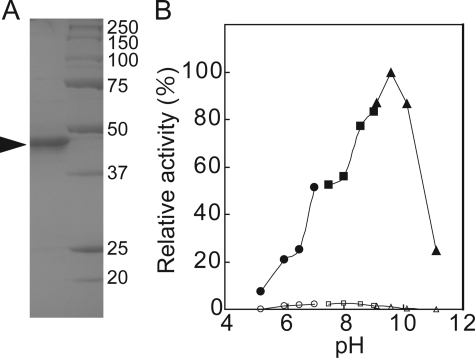
NAD Kinase and NADH Kinase Activity of Purified Pos5p (Pos5ΔMTSp)—Despite the significance of Pos5p, its biochemical properties have not been definitively determined (6, 9). By removing MTS, soluble Pos5p (Pos5ΔMTSp) was expressed in E. coli, as reported previously (9), and purified (Fig. 6A). The purified Pos5p exhibited considerably higher NADH kinase than NAD kinase activity within a range of pH 5 to pH 11 (Fig. 6B). The optimum pH of the activities of NAD kinase and NADH kinase were pH 8.0 and pH 9.5, respectively. The kinetic values of Pos5p were determined and compared with those of Utr1p, Yef1p, and NADK3 from A. thaliana (Tables 2 and 3). NADK3 is also an NAD kinase exhibiting high NADH kinase activity (27). Comparison of the values of Pos5p with those of Utr1p and Yef1p clearly indicated that Pos5p exhibits a preference for NADH over NAD+, whereas Utr1p exhibits a preference for NAD+ over NADH, and also that the activities of Yef1p toward both NAD+ and NADH were low. The high Km (5.3 mm) for the NAD+ of Pos5p could explain the low NAD kinase activity (shown in Fig. 6B), which was assayed in the presence of 2.0 mm NAD+. It should be noted that NAD kinase activity in the mitochondrial fraction from the utr1yef1pos5 triple mutant carrying POS5 was assayed in the presence of 5.0 mm NAD+ (Fig. 4A). In addition, Pos5p exhibited a kinetic behavior that was different to that of NADK3 (Tables 2 and 3). In particular, the Km of NADK3 for NADH is lower than that of Pos5p.
FIGURE 6.
Properties of purified Pos5p (Pos5pΔMTSp). A, purification of Pos5p. Lane 1, purified Pos5p (1.8μg); lane 2, marker (Bio-Rad). The arrowhead indicates the purified Pos5p. B, optimum pH of the NAD kinase (open symbol) and NADH kinase (closed symbol) activities of Pos5p. The activity of NADH kinase at pH 9.5 was taken as 100%. Sodium acetate (circles), Tris-HCl (squares), and glycine-HCl (triangles).
TABLE 3.
Kinetic values for the ATP of Pos5p and NADK3
Values were determined by varying the concentration of ATP in the presence of 2.0 mm NAD+ or NADH (Pos5p) and in the presence of 4.0 mm NAD+ or 0.8 mm NADH (NADK3).
|
Km
|
Vmax
|
Vmax/Km
|
||||||
|---|---|---|---|---|---|---|---|---|
| ATP (NAD+) | ATP (NADH) | ATP (NAD+) | ATP (NADH) | ATP (NAD+) | ATP (NADH) | |||
| mm | μmol.min-1.mg-1 | |||||||
| Pos5p | 0.89 | 0.45 | 3.5 | 44.6 | 3.9 | 99.1 | ||
| NADK3a | 0.19 | 0.062 | 20.3 | 39.5 | 106.8 | 637 | ||
Data are from Ref. 27.
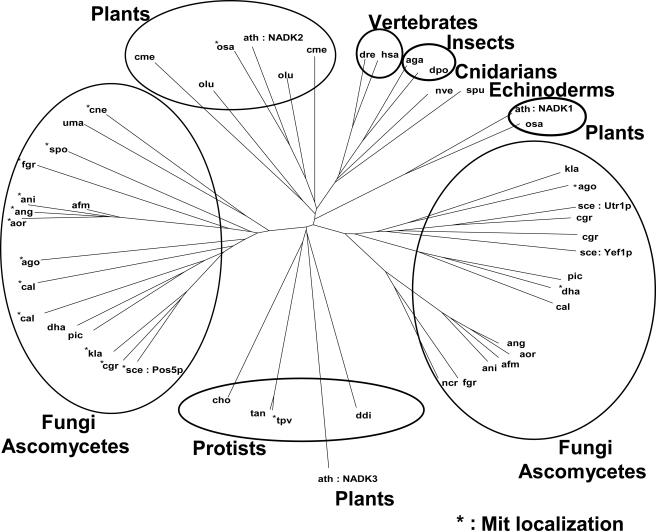
BLAST analysis using the primary structure of Pos5p indicated that Pos5p shows highest homology to the NAD kinase homologs from fungi, plants, and other higher eukaryotes. Pos5p is not, however, homologous to Arabidopsis NADK3, which is only approximately the 750th homologous protein. Using the Pos5p-homologous proteins (50 proteins) plus NADK3, a phylogenic tree was constructed (Fig. 7). The tree indicates that Pos5p belongs to the fungal NAD kinase homolog group, which is distinguishable from another fungal group containing Utr1p and Yef1p. The proteins, which were predicted (using TargetP) to reside in the mitochondria, were concentrated in the group to which Pos5p belongs. The tree suggests that the proteins in the Pos5p fungal group are mitochondrial NAD kinases displaying high NADH kinase activity. NADK3 is located distant from Pos5p in the tree.
FIGURE 7.
The phylogenic tree constructed using Pos5p-homologous proteins plus NADK3 from A. thaliana. The three-letter code for each organism in the KEGG database is shown to represent each NAD kinase homolog. Proteins predicted to reside in the mitochondria are denoted by an asterisk. S. cerevisiae (sce) NAD kinases (Pos5p, Utr1p, and Yef1p), and Arabidopsis (ath) NAD kinases (NADK1, NADK2, and NADK3) are specified.
DISCUSSION
This study has demonstrated that the mitochondrial NAD kinase reaction followed by the NADP+-dependent acetaldehyde dehydrogenase reaction catalyzed by Ald4/Ald5p, and in particular Ald4p, serves as a source of mitochondrial NADPH in S. cerevisiae. Although the NAD kinase activity of Pos5p was demonstrated to be lower than its NADH kinase activity (Tables 2 and 3 and Fig. 4), we assume that the former should be functional in mitochondria. It has been proposed that, when human cells are exposed to oxidative challenges, they rely on the existing NADP+ pool and enhance the capacity to retain it in a reduced state by increasing the activity of NADP+-dependent dehydrogenase (32). A single S. cerevisiae S288C cell is estimated to contain 22,200 molecules of Ald4p and 23,300 molecules of Ald5p, a substantial excess compared with the 4,650 molecules of Pos5p (33). Furthermore, a published microarray data base found in the Saccharomyces Genome Database reveals that ALD4 is up-regulated by H2O2 treatment, whereas MAE1 is strongly down-regulated, ALD5 is slightly down-regulated, and IDP1 is slightly up-regulated. The different responses of ALD4 and ALD5 to H2O2 may explain the reason why ALD4 is more important than ALD5 (Fig. 5C). We assume that the two successive reactions (the NAD kinase reaction followed by the acetaldehyde dehydrogenase reaction) can produce NADPH even when the amount of mitochondrial NADH is insufficient, e.g. when cell growth is solely dependant on fermentation, and even when the NADP+ pool is limited due to the lower NAD kinase activity of Pos5p. Moreover, taken together with the significance of Ald6p as a cytosolic NADPH source (3), the present results confirm the importance of the acetaldehyde dehydrogenase reaction as a source of both cytosolic and mitochondrial NADPH.
In the case of mammals and humans, NAD kinase is located in the cytosol, not the mitochondria, and human NAD kinase appears to exhibit only limited NADH kinase activity (32). This indicates that the supply of mitochondrial NADPH in mammals is critically dependent on mitochondrial NADP+-dependent dehydrogenase. Indeed, the significant role of mitochondrial NADP+-dependent isocitrate dehydrogenase has been reported for the mammalian system (11). Together with the present results, these observations suggest that mitochondrial NADP+-dependent acetaldehyde dehydrogenase might also be significant in mammals.
On the basis of the phenotypes of pos5 and the higher NADH kinase activity than NAD kinase activity of Pos5p, the NADH kinase reaction has been proposed to be significant as a mitochondrial NADPH source (6), although no evidence demonstrating the physiological importance of the mitochondrial NADH kinase reaction has previously been presented. Here, on the basis of the H2O2r phenotypes of ald4pos5 carrying POS5 or 62MTS+UTR1 (Fig. 5C), we present evidence for the physiological significance of the mitochondrial NADH kinase reaction. Although the NADH kinase activity of purified Utr1p is lower than that of Pos5p (Table 2) (7) and the mitochondrial NADH kinase activity of 62MTS-tagged Utr1p was below the limits of detection (Fig. 4B), we also demonstrate that the lower NADH kinase activity of 62MTS-tagged Utr1p is sufficient to protect cells against H2O2.
Although the physiological role of the “higher” NADH kinase activity of Pos5p remains to be determined, this elevated activity may be related to the protection of cells against ROS. In cells dependent on mitochondrial respiration, the mitochondrial matrix would be replete with NADH due to the NADH-generating TCA cycle. Respiration itself is a primary source of ROS (34). It is possible that the mitochondrial NADH kinase reaction converts this abundant mitochondrial NADH directly into NADPH, without the help of the dehydrogenase reaction, thereby protecting the cells from ROS. In addition, the phylogenic tree suggests that fungi might have Utr1p-like cytosolic NAD kinase and mitochondrial Pos5p-like NAD kinase exhibiting higher NADH kinase activity (Fig. 7). Taken together with the advantage of the NADH kinase reaction in respiring cells, it is tempting to speculate that the fungal mitochondrial “higher” NADH kinase reaction enables fungi to grow rapidly on various non-fermentable carbon sources.
With regard to Idp1p, no phenotype of idp1 was reported in the presence of 0.5 mm H2O2, whereas zwf1 exhibits H2O2s at 0.5 mm H2O2 (12). Although it was found that idp1 exhibits H2O2s at 1.0 or 2.0 mm H2O2 (Fig. 5A), this sensitivity was compensated for by plasmid-derived, but not chromosome-derived, Pos5p, and even plasmid-derived mitochondrial YfjB (62MTS-tagged YfjB) (Fig. 5B). Pos5p derived from the chromosome may therefore be inactivated via an unrevealed mechanism in idp1. Nevertheless, because the idp1pos5 carrying 62MTS+yfjB was H2O2r, whereas the ald4pos5 carrying 62MTS+yfjB was H2O2s (Fig. 5C), it is concluded that Idp1p is dispensable, but that Ald4p is indispensable, in the absence of the mitochondrial NADH kinase reaction.
Supplementary Material
This work was supported by Grant-in-Aid 19780057 from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to S. K.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Tables S1 and S2.
Footnotes
The abbreviations used are: ROS, reactive oxygen species; 62MTS, the DNA encoding a Pos5p-specific N-terminal additional sequence consisting of 62 amino acid residues; MTS, mitochondrial targeting sequence; MTS, the DNA encoding the MTS; PMS, post-mitochondrial supernatant; YfjB, E. coli NAD kinase; H2O2r, resistance to H2O2; H2O2s, sensitivity to H2O2; Pos5ΔMTSp, Pos5p lacking the MTS.
References
- 1.Pollak, N., Dolle, C., and Ziegler, M. (2007) Biochem. J. 402 205–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Minard, K. I., and McAlister-Henn, L. (2005) J. Biol. Chem. 280 39890–39896 [DOI] [PubMed] [Google Scholar]
- 3.Grabowska, D., and Chelstowska, A. (2003) J. Biol. Chem. 278 13984–13988 [DOI] [PubMed] [Google Scholar]
- 4.Kawai, S., Suzuki, S., Mori, S., and Murata, K. (2001) FEMS Microbiol. Lett. 200 181–184 [DOI] [PubMed] [Google Scholar]
- 5.Shi, F., Kawai, S., Mori, S., Kono, E., and Murata, K. (2005) FEBS J. 272 3337–3349 [DOI] [PubMed] [Google Scholar]
- 6.Outten, C. E., and Culotta, V. C. (2003) EMBO J. 22 2015–2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bieganowski, P., Seidle, H. F., Wojcik, M., and Brenner, C. (2006) J. Biol. Chem. 281 22439–22445 [DOI] [PubMed] [Google Scholar]
- 8.Mori, S., Kawai, S., Shi, F., Mikami, B., and Murata, K. (2005) J. Biol. Chem. 280 24104–24112 [DOI] [PubMed] [Google Scholar]
- 9.Strand, M. K., Stuart, G. R., Longley, M. J., Graziewicz, M. A., Dominick, O. C., and Copeland, W. C. (2003) Eukaryot. Cell 2 809–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shianna, K. V., Marchuk, D. A., and Strand, M. K. (2006) Mitochondrion 6 94–101 [DOI] [PubMed] [Google Scholar]
- 11.Jo, S. H., Son, M. K., Koh, H. J., Lee, S. M., Song, I. H., Kim, Y. O., Lee, Y. S., Jeong, K. S., Kim, W. B., Park, J. W., Song, B. J., and Huh, T. L. (2001) J. Biol. Chem. 276 16168–16176 [DOI] [PubMed] [Google Scholar]
- 12.Minard, K. I., Jennings, G. T., Loftus, T. M., Xuan, D., and McAlister-Henn, L. (1998) J. Biol. Chem. 273 31486–31493 [DOI] [PubMed] [Google Scholar]
- 13.Outten, C. E., Falk, R. L., and Culotta, V. C. (2005) Biochem. J. 388 93–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boles, E., de Jong-Gubbels, P., and Pronk, J. T. (1998) J. Bacteriol. 180 2875–2882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saint-Prix, F., Bonquist, L., and Dequin, S. (2004) Microbiology 150 2209–2220 [DOI] [PubMed] [Google Scholar]
- 16.Evans, T. C., Mackler, B., and Grace, R. (1985) Arch. Biochem. Biophys. 243 492–503 [DOI] [PubMed] [Google Scholar]
- 17.Guthrie, C., and Fink, G. R. (1991) in Guide to Yeast Genetics and Molecular Biology (Abelson, J. N., and Simon, M. I., eds) pp. 3–21, Academic Press, San Diego, CA
- 18.Goldstein, A. L., and McCusker, J. H. (1999) Yeast 15 1541–1553 [DOI] [PubMed] [Google Scholar]
- 19.Wach, A., Brachat, A., Alberti-Segui, C., Rebischung, C., and Philippsen, P. (1997) Yeast 13 1065–1075 [DOI] [PubMed] [Google Scholar]
- 20.Boeke, J. D., Trueheart, J., Natsoulis, G., and Fink, G. R. (1987) Methods Enzymol. 154 164–175 [DOI] [PubMed] [Google Scholar]
- 21.Daum, G., Bohni, P. C., and Schatz, G. (1982) J. Biol. Chem. 257 13028–13033 [PubMed] [Google Scholar]
- 22.Bradford, M. M. (1976) Anal. Biochem. 72 248–254 [DOI] [PubMed] [Google Scholar]
- 23.Ochiai, A., Mori, S., Kawai, S., and Murata, K. (2004) Protein Expression Purif. 36 124–130 [DOI] [PubMed] [Google Scholar]
- 24.Noltmann, E. A., Gubler, C. J., and Kuby, S. A. (1961) J. Biol. Chem. 236 1225–1230 [PubMed] [Google Scholar]
- 25.Kawai, S., Mori, S., Mukai, T., Hashimoto, W., and Murata, K. (2001) Eur. J. Biochem. 268 4359–4365 [DOI] [PubMed] [Google Scholar]
- 26.Altschul, S. F., Gish, W., Miller, W., Myers, E. W., and Lipman, D. J. (1990) J. Mol. Biol. 215 403–410 [DOI] [PubMed] [Google Scholar]
- 27.Turner, W. L., Waller, J. C., and Snedden, W. A. (2005) Biochem. J. 385 217–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thompson, J. D., Higgins, D. G., and Gibson, T. J. (1994) Nucleic Acids Res. 22 4673–4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Emanuelsson, O., Nielsen, H., Brunak, S., and von Heijne, G. (2000) J. Mol. Biol. 300 1005–1016 [DOI] [PubMed] [Google Scholar]
- 30.Iwahashi, Y., Hitoshio, A., Tajima, N., and Nakamura, T. (1989) J. Biochem. (Tokyo) 105 588–593 [DOI] [PubMed] [Google Scholar]
- 31.Haselbeck, R. J., and McAlister-Henn, L. (1991) J. Biol. Chem. 266 2339–2345 [PubMed] [Google Scholar]
- 32.Pollak, N., Niere, M., and Ziegler, M. (2007) J. Biol. Chem. 282 33562–33571 [DOI] [PubMed] [Google Scholar]
- 33.Ghaemmaghami, S., Huh, W. K., Bower, K., Howson, R. W., Belle, A., Dephoure, N., O'Shea, E. K., and Weissman, J. S. (2003) Nature 425 737–741 [DOI] [PubMed] [Google Scholar]
- 34.Turrens, J. F. (1997) Biosci. Rep. 17 3–8 [DOI] [PubMed] [Google Scholar]
- 35.Thomas, D., Cherest, H., and Surdin-Kerjan, Y. (1991) EMBO J. 10 547–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Slekar, K. H., Kosman, D. J., and Culotta, V. C. (1996) J. Biol. Chem. 271 28831–28836 [DOI] [PubMed] [Google Scholar]
- 37.Sikorski, R. S., and Hieter, P. (1989) Genetics 122 19–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.