Abstract
Scatter factor (SF) (hepatocyte growth factor) is a pleiotrophic cytokine that accumulates in tumors, where it may induce invasion, angiogenesis, and chemoresistance. We have studied the mechanisms by which SF and its receptor (c-Met) protect cells against the DNA-damaging agent adriamycin (ADR) as a model for chemoresistance of SF/c-Met-overexpressing tumors. Previous studies identified a phosphatidylinositol 3-kinase/c-Akt/Pak1/NF-κB cell survival pathway in DU-145 prostate cancer and Madin-Darby canine kidney epithelial cells. Here we studied Src signaling pathways involved in SF cell protection. Src enhanced basal and SF stimulated NF-κB activity and SF protection against ADR, in a manner dependent upon its kinase and Src homology 3 domains; and endogenous Src was required for SF stimulation of NF-κB activity and cell protection. The ability of Src to enhance SF stimulation of NF-κB activity was due, in part, to its ability to stimulate Akt and IκB kinase activity; and Src-mediated stimulation of NF-κB was due, in part, to a Rac1/MKK3/6/p38 pathway and was Akt-dependent. SF caused the activation of Src and the Rac1 effector Pak1. Furthermore, SF induced activating phosphorylations of MKK3, MKK6, and p38 within the c-Met signalsome in an Src-dependent manner. The NF-κB-inducing kinase was found to act downstream of TAK1 (transforming growth factor-β-activated kinase 1) as a mediator of SF- and Src-stimulated NF-κB activity. Finally, the Src/Rac1/MKK3/6/p38 and Src/TAK1/NF-κB-inducing kinase pathways exhibited cross-talk at the level of MKK3. These findings delineate some novel signaling pathways for SF-mediated resistance to ADR.
Scatter factor (SF)2 (hepatocyte growth factor) is a pleiotrophic cytokine that can stimulate cell motility, invasion, proliferation, morphogenesis, and differentiation in different cell types and contexts. It is postulated to play roles in development, tissue repair and remodeling, tumorigenesis, and angiogenesis (1). Although SF was also identified as a “tumor cytotoxic factor” for hepatocarcinoma cells (2), in most cell types and contexts, SF inhibits apoptosis and promotes cell survival (3–6). We have studied the anti-apoptotic activity of SF against cancer therapy agents, particularly DNA-damaging agents (5–7). These studies showed that SF protects epithelial, carcinoma, and glioma cells against adriamycin (ADR) (a topoisomerase IIα inhibitor), ionizing radiation (which causes breakage of the sugar-phosphate backbone of DNA), cis-platinum (which cross-links DNA), and other agents. SF and its receptor c-Met are often overexpressed in tumors (8, 9), and their overexpression is associated with a poor prognosis in breast cancer and other tumor types (10–13). Thus, besides promoting invasion and metastasis, SF and c-Met overexpression in tumors may contribute to chemo/radioresistance.
Previous studies have identified some of the pathways through which SF protects epithelial and cancer cells against DNA-damaging agents. The core protective pathway involves signaling from c-Met to PI 3-kinase to the survival-promoting serine/threonine kinase c-Akt (5–7). In addition to apoptosis inhibition, this pathway enhances the repair of DNA strand breaks (6, 7). In this pathway, c-Akt acts upstream of Pak1 (p21-associated kinase 1) to stimulate NF-κB transcriptional activity and induce the expression of anti-apoptotic genes (TRAF2, cIAP1, and cIAP2) (7, 14) (7, 14). Gab1 (Grb2-associated binder-1), a multisubstrate adaptor protein, and the tumor suppressor phosphatase and tensin homolog (PTEN) act upstream of c-Akt as negative regulators of SF protection (7, 14).
c-Src, the homolog of the Rous sarcoma virus oncogene (v-src), is a member of a family of cytoplasmic tyrosine kinases. Src can function as a signal transducer for growth factor-stimulated cell migration, invasion, proliferation, survival, and tumorigenesis (15). However, its roles in cell motility and proliferation are better understood than its role in cell survival. One mechanism by which v-src or activated c-Src mutants can stimulate cell survival (and other functions) is through a PI 3-kinase/Akt pathway (16, 17). Because it is frequently overexpressed and/or activated in human cancers, Src (and other Src family kinases) is a potential target for cancer therapy (18).
Src and Src family kinases (e.g. Fyn) interact with the activated c-Met receptor, in part, via a “multifunctional docking site” (1349YVHVXXXYVNV) in the carboxyl terminus of the receptor (19). This interaction causes activation of Src (19, 20). In some settings, Src can mediate activation of c-Met or activation of the SF promoter (21, 22). Previously, we showed that Src can stimulate NF-κB activity and that inhibition of Src (e.g. using the pharmacologic inhibitor PP1) blocks the ability of SF to stimulate NF-κB activity (14). Here we demonstrate that Src is required for SF cell protection, and we identify pathways by which Src stimulates NF-κB activity and cell survival.
EXPERIMENTAL PROCEDURES
Cell Lines and Culture—DU-145 human prostate cancer and Madin-Darby canine kidney (MDCK) epithelial cells were obtained from the American Type Culture Collection (Manassas, VA). The cells were cultured in DMEM containing 5% fetal calf serum, nonessential amino acids (100 mm), l-glutamine (5 mm), streptomycin (100 μg/ml), and penicillin (100 units/ml) (from BioWhittaker, Walkersville, MD). The cells were grown at 37 °C in a humidified atmosphere of 95% air and 5% CO2.
Reagents—Recombinant human two-chain SF was a gift from Dr. Ralph Schwall (Genentech, South San Francisco, CA). Adriamycin (doxorubicin) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) dye were obtained from Sigma. The p38 inhibitor SB-202190 was purchased from Calbiochem. Antibody reagents are described below.
Expression Vectors—Vectors encoding wild-type (WT), activated mutant (Y527F and Y530F), kinase-dead (K295R), and SH3 domain mutant (D99N) chicken Src were provided by Dr. D. Shalloway (Cornell University, Ithaca, NY) (23). The Src cDNAs were cloned into the pcDNA3 vector (Clontech) using a PCR-based strategy, and the success of cloning was confirmed by sequence analysis. A dominant negative (DN) kinase-dead Akt mutant (K179A) in the pCIS2 vector was provided by Dr. M. J. Quon (NHLBI, National Institutes of Health, Bethesda) (24). WT-PTEN and a phosphatase-defect mutant (C124S) in the pFLAG-CMV vector were provided by Dr. M. Georgescu (Rockefeller University, New York) (25). WT-MKK3, DN-MKK3 (S189A/T193E), constitutively active (ca) MKK3 (S189E/T193E), WT-MKK6, DN-MKK6 (K82A), and ca-MKK6 (K82E) vectors were provided by Dr. R. Davis (University of Massachusetts Medical School, Worcester, MA) (26). WT-Rac1 and DN-Rac1 (RacN17) in the pCEFL vector were provided by Dr. J. S. Gutkind (NIDCR, NIH, Bethesda). WT-NIK and DN-NIK cDNAs in the pcDNA3 vector were provided by Dr. D. Wallach (Weizmann Institute of Science, Rehovot, Israel) (27). The DN-NIK vector encodes a kinase-dead mutant NIK with a KK → AA substitution within the ATP-binding domain. TAK1 and DN-TAK1 cDNAs in the pcDNA3 vector were provided by Dr. Hongbing Shu (National Jewish Medical Center, Denver, CO) (28).
Transient Transfections—Subconfluent proliferating cells were transfected overnight with the vector(s) of interest or the empty pcDNA3 vector (Invitrogen) (10 μg of plasmid DNA per 100-mm dish or 5 μg per well in 6-well dishes) using Lipofectamine™ (Invitrogen) and washed to remove the excess vector and Lipofectamine.
Small Interfering (si) RNA—Human NIK-siRNA (sc-36065) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Src-siRNA (M-005196) and nontargeting siRNA pools (Control siRNA, D-001206-13-01) were obtained from Upstate (Charlottesville, VA). siRNAs were transfected into cells using Oligofectamine® (Invitrogen) according to the manufacturer's instructions. Treatment of cells with 50 nm gene-specific siRNA for 48 h was sufficient to significantly knock down the target protein levels.
Measurement of NF-κB Transcriptional Activity—The NF-κB-Luc reporter (Stratagene, La Jolla, CA) contains five copies of the NF-κB enhancer element upstream of a TATA box and luciferase. Briefly, subconfluent proliferating cells in 2-cm2 wells were transfected overnight with 0.25 μgofNF-κB-Luc and 0.25 μg of each indicated vector, using Lipofectamine™. The cells were washed, allowed to recover for several hours, treated ± SF (100 ng/ml) for 24 h, and harvested for measurement of luciferase activity, using the dual luciferase reporter assay system (Promega, Piscataway, NJ). Relative transfection efficiency was determined using the Galacto-Star mammalian reporter gene assay system (Applied Biosystems, Foster City, CA) according to manufacturer's protocol. Luciferase values were means ± S.E. of quadruplicate wells.
Adriamycin Treatment—Subconfluent proliferating cells in 6-well dishes were transfected overnight with the indicated vector(s) (5 μg of plasmid DNA per well), washed, and allowed to recover in fresh culture medium for several hours. The cells were harvested, inoculated into 96-well dishes in complete culture medium, allowed to attach overnight, washed, incubated ± SF (100 ng/ml for 48 h) in serum-free DMEM, exposed to different doses of ADR (2 h at 37 °C), and post-incubated for 48 h in fresh drug-free complete culture medium (again ± SF). They were then analyzed for cell viability using MTT assays. For each assay condition, cell viability was determined in 10 replicate wells.
MTT Cell Viability Assay—This assay is based on the ability of viable mitochondria to convert MTT, a soluble tetrazolium salt, into an insoluble formazan precipitate, which is dissolved in DMSO and quantitated by spectrophotometry (29). After ADR treatment as above, cells in 96-well dishes were tested for MTT dye conversion. The cell viability was calculated as the amount of MTT dye conversion relative to sham-treated control cells.
Measurement of Src Kinase Activity—Whole cell lysates were prepared, and equal aliquots of total cellular protein (500 μg) were immunoprecipitated with an anti-Src antibody, as described previously (23). The precipitates were incubated with 10 μl of reaction buffer (20 mm PIPES (pH 7.0), 10 mm MnCl2, 10 μm Na3VO4), 1 μl of freshly prepared acid-denatured enolase (Sigma) (5 μg of enolase plus 1 μl of 50 mm HCl incubated at 30 °C for 10 min then neutralized with 1 μl of 1 m PIPES (pH 7.0)), and 10 μCi of [32P]ATP. After 10 min of incubation at 30 °C, reactions were terminated by addition of 2× SDS sample buffer, and samples were analyzed on 8% SDS-polyacrylamide gels. Serine and threonine phosphorylations were hydrolyzed by incubating the gel in 1 m KOH at 45 °C for 30 min, followed by fixing in 45% methanol and 10% acetic acid for 30 min at 25 °C, and vacuum drying for 2 h at 80 °C. Autoradiograms were produced and quantitated using a Storm PhosphorImager (GE Healthcare).
Pak1 Kinase Activity—Rac1 activity was assessed by measuring the kinase activity of the Rac1 effector Pak1 (30). DU-145 cells were harvested, and whole cell lysates were prepared (see below). Pak1 was immunoprecipitated from 150 to 250 μgof cell lysate in lysis buffer (see below) with a rabbit polyclonal anti-Pak1 antibody (ab3844, Chemicon) and dissolved in SDS-PAGE sample buffer. As a control, one-fifth of each IP sample was Western blotted for Pak1, using a polyclonal Pak1 antibody (N-19, 1:500 dilution, Santa Cruz Biotechnology). Pak1 kinase activity was determined using an in-gel kinase assay (31). Briefly, the precipitates were run on 10% SDS-polyacrylamide gels containing 0.5 mg/ml myelin basic protein. Proteins in the gel were then renatured; and their kinase activity toward myelin basic protein was initiated by soaking the gels in kinase buffer containing 25 μCi/ml [32P]ATP and 10 μm unlabeled ATP. The gels were washed extensively and autoradiographed to detect 32P-labeled proteins. Pak1 activity was quantitated by densitometry, using an AlphaImager 2000 (Alpha Innotech, San Leandro, CA).
IKK-β Kinase Activity—IKK-β kinase activity was measured using the HTScan™ IKK-β kinase assay kit (Cell Signaling Technology, Danvers, MA). This assay measures the ability of a cell lysate to phosphorylate a biotinylated IκB-α (Ser-32) substrate peptide. A phospho-IκB-α (Ser-32/36)-specific mouse monoclonal antibody was used to detect the phosphorylated substrate peptide. For assay standardization, the kit provides an active GST-IKK-β kinase fusion protein. Each assay condition was tested in five replicate wells, and IKK-β activity was expressed relative to untransfected, non-SF-treated controls.
Immunoprecipitation—Subconfluent proliferating DU-145 cells were washed and incubated in serum-free DMEM ± SF (100 ng/ml) for 20 min. The cells were then scraped into lysis buffer (20 mm Tris (pH 7.5), 150 mm NaCl, 10% glycerol, 1% Nonidet P-40, and protease inhibitor mixture set I (Calbiochem)) (1 ml per 100-mm dish). The lysates were incubated for 30 min at 0 °C and centrifuged at 17,000 × g for 15 min at 4 °C. After protein quantitation, 500 μg of supernatant protein was pre-cleared for 1 h by addition of Bio-Mag beads (Qiagen) and incubated with 5 μg of IP antibody plus Bio-Mag beads at 4 °C overnight. The IP antibodies were rabbit polyclonal Src antibody (sc-6096, Santa Cruz Biotechnology) or rabbit polyclonal c-Met antibody (ab14570, Abcam, Inc., Cambridge, MA). The beads were washed four times with 0.5 ml of lysis buffer; and 1× loading buffer (25 mm Tris-HCl (pH 6.5), 5% glycerol, 1% SDS, 1% 2-mercaptoethanol, and 0.05% bromphenol blue) was added. The samples were boiled for 3 min before Western blotting.
Western Blotting—After treatment, the cells were collected using trypsin, washed twice with phosphate-buffered saline, and pelleted by centrifugation. The pellet was resuspended in RIPA buffer, placed on ice for 30 min, and spun for 15 min at 14,000 rpm at 4 °C to remove the insoluble material. Western blotting was performed as described earlier (14). Briefly, aliquots of cell protein (50 μg) were electrophoresed in 4–20% SDS-polyacrylamide gradient gels and transferred to nitrocellulose membranes. Alternatively, immunoprecipitated proteins (see above) were subjected to SDS-PAGE and transferred to nitrocellulose membranes.
The membranes were blotted using the following primary antibodies: phospho-Akt (serine 473) (9271S, Cell Signaling Technology; 1:500 dilution); phospho-Akt (threonine 309) (monoclonal 244F9, Cell Signaling Technology); total Akt (9271, Cell Signaling Technology; 1:500); c-Met (H-190, Santa Cruz Biotechnology; 1:1000); phospho-c-Met (tyrosine 1349) (BIOSOURCE); IκB-α (C-15, Santa Cruz Biotechnology; 1:500); IKK-α (rabbit polyclonal IKK-α, Sigma; 1:1000); IKK-β (mouse monoclonal 62AT216, Abgent, San Diego; 1:500); phospho-IKK-α/β (sc-21661, Santa Cruz Biotechnology; 1:1000); MKK3 (rabbit polyclonal, 9232, Cell Signaling Technology; 1:1000); MKK6 (rabbit polyclonal, 9264, Cell Signaling Technology; 1:1000); NIK (rabbit polyclonal, H248, Santa Cruz Biotechnology; 1:200); phospho-p38 (threonine 180/182) (rabbit polyclonal, 9211, Cell Signaling Technology, 1:1000); total p38 (mouse monoclonal 9228, Cell Signaling Technology, 1:1000); phospho-Src (tyrosine 418) (2101S, Cell Signaling Technology); Src (mouse monoclonal, AH01152, BIOSOURCE; 1:1000); TAK1 (rabbit polyclonal, M579, Santa Cruz Biotechnology, 1:500); and actin (goat polyclonal, Santa Cruz Biotechnology; 1:1000). The membranes were then blotted with the appropriate secondary antibodies (Santa Cruz Biotechnology; 1:1000); and the blotted proteins were visualized using the enhanced chemiluminescence detection system (Amersham Biosciences), with colored molecular size markers (Bio-Rad).
Anoikis Assays—Assays of anoikis (apoptotic cell death triggered by detachment of the cell from its substratum) were performed by a modification of previously described protocols (3, 32). Briefly, subconfluent proliferating MDCK cells were transiently transfected overnight with the indicated vector(s), washed, and allowed to recover for several hours. Plastic 100-mm Petri dishes were coated with 8 ml of a 10 mg/ml solution of polyhydroxyethyl methacrylate (poly-HEMA), a polymer to which the cells cannot attach. The transfected cells were then suspended using trypsin, counted, and inoculated into poly-HEMA-coated dishes (3 × 106 cells per dish) in cell growth medium ± SF (100 ng/ml). At 48 h after inoculation, the cells were harvested, and any cell aggregates were dispersed using trypsin. Cell viability was determined by trypan dye exclusion analysis (33). For each dish, at least 200 cells were counted. Each assay was performed in duplicate, and the results represent the means ± ranges from two independent experiments.
Statistical Analyses—Where appropriate, comparisons were made using the two-tailed Student's t test
RESULTS
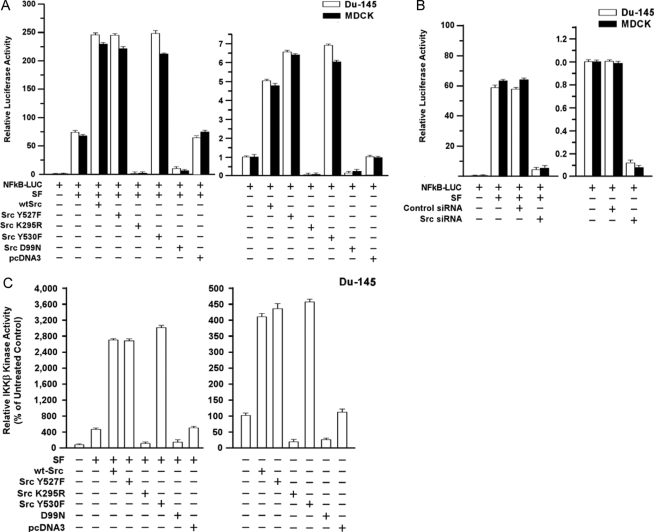
Src Modulates SF Stimulation of NF-κB Transcriptional Activity and IKK-β Kinase Activity—We tested the role of Src in SF stimulation of NF-κB activity, using a set of Src mutant expression vectors. DU-145 or MDCK cells were transiently transfected with different vectors plus the NF-κB-Luc reporter, treated ± SF (100 ng/ml) for 24 h, and assayed for luciferase activity. Typically, SF caused a 50–70-fold increase in NF-κB activity. With SF present, WT-Src caused a 3–4-fold increase in NF-κB activity (p < 0.001, two-tailed t test) beyond that observed with SF alone or SF + empty vector (Fig. 1A). Two activated Src mutants (Y527F and Y530F) caused increases in SF-stimulated NF-κB activity (p < 0.001) similar to WT-Src. In contrast, a DN kinase-dead Src mutant (K295R) (23) abrogated basal and SF-stimulated NF-κB activity; and an SH3 domain mutant (D99N) (34) reduced the SF-stimulated NF-κB activity to about 20% of that due to SF alone (p < 0.001). DU-145 and MDCK cells gave similar responses.
FIGURE 1.
Ability of Src to modulate SF stimulation of NF-κB and IKK-β kinase activity. A, effect of Src mutant proteins on basal and SF-stimulated NF-κB activity. Subconfluent proliferating DU-145 or MDCK cells in 2-cm2 wells were co-transfected overnight with the indicated Src vector and NF-κB-Luc reporter using Lipofectamine™ (0.25 μg of plasmid DNA per vector). The wells were washed, post-incubated in fresh culture medium ± SF (100 ng/ml) for 24 h, and harvested for luciferase assays. Luciferase values are expressed relative to control cells (no vector, 0 SF) and are means ± S.E. of four replicate wells. The data shown are representative of at least two independent experiments. B, effect of Src knockdown on basal and SF-stimulated NF-κB activity. Proliferating cells were preincubated with control-siRNA or Src-siRNA (50 nm for 48 h) and then assayed for basal and SF-stimulated NF-κB-Luc activity as above. C, Src enhances and is required for SF stimulation of IKK-β kinase activity. DU-145 cells were transfected with the indicated vector, washed, allowed to recover for several hours, and treated ± SF (100 ng/ml) for 20 min. The cells were harvested; and IKK-β activity was determined using a commercial assay kit (see under “Experimental Procedures”). The IKK-β activity values are means ± S.E. of five replicate wells and are expressed as a percent of the control (no SF, mock transfection).
In the absence of SF, WT-Src and the activated mutants stimulated NF-κB activity by about 5–6-fold, compared with untransfected or empty vector (pcDNA3)-transfected controls (p < 0.001) (Fig. 1A). The K295R (DN) and D99N mutants severely attenuated or abrogated the basal NF-κB activity in the absence of SF (p < 0.001). Because DN-Src might have other effects than inhibition of Src, we also tested the effects of Src knockdown. A control-siRNA had no effect on basal or SF-stimulated NF-κB activity, but Src-siRNA caused a ≥10-fold reduction in SF-stimulated as well as basal NF-κB activity (p < 0.001) (Fig. 1B). These findings suggest that Src stimulates basal and SF-induced NF-κB activity in a manner requiring its kinase and SH3 domains and that Src is required for SF-induced NF-κB activity.
IκB kinase (IKK) stimulates NF-κB activity by causing phosphorylation and degradation of IκB proteins, the cytoplasmic inhibitors of NF-κB (35). We tested the effects of Src on the kinase activity of IKK-β, the catalytic subunit, which phosphorylates IκB-α and IκB-β on serine residues. SF caused a 5-fold increase in IKK-β activity in DU-145 cells (p < 0.001) (Fig. 1C). WT-Src, Y527F, and Y530F enhanced IKK-β activity ≥6-fold beyond that due to SF alone or SF+pcDNA3 (p < 0.001), whereas K295R and D99N blocked the SF-stimulated increases in IKK-β activity (p < 0.001). Without SF, WT-Src caused a 4-fold increase in IKK-β activity (p < 0.001), whereas K295R and D99N inhibited IKK-β activity (Fig. 1C). These findings suggest that Src contributes to SF-stimulated (and basal) IKK-β.
Effects of SF and Src Mutant Proteins on Src Activity—We performed IP kinase assays using enolase as the substrate (23) to confirm that SF stimulates endogenous Src kinase activity. Here, a 20-min exposure to SF stimulated Src activity in DU-145 and MDCK cells by 4.5–6.5-fold (supplemental Fig. 1A). In DU-145 cells, expression of WT-Src or activated Src mutants (Y527F or Y530F) further enhanced the SF-stimulated Src activity (supplemental Fig. 1B). In contrast, the K295R and D99N mutants abrogated the SF-stimulated Src activity, consistent with a DN action. We also tested the effects of exogenous Src proteins on Src activation, indicated by phosphorylation of tyrosine 418 in the activation loop (36). DU-145 cells were transfected with Src vectors, treated ± SF for 20 min, and Western blotted for phospho-Src (tyrosine 418). All Src proteins were well expressed. WT-Src, Y527F, and Y530F increased the levels of phospho-Src without or with SF present (supplemental Fig. 1, C and D). In contrast, cells expressing K295R or D99N showed little or no basal phospho-Src or SF-induced Src phosphorylation, suggesting that these mutants block endogenous Src activation.
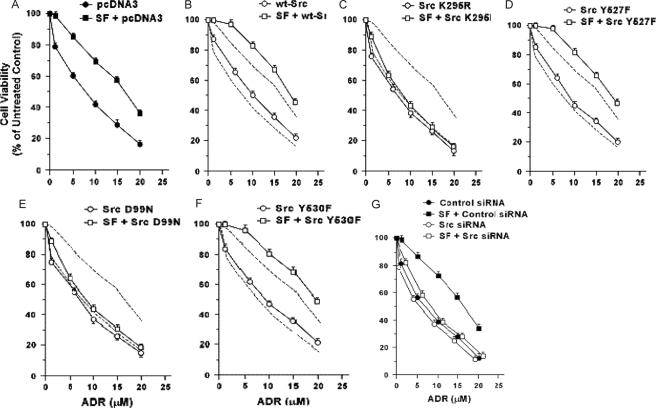
Role of Src in SF Cell Protection—We tested the ability of Src mutants to modulate SF protection of DU-145 cells against ADR. Survival was assessed using MTT assays in cells pretreated ± SF at 48 h after exposure to ADR. Here, SF enhanced the survival of cells empty vector transfected with at all ADR doses (p < 0.001–0.01) (Fig. 2A). Without SF, WT-Src caused modest but consistent increases in survival; but with SF, WT-Src caused larger increases in survival at all doses except 1 μm (p < 0.01) (Fig. 2B).
FIGURE 2.
Effect of Src proteins on basal and SF-stimulated survival in response to adriamycin. A–F, effect of Src expression vectors. Subconfluent proliferating DU-145 cells in 6-well dishes were transfected overnight with the indicated Src vector (5 μg of plasmid DNA per well), washed, and allowed to recover for several hours. The cells were then harvested, plated into 96-well dishes, allowed to attach overnight, incubated ± SF (100 ng/ml for 48 h), exposed to different doses of ADR (2 h at 37 °C), post-incubated for 48 h in fresh drug-free medium, and analyzed for cell viability using MTT assays. Values are expressed relative to non-ADR-treated control cells and are means ± S.E. of 10 replicate wells. The results shown are representative of two independent experiments. The vectors tested were as follows: empty pcDNA3 vector (A), WT-Src (B), Src-K295R (C), Src-Y527F (D), Src-D99N (E), and Src-Y530F (F). The dashed lines in B–F show empty vector-transfected cells. G, effect of Src knockdown. Proliferating cells were preincubated with control versus Src siRNA (50 nm for 48 h), incubated ± SF (100 ng/ml for 48 h), exposed to different doses of ADR (2 h at 37 °C), and post-incubated for 48 h in fresh drug-free medium. Cell viability values are means ± S.E. of 10 replicate wells.
DN-Src (K295R) had no effect on survival in the absence of SF but severely attenuated the SF-stimulated survival at all ADR doses (p < 0.01) (Fig. 2C). Src mutants Y527F (Fig. 2D) and Y530F (Fig. 2F) caused modest increases in survival without SF but caused stronger increases in SF-stimulated survival (p < 0.01 at all ADR doses except 1 μm). The SH3 mutant (D99N) did not alter basal cell survival but strongly attenuated SF-stimulated survival (p < 0.01) (Fig. 2E). These findings suggest that the Src kinase and SH3 domains are required for protection by SF, and the SF protection pathway can be further activated by exogenous Src. We tested the role of endogenous Src in modulating survival by pretreating the cells with Src versus control-siRNA. With control-siRNA, SF protected the cells against ADR as expected, whereas Src-siRNA abrogated SF protection (p < 0.001) (Fig. 2G). Without SF, the survival of cells treated with Src-siRNA was similar to or slightly less than that of cells treated with control-siRNA. These findings suggest that Src is required for SF protection against ADR.
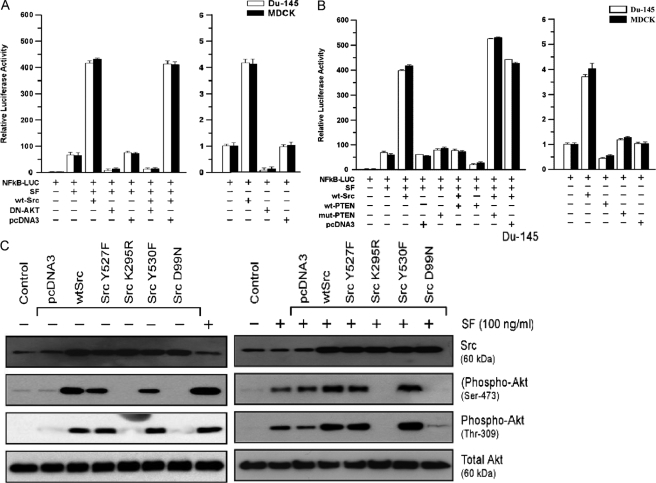
Src-stimulated NF-κB Activity Is Akt-dependent—Here, DU-145 or MDCK cells were transfected with WT-Src and/or DN-Akt and tested for SF stimulation of NF-κB activity. DN-Akt nearly abrogated NF-κB activity in the presence of SF alone; and in the presence of (SF+WT-Src), it reduced NF-κB activity to below that found with SF alone (p < 0.001) (Fig. 3A). PTEN, an inositol polyphosphate-3-phosphatase, acts upstream of Akt to block its activation (35). We tested the effects of WT-PTEN and a phosphatase-defective mutant (mut-PTEN) on Src/SF-stimulated NF-κB activity. WT-PTEN reduced SF-stimulated NF-κB activity to about 30% that with SF alone (p < 0.001), whereas mut-PTEN caused modest increases in NF-κB activity (Fig. 3B). WT-Src caused a 5.6–7.1-fold increase in SF-stimulated NF-κB activity (p < 0.001), whereas its co-expression with WT-PTEN reduced the NF-κB activity to about that due to SF alone (p < 0.001). SF-stimulated NF-κB activity was slightly higher in the presence of WT-Src+mut-PTEN than WT-Src alone. Consistent with these findings, WT-PTEN abrogated the ability of WT-Src to enhance survival of ADR-treated MDCK cells; and in the presence of SF, cells transfected with WT-Src+WT-PTEN showed lower survival than cells transfected with empty vector only (data not shown). These findings suggest that the ability of Src to stimulate NF-κB activity and cell protection is dependent upon Akt.
FIGURE 3.
Role of Akt in Src modulation of SF-stimulated NF-κB activity. A, DN-Akt blocks SF+WT-Src-stimulated NF-κB activity. Proliferating DU-145 cells in 2-cm2 wells were co-transfected overnight with the indicated vectors, washed, post-incubated in fresh culture medium ± SF (100 ng/ml) for 24 h, and harvested for luciferase assays. Relative luciferase values are means ± S.E. of four replicate wells. B, PTEN inhibits SF+WT-Src-stimulated NF-κB activity in DU-145 and MDCK cells. Assays were performed as described in A. C, effect of Src mutant proteins on the basal activation state and SF-stimulated activation of Akt. Proliferating DU-145 cells were transfected overnight with the indicated vector, washed, post-incubated for 24 h to allow gene expression, and treated ± SF (100 ng/ml) for 20 min. The cells were then harvested for Western blotting for Src, phospho-Akt (serine 473), phospho-Akt (tyrosine 309), and total Akt.
Role of Src in Akt Activation—To test the ability of Src to modulate activating phosphorylations of Akt, DU-145 cells were transfected with different Src vectors, treated ± SF (20 min), and Western blotted using phospho-specific antibodies. Without SF, there was little phospho-Akt in untransfected or vector-transfected cells. WT-Src and both active Src mutants induced Akt phosphorylation on serine 473 and threonine 309 (Fig. 3C, left) and also enhanced the SF-stimulated Akt phosphorylation on both sites (Fig. 3C, right), whereas K295R and D99N abrogated the SF-induced phosphorylation. These findings suggest that Src overexpression can activate Akt and Src activity is required for Akt activation by SF.
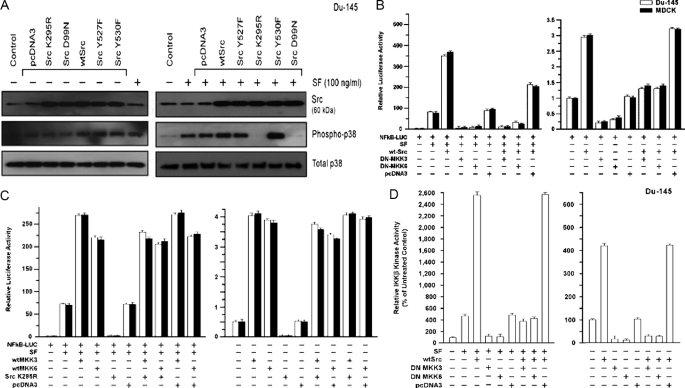
Role of MKK3/6/p38 Pathway in Src Stimulation of NF-κB Activity—Previously, we showed that an MKK3/6/p38 pathway contributes to SF stimulation of NF-κB activity (see under “Discussion”). Here, we tested the ability of different Src proteins to regulate this pathway. We used an anti-phospho-p38 (threonine 180/182) antibody to detect activated p38 (26). WT-Src and the active mutants (but not the inactive mutants) enhanced the basal phospho-p38 levels, whereas total p38 levels were unchanged (Fig. 4A, left). Each Src protein was well expressed. A 20-min exposure to SF stimulated phospho-p38 formation, and the three active Src proteins enhanced SF-stimulated p38 phosphorylation (Fig. 4A, right). These findings suggest roles for Src in basal and SF-stimulated p38 activation. MKK3 and MKK6 are selective p38 activators (38). We tested the requirement for MKK3/6 in Src stimulation of NF-κB activity, using DN expression vectors. Here we found that expression of a DN-MKK3 or DN-MKK6 protein strongly inhibited both WT-Src+SF-stimulated and WT-Src-stimulated NF-κB activity (p < 0.001) (Fig. 4B).
FIGURE 4.
Role of MAPK pathway in Src-mediated activation of NF-κB. A, Src causes activation (phosphorylation) of p38 and is required for SF-stimulated p38 activation. DU-145 cells were transfected with empty vector (pcDNA3), WT-Src, or a dominant negative Src (K295R); washed; post-incubated for 24 hto allow gene expression; treated ± SF (100 ng/ml) for 20 min; and harvested for Western blotting to detect Src, phospho-p38, total p38, or α-actin. B, requirement of MKK3/6 for Src stimulation of NF-κB activity. Proliferating cells in 2-cm2 wells were transiently co-transfected overnight with the indicated vector(s) and the NF-κB-Luc reporter, washed, post-incubated ± SF (100 ng/ml) for 24 h, and harvested for luciferase assays. Luciferase values are expressed relative to the negative control (no vector, 0 SF) and are means ± S.E. of four replicate wells. C, ability of exogenous MKK3 and MKK6 to activate NF-κB in the absence of Src activity. Assays were performed as described for C. D, role of MKK3 and MKK6 in Src-mediated stimulation of IKK-β kinase activity. DU-145 cells were transfected as indicated, washed, allowed to recover for several hours, and treated ± SF (100 ng/ml) for 20 min. The cells were then harvested, and IKK-β activity was determined as described under “Experimental Procedures.” The IKK-β kinase activity values are means ± S.E. of five replicate wells and are expressed as a percentage of the control value (no SF, mock transfection).
Next, we tested if Src is required for MKK3/6-mediated NF-κB activation. WT-MKK3 and WT-MKK6 enhanced SF-stimulated NF-κB activity by 3–4-fold in DU-145 and MDCK cells (p < 0.001). As expected, DN-Src (K295R) blocked SF-stimulated NF-κB activation (Fig. 4C). However, WT-MKK3 and WT-MKK6 each rescued the inhibition of NF-κB activity by DN-Src (p < 0.001). In fact, DN-Src had little or no effect on NF-κB activity in the presence of WT-MKK3/6 ± SF. These findings suggest that MKK3/6 act downstream of Src to activate NF-κB, in part, through p38. We further found that DN-MKK3 and DN-MKK6 inhibited basal and SF/WT-Src/(SF+WT-Src)-stimulated IKK-β activity (Fig. 4D).
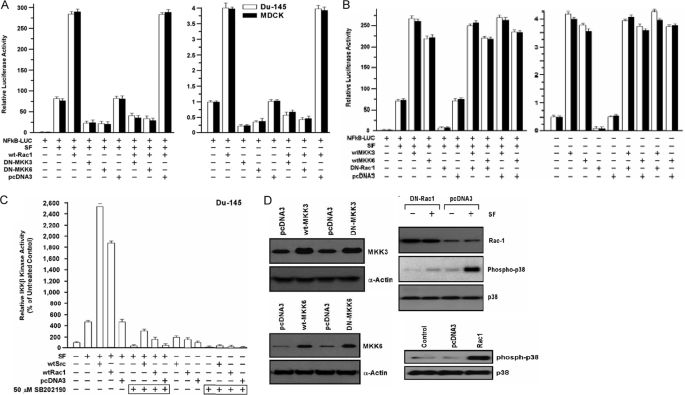
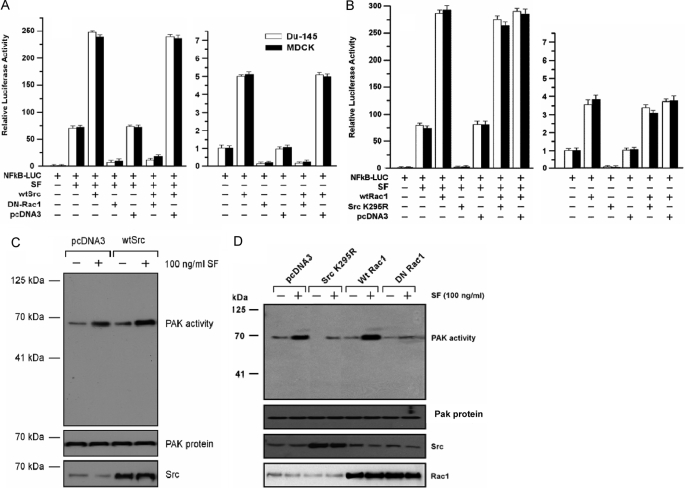
Role of MKK3/6 in Stimulation of NF-κB Activity by Rac1—Several studies suggest that Rac1, a member of the Ras superfamily of small GTPases, can activate MKK3/MKK6/p38 signaling (25, 39). We found that exogenous WT-Rac1 enhanced SF-stimulated NF-κB activity by 3–4-fold in DU-145 and MDCK cells (p < 0.001), and co-expression of DN-MKK3 or DN-MKK6 strongly attenuated the SF+WT-Rac1-stimulated NF-κB activity (p < 0.001) (Fig. 5A). Next, we tested if WT-MKK3 or WT-MKK6 could stimulate NF-κB activity in cells co-transfected with a DN-Rac1 (RacN17). DN-Rac1 blocked SF-stimulated NF-κB activity but was unable to block NF-κB activity stimulated by WT-MKK3/6 ± SF (Fig. 5B). These findings suggest that Rac1 acts upstream of MKK3/6 in the NF-κB activation pathway.
FIGURE 5.
Rac1 stimulates NF-κB activity through MKK3 and MKK6. A, Rac1 stimulation of NF-κB requires MKK3 and MKK6. Cells were co-transfected overnight with the indicated vector(s), washed, post-incubated ± SF (100 ng/ml) for 24 h, and harvested for luciferase assays. Values are expressed relative to the negative control (no vector, 0 SF) and are means ± S.E. of four replicate wells. B, ability of wild-type MKK3 and MKK6 to activate NF-κB in the absence of Rac1 activity. Assays were performed as described above. C, inhibition of p38 blocks IKK-β kinase activity stimulated by Rac1 and Src. DU-145 cells were transfected with the indicated vectors, washed, treated ± p38 inhibitor SB-202190 (50 μm) for 24 h, treated ± SF (100 ng/ml) for 20 min, and then assayed for IKK-β kinase activity. Values are means ± S.E. of five replicate wells. D, Western blot assays. Left, DU-145 cells were transfected with the indicated wild-type (wt) or dominant negative (DN) vectors, washed, post-incubated for 24 h to allow gene expression, and Western blotted for MKK6, MKK3, or α-actin (loading control). Right, cells were transfected with empty vector, WT-Rac1, or DN-Rac1 (RacN17); washed; post-incubated for 24 h; treated ± SF (100 ng/ml) for 20 min; and Western blotted for Rac1, phospho-p38, and total p38.
We tested the role of p38 in Rac1- and Src-stimulated IKK-β activity, using a selective p38 inhibitor (SB-202190). WT-Rac1 and WT-Src enhanced SF-stimulated IKK-β activity by 4–5.4-fold and stimulated basal IKK-β activity to a lesser extent (Fig. 5C). SB-202190 abrogated the SF-stimulated IKK-β activity, and reduced SF+WT-Rac1 and SF+WT-Src stimulated IKK-β activity to less than that of SF alone (p < 0.001). SB-202190 also inhibited the WT-Rac1 and WT-Src stimulated IKK-β kinase activity in the absence of SF. The expression of wild-type and DN MKK proteins is shown in Fig. 5D (right panels). Like DN-Src, DN-Rac1 blocked SF-induced p38 phosphorylation, and WT-Rac1 induced p38 phosphorylation in the absence of SF (Fig. 5D, left panels). These findings suggest that p38 is required for IKK-β activation by SF, Rac1, and Src, individually or in combination.
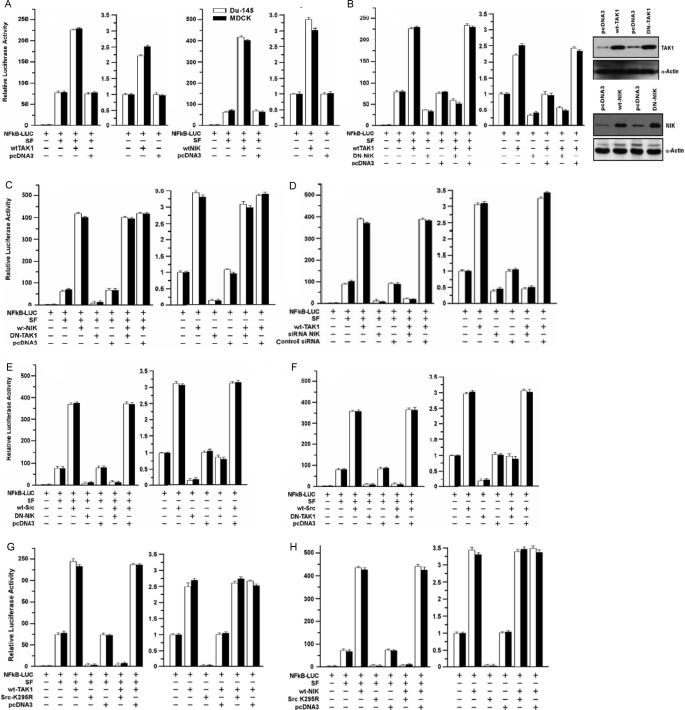
Relationship of TAK1/NIK to Src and MKK3/6 Pathways of NF-κB Activation—TAK1 (transforming growth factor-β-activated kinase 1) is an MAPKKK family protein that can stimulate NF-κB activity by activating NIK (NF-κB inducing kinase), MKK3, and MKK6 (40, 41). We tested the roles of TAK1 and NIK in SF/Src-mediated NF-κB activation. WT-TAK1 and WT-NIK enhanced SF-stimulated NF-κB activity by ≅3- and 6-fold, respectively (p < 0.001), and stimulated basal NF-κB activity to a lesser extent (Fig. 6A). DN-NIK inhibited stimulation of NF-κB activity by SF alone and (SF+WT-TAK1) (p < 0.001) (Fig. 6B). DN-TAK1 also inhibited basal and SF-stimulated NF-κB activity (p < 0.001) but had little effect on WT-NIK- or SF+WT-NIK-stimulated NF-κB activity (Fig. 6C). Expression of the exogenous TAK1 and NIK proteins is shown in Fig. 6B (right panels). Consistent with these data, NIK-siRNA inhibited SF-stimulated NF-κB activity and abrogated the ability of WT-TAK1 to enhance SF-induced NF-κB activity (p < 0.001) (Fig. 6D), suggesting that NIK is downstream of TAK1 and contributes to SF-stimulated NF-κB activity.
FIGURE 6.
Role of TAK1 and NIK in SF- and Src-mediated NF-κB activation. A, effects of TAK1 and NIK on NF-κB activity. Proliferating cells were transfected overnight with the indicated vectors, washed, post-incubated ± SF (100 ng/ml) for 24 h, and harvested for luciferase assays. Luciferase values are expressed relative to control cells (no vector, 0 SF) and are means ± S.E. of four replicate wells. B, DN-NIK inhibits SF and WT-TAK1 stimulated NF-κB activity. Left, NF-κB transcriptional assays were performed as described in A. Right, DU-145 cells were transfected with the indicated expression vectors, washed, post-incubated for 24 h to allow gene expression, and Western blotted to detect NIK, TAK1, or α-actin. C, DN-TAK1 does not inhibit SF+WT-NIK-stimulated NF-κB activity. Assays were performed as described for A. D, knockdown of NIK inhibits SF and WT-TAK1 stimulated NF-κB activity. DU-145 cells were preincubated with control or Src siRNA (50 nm for 48 h), after which the assays were performed as in A. E, role of NIK in SF+Src-stimulated NF-κB activity. Assays were performed as in A. F, role of TAK1 in SF+Src-stimulated NF-κB activity. Assays were performed as in A. G and H, role of Src in SF/TAK1 (G) and SF/NIK (H) stimulated NF-κB activity. Assays were performed in A.
Next, we tested the roles of TAK1/NIK in SF+Src-induced NF-κB activation. Again, DN-NIK (Fig. 6E) and DN-TAK1 (Fig. 6F) inhibited basal and SF-stimulated NF-κB activity, but the degree of inhibition was greater than in the experiments shown in Fig. 6, B and C. Both DN-NIK and DN-TAK1 blocked SF+WT-Src-stimulated NF-κB activity (p < 0.001), and both DN vectors reduced the NF-κB activity due to WT-Src alone to about the basal activity without WT-Src or SF (p < 0.001). In the absence of SF, DN-Src failed to inhibit the ability of WT-TAK1 (Fig. 6G) or WT-NIK (Fig. 6H) to stimulate NF-κB activity. However, DN-Src blocked SF+WT-TAK1- and SF+WT-NIK-stimulated NF-κB activity. Stated otherwise, neither WT-TAK1 nor WT-NIK rescued the inhibition of SF-stimulated NF-κB activity by DN-Src. These findings suggest that whereas Src is upstream of TAK1/NIK, SF stimulates an additional Src-dependent but TAK1/NIK-independent pathway(s) essential for SF activation of NF-κB.
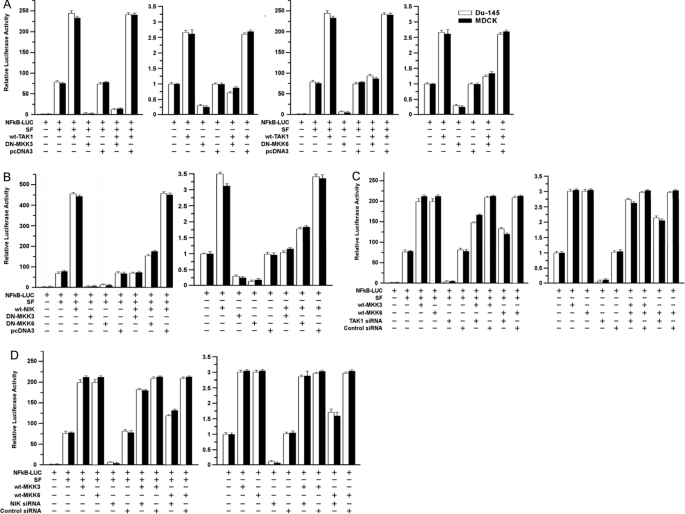
Next, we tested if TAK1/NIK and MKK3/6 interact in regulating NF-κB. As above, DN-MKK3 and DN-MKK6 inhibited and WT-TAK1 and WT-NIK enhanced basal and SF-induced NF-κB activity. The combination (WT-TAK1+DN-MKK3) reduced basal and SF-stimulated NF-κB activity to levels similar to or lower than those observed in the absence of WT-TAK1 (Fig. 7A). Similar results were observed for the combination (WT-NIK+DN-MKK3) (Fig. 7B). DN-MKK6 also inhibited WT-TAK1, and WT-NIK stimulated NF-κB activity but to a lesser extent than did DN-MKK3.
FIGURE 7.
Interactions of TAK1/NIK and MKK3/6 pathways in mediating NF-κB activation. A, effects of DN-MKK3 and DN-MKK6 on SF/TAK1-stimulated NF-κB activity. Assays were performed as described for Fig. 6A. B, effects of DN-MKK3/6 on SF/NIK-stimulated NF-κB activity. Assays were performed as described above. C and D, effects of TAK1-siRNA (C) and NIK-siRNA (D) on SF/MKK3/6-stimulated NF-κB activity. Assays were performed as described above for Fig. 6E.
We performed the converse study using WT-MKK3/6 and siRNAs to knock down TAK1 or NIK. TAK1- and NIK-siRNA strongly inhibited basal and SF-stimulated NF-κB activity but had only a modest effect on WT-MKK3-stimulated activity (Fig. 7, C and D). The siRNAs caused a moderate reduction of WT-MKK6-stimulated NF-κB activity (±SF), but neither siRNA reduced NF-κB activity close to that observed in the absence of WT-MKK6. These findings suggest that MKK3 acts, in part, downstream of TAK1/NIK to stimulate NF-κB signaling. We note that although DN-MKK3 inhibited NF-κB under all conditions, WT-TAK1 and WT-NIK were still able to stimulate (partially rescue) NF-κB activity in the presence of DN-MKK3, suggesting that TAK1/NIK also stimulate NF-κBbyan MKK3-independent mechanism. The relationship between TAK1/NIK and MKK6 is less clear than for MKK3, but our results suggest that TAK1 and NIK signal to NF-κB by MKK6-dependent and MKK6-independent pathways.
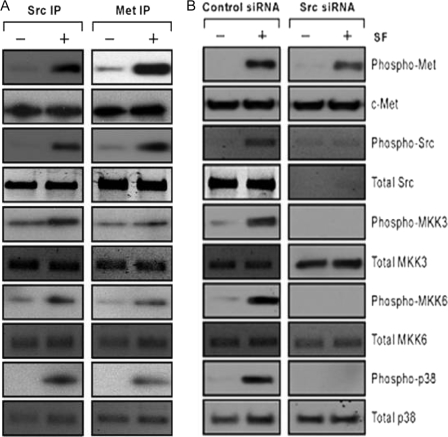
Association of MKK3/6 and p38 with Src and c-Met—DU-145 cells were incubated ± SF (100 ng/ml for 20 min) and subjected to IP-Western blotting using IP antibodies against Src or Met. We found that Src and Met associated with each other in bi-directional IPs in an SF-independent manner (Fig. 8A). Although the total quantity of Src in the Met IP and vice versa did not change, SF increased the amount of phosphorylated Src associated with Met and vice versa. Both Src and Met associated with MKK3, MKK6, and p38; and the levels of phosphorylated MKK3, MKK6, and p38 in the Src and Met IPs were increased by SF. These findings suggest that SF causes activation of Src, MKK3, MKK6, and p38 within the c-Met signalsome.
FIGURE 8.
Interaction of Src and c-Met with MAPK pathway proteins. A, association of Src and Met with MKK3, MKK6, and p38. Proliferating DU-145 cells were treated ± SF (100 ng/ml for 20 min), harvested, and subjected to IP-Western blotting. Whole cell lysates were immunoprecipitated using antibodies against total Src or total c-Met and Western blotted to detect the indicated protein species. B, effect of Src knockdown on activation of MKK3, MKK6, and p38. DU-145 cells were preincubated with control or Src siRNA (50 nm for 48 h), treated ± SF (100 ng/ml) for 20 min, and harvested for Western blotting to detect the indicated protein species.
To test the dependence of MKK3/6/p38 phosphorylations upon Src, DU-145 cells were pretreated with Src-versus control-siRNA, incubated ± SF, and Western blotted. Src-siRNA knocked down Src protein levels but had little effect on the total levels of Met, MKK3/6, or p38 (Fig. 8B). SF induced phosphorylation of Src, MKK3, MKK6, and p38 in the control-siRNA-treated cells but not the Src-siRNA-treated cells. SF was still able to induce Met phosphorylation in Src-siRNA-treated cells. These findings suggest that Src is required for activation of the MKK3/6/p38 pathway by SF but is not required for Met activation, and they suggest an SF/c-Met/Src/MKK3/6/p38/NF-κB activation scheme.
Src Acts Upstream of Rac1 in NF-κB Activation—Here we tested the ability of DN-Rac1 to block WT-Src-stimulated NF-κB activity and vice versa. Consistent with earlier findings, SF stimulated NF-κB activity by 70-fold in DU-145 and MDCK cells; and WT-Src enhanced the SF-stimulated NF-κB activity by about 3.5-fold (Fig. 9A). DN-Rac1 blocked the increase in NF-κB activity due to SF alone and reduced the NF-κB activity of cells treated with SF+WT-Src to below that due to SF alone (p < 0.001). DN-Rac1 also inhibited basal and WT-Src-stimulated NF-κB activity in the absence of SF. In contrast, DN-Src did not block the ability of WT-Rac1 to stimulate NF-κB activity in the presence or absence of SF (Fig. 9B). As positive controls, DN-Src inhibited basal and SF-stimulated NF-κB activity in the absence of WT-Rac1 (p < 0.001). These findings suggest that Rac1 is required for stimulation of NF-κB activity by SF and WT-Src, and they place Src upstream of Rac1 for NF-κB activation. We next tested the ability of SF and Src to stimulate Rac1 activity, which was assessed indirectly, by measuring the kinase activity of Pak1, a target of Rac1 (30). DU-145 cells were transfected as indicated, treated ± SF (20 min), and harvested for a Pak1 IP kinase assay, using myelin basic protein as the substrate. SF stimulated Pak1 activity, and WT-Src further enhanced the SF-stimulated Pak1 activity (Fig. 9C). DN-Src inhibited both basal and SF-stimulated Pak1 activity (Fig. 9D). Similarly, WT-Rac1 enhanced and DN-Rac1 inhibited SF-stimulated Pak1 activity. As controls, the exogenous Src and Rac proteins were well expressed, and the total Pak1 protein levels remained constant. These findings suggest that SF and Src stimulate Rac1 activity.
FIGURE 9.
Src acts upstream of Rac1 for NF-κB activation. A, DN-Rac1 blocks WT-Src stimulated NF-κB activation. Assays were carried out as in Fig. 6A. B, DN-Src (K295R) does not inhibit Rac1-stimulated NF-κB activation. Assays were performed as described above. C and D, regulation of Pak1 kinase activity by SF, Src, and Rac1. DU-145 cells were transfected with the indicated vectors, washed, post-incubated for 24 h, and treated ± SF (100 ng/ml for 20 min). The cells were then assayed for Pak1 activity using an IP kinase assay with myelin basic protein as the substrate. Aliquots of cell lysates were also used to Western blot for Src, Pak1, and Rac1. C shows the effects of SF and WT-Src on Pak1 activity. D shows the effects of SF, DN-Src (K295R), WT-Rac1, and DN-Rac1 (RacN17) on Pak1 activity.
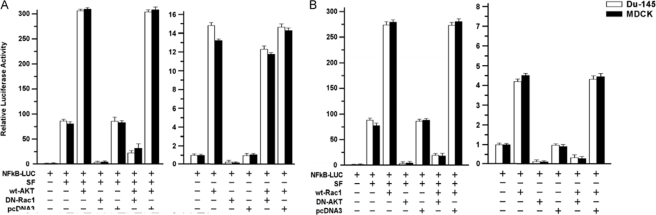
Relationship of Akt and Rac1 Signaling—We tested the effects of simultaneously stimulating Akt and inhibiting Rac1 and vice versa on NF-κB activity in DU-145 and MDCK. In SF-treated cells, WT-Akt alone further stimulated NF-κB activity by about 4-fold, whereas DN-Rac1 alone nearly abrogated the SF-stimulated NF-κB activity (Fig. 10A). In the presence of SF+WTAkt+DN-Rac1, the NF-κB activity was lower than in the presence of SF alone. In the converse experiment, WT-Rac1 alone enhanced and DN-Akt alone nearly abrogated the SF-stimulated NF-κB activity (Fig. 10B). The combination (SF+WTRac1+DN-Akt) gave significantly lower NF-κB activity than SF alone (p < 0.001). Interestingly, in the absence of SF, DN-Akt was much more effective at inhibiting WT-Rac1-stimulated NF-κB activity than vice versa. These findings suggest that in the c-Met/NF-κB pathway, Akt and Rac1 do not act in a simple series model but interact in a more complex manner (see “Discussion”).
FIGURE 10.
Interactions of Akt and Rac1 signaling pathways in mediating NF-κB activation. A, effect of DN-Rac1 on SF/WT-Akt stimulated NF-κB activity. B, effect of DN-Akt on SF/WT-Rac stimulated NF-κB activity. Assays were carried out as described above for Fig. 6A.
Effect of ADR on NF-κB Signaling—As described earlier, DN-Src and Src-siRNA decreased NF-κB activity in the absence of SF (Fig. 1). However, DN-Src (Fig. 2C) and Src-siRNA (Fig. 2G) caused little or no reduction of survival of ADR-treated cells in the absence of SF, suggesting that NF-κB does not contribute significantly to the basal survival of ADR-treated cells in our assay system. To further examine this issue, we tested the effect of ADR on NF-κB activity. DU-145 cells were transfected with the NF-κB-Luc reporter, treated with ADR for 2 h, and treated ± SF for different times up to 24 h prior to harvesting for luciferase assays. ADR did not cause any NF-κB activation but did cause some reduction of NF-κB activity to below base line (supplemental Fig. 2). SF caused time-dependent increases in NF-κB activity, with the increases being smaller in the presence of ADR than in its absence at most time points (p < 0.01). The finding that ADR does not activate NF-κB is consistent with the observation that inhibition of Src had very little effect on the survival of ADR-treated cells.
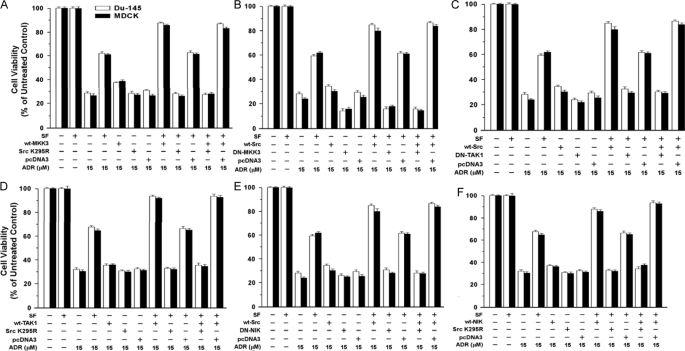
Effect of Signaling Proteins on Cell Survival—Because NF-κB may not be the only Src target for protection against ADR, NF-κB reporter assays may not always reflect cell survival. Here, we used survival (MTT) assays as a second biologic readout for various signaling proteins. Studies were carried out at two doses of ADR (15 and 20 μm). The results were qualitatively similar at each dose and in DU-145 versus MDCK cells. First, we tested combinations of WT-MKK3 (or WT-MKK6) and DN-Src and vice versa. WT-MKK3 yielded moderate increases in the survival of ADR-treated cells by itself but gave much larger increases in survival (up to +27%) with SF present (p < 0.001) (Fig. 11A). Increases in survival because of SF or SF+WT-MKK3 were abolished by DN-Src. DN-MKK3 reduced survival to below control levels (±SF) and abrogated the ability of WT-Src to enhance SF protection (Fig. 11B). Similar results were obtained in parallel studies using WT-MKK6 and DN-MKK6 (data not shown). These results are generally consistent with the NF-κB assays and suggest that MKK3/6 contribute to basal and SF-stimulated cell survival.
FIGURE 11.
Effects of signal transducers and inhibitors on SF protection against ADR. A, effects of DN-Src (K295R) on protection by SF and MKK3. Subconfluent proliferating DU-145 cells in 6-well dishes were transiently transfected overnight with the indicated vector(s) (5 μg of plasmid DNA per vector per well); and the assays were carried out as described in the Fig. 2 legend. Cell viability values are expressed relative to non-ADR-treated control cells and are means ± S.E. of 10 replicate wells. The results shown are representative of two independent experiments. B, effect of DN-MKK3 on protection by SF and WT-Src. Assays were carried out as described for A. C and E, effects of DN-TAK1 (C) and DN-NIK (E) on protection by SF and WT-Src. Assays were performed as described above. D and F, effects of DN-Src on protection by WT-TAK1 (D) and WT-NIK (F). Assays were performed as described above.
As expected, WT-Src caused larger increases in survival in the presence of SF than in its absence (Fig. 11C). DN-TAK1 caused small reductions in survival in the absence of SF but strongly inhibited the ability of SF to protect cells (p < 0.001), and DN-TAK1 abolished the protective activity of SF+WT-Src (Fig. 11C). Like WT-Src, WT-TAK1 alone caused only a slight increase in survival but significantly enhanced SF-stimulated cell survival (Fig. 11D)(p < 0.001). As before, DN-Src alone caused little or no reduction in cell survival, but DN-Src essentially abrogated the protection because of SF or SF+WT-TAK1 (p < 0.001). Experiments using WT-Src/DN-NIK (Fig. 11E) and WT-NIK/DN-Src (Fig. 11F) gave similar results to those using WT-Src/DN-TAK1 and WT-TAK1/DN-Src.
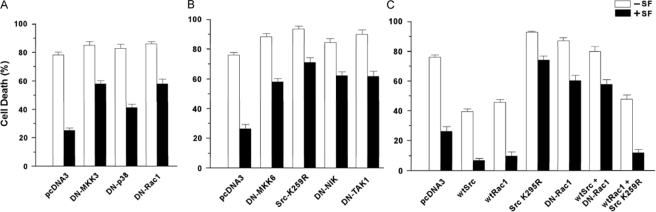
Effects of Inhibition of Signal Transduction on SF Protection against Anoikis—Previous studies show that SF protects cells against apoptosis caused by loss of attachment to a substratum (anoikis) (3, 32). Here we tested whether the signal transduction pathways that regulate SF protection against ADR also modulate SF protection against anoikis. Briefly, MDCK cells were transfected with DN and/or wild-type expression vectors, inoculated into dishes coated with a compound to which the cells cannot attach (poly-HEMA), incubated ± SF for 48 h, and assayed for cell death by the failure to exclude trypan blue dye. Typically, MDCK cells transfected with empty pcDNA3 vector showed 75–80% cell death in the absence of SF, and the cell death rates were reduced to 25% with SF present (p < 0.001) (Fig. 12). Without SF, cell death rates were increased modestly (+3–8%) by DN-MKK3, DN-p38, and DN-Rac1. However, each of these DN proteins increased cell death more substantially (by +34, +17, and +34%, respectively) with SF present (Fig. 12A). Similarly, DN-MKK6, DN-Src, DN-NIK, and DN-TAK1 strongly attenuated SF protection against anoikis (Fig. 12B). Of the vectors tested, DN-Src caused the largest increase in cell death in the absence of SF (+17%), suggesting that Src contributes to basal survival in this assay.
FIGURE 12.
Modulation of SF-mediated protection against anoikis by inhibition of signal transduction. MDCK cells were transfected overnight with the indicated vector(s), inoculated into 100-mm poly-HEMA-coated dishes (3 × 106 cells per dish), and incubated ± SF (100 ng/ml) for 48 h. The cells were then harvested and assayed for cell death based on the failure to exclude trypan blue dye. The values shown are the means ± ranges from two independent experiments, with each assay performed in duplicate. A shows the effects of DN MKK3, DN-p38, and DN-Rac1 on SF-mediated protection against anoikis; and B shows the effects of DN-MKK6, DN-Src (Src-K359R), DN-NIK, and DN-TAK1 on the protection. C, combinations of wild-type (wt) and DN Src and Rac1 vectors were tested for their effects on SF protection against anoikis.
WT-Src and WT-Rac1 inhibited anoikis with or without SF (Fig. 10C). In tests of combinations of vectors, WT-Src+DN-Rac1-transfected cells showed little or no reduction in cell death compared with cells transfected with DN-Rac1 alone. In contrast, death rates in WT-Rac1+DN-Src-transfected cells were similar to those of cells transfected with WT-Rac1 alone. These findings suggest that a Src/Rac1 pathway contributes to protection against anoikis, with Rac1 acting downstream of Src.
DISCUSSION
We studied the role of Src in SF protection and survival signaling. In DU-145 cells, WT-Src and activated mutants enhanced survival of ADR-treated cells without or with SF, but the increased survival due to Src was greater with SF present. Conversely, Src knockdown abrogated SF protection against ADR. Kinase-dead and SH3 domain mutants behaved like dominant negatives and blocked SF protection. These findings suggest that Src kinase activity and its SH3 domain (a signaling module that interacts with proline-rich amino acid sequences) (40, 42) are required for protection. Having previously shown that NF-κB is required for SF protection, we used NF-κB reporter assays to probe the mechanisms by which Src signals cell survival.
The IKK complex regulates NF-κB activity by phosphorylating the cytoplasmic inhibitors of NF-κB(IκB proteins), leading to their degradation. Several studies describe a role for Src in IKK activation by the inflammatory cytokines interleukin-1 and tumor necrosis factor-α (43, 44), and another study suggests that Src is a component of the IKK complex and associates with the regulatory subunit IKK-γ (NEMO) (45). We established that Src modulates SF-stimulated IKK-β activity. Thus, active Src proteins enhanced and DN mutant Src proteins blocked SF-stimulated IKK-β activity.
As expected, SF caused an activating phosphorylation of Src (tyrosine 418) and stimulated Src kinase activity. In DU-145 and MDCK cells, active Src proteins stimulated NF-κB activity without or with SF. However, the impact of Src was greater with SF present, because SF stimulated NF-κB activity by 50–70-fold over the basal activity. These findings suggest that although Src is required for SF protection, Src alone cannot fully activate NF-κB signaling.
NF-κB activation by SF+WT-Src or WT-Src alone was strongly attenuated by DN-Akt, indicating that Src activation of NF-κB is Akt-dependent. We previously found that Akt is required for NF-κB activation by SF (14). Roles for Akt in NF-κB activation have been described in other contexts (46, 47). Thus, in response to tumor necrosis factor-α, Akt mediates IKK-α phosphorylation on serine 23, suggesting that Akt activates the IKK kinase (47). PTEN inhibits Akt activation via its lipid phosphatase activity (37). Consistent with this finding, wild-type (but not mutant) PTEN blocked SF+Src-induced NF-κB activity.
We identified MKK3 and MKK6 (activators of p38 (26, 38)) as mediators of SF activation of NF-κB in DU-145 and MDCK cells (48). Inhibition of p38 blocked SF-stimulated NF-κB activity, suggesting that p38 is the relevant target of MKK3/6. p38 may stimulate NF-κB by enhancing the activity of the p65/RelA subunit (49) and/or stimulating IKK activity. Here, we identified Src and Rac1 as upstream activators of an MKK3/6/p38 pathway that mediates SF activation of the IKK-β kinase and NF-κB. Stimulation of IKK-β activity by SF, Src, and Rac1 was abrogated by p38 inhibition. Biochemical studies revealed that SF caused activating phosphorylations of Src, MKK3, MKK6, and p38 in protein complexes associated with c-Met and with Src and that the phosphorylation of MKK3, MKK6, and p38 were Src-dependent. These findings suggest that SF stimulates Src/MKK3/6/p38 signaling in the c-Met signalsome.
Our findings place Src and Rac1 upstream of MKK3/6/p38 for NF-κB activation by SF and are consistent with reports that Src can activate p38 (50) and stimulate NF-κB (51, 52) in other contexts. Mechanisms by which Src stimulates NF-κB include tyrosine phosphorylation and activation of IKK (43) or inhibition of IκB-α (51). Several studies suggest a role for a Rac1/MKK3/p38 pathway in cell invasion (45, 52). Our study suggests that this pathway also mediates NF-κB activity and cell survival.
NIK associates with and activates IKK, leading to phosphorylation of IκB and release of the NF-κB transcriptional subunits (53, 54). Analysis of Nik–/– mice suggests that NIK is not a universal IKK activator but acts in a receptor-specific manner (55). In our study, WT-NIK enhanced and DN-NIK or NIK-siRNA inhibited SF-stimulated NF-κB activity, suggesting that NIK contributes to NF-κB activation by SF. TAK1, a potential upstream activator of IKK-β and MKK3/6 (40, 41), enhanced SF-stimulated NF-κB activity in an NIK-dependent manner, suggesting that a TAK1/NIK/NF-κB pathway operates in DU-145 and MDCK. Further studies revealed that WT-Src and Rac1 enhanced whereas DN-Src and DN-Rac1 blocked SF-stimulated Pak1 activity. Previously, we showed that Pak1 is required for SF stimulation of NF-κB and protection against ADR (7, 14). This study validates a role for Rac1 and Pak1 in SF survival signaling and implicates Src and Rac1 as Pak1 activators in this pathway. However, because Pak1 is a target of other signaling pathways, Rac1 may not be the only mediator of Pak1 activation by SF.
Although DNA-damaging agents, including anthracyclines, may cause activation of NF-κB (58, 59), the effects of NF-κBon cell survival are variable; and NF-κB may promote survival, have no effect, or even promote apoptosis in some contexts (60–62). Although inhibition of Src caused a reduction of basal NF-κB activity, it had little or no effect on the basal cell survival response to ADR in the absence of SF. This finding is consistent with our previous observation that inhibition of NF-κB using an IκB super-repressor caused little or no reduction of basal survival (14). Consistent with these data, ADR did not stimulate NF-κB signaling. These considerations suggest that in our assays, the basal NF-κB activity is sufficiently low (even though it is detected by reporter assays) and that reducing it further with DN-Src or Src-siRNA has a negligible effect on survival.
To determine whether Akt and Rac1 act in series to stimulate NF-κB activity as do Src and Rac1, we tested the effects of simultaneously stimulating Akt and inhibiting Rac1 and vice versa. Here, DN-Rac1 strongly inhibited the SF+WT-Akt-stimulated NF-κB activity; and conversely, DN-Akt strongly inhibited the SF+WT-Rac1-stimulated NF-κB activity. These findings suggest that Akt and Rac1 do not act in series. One possible model is that these proteins act in parallel pathways, both of which are required to activate NF-κB. Alternatively, Akt and Rac1 may interact to confer maximal NF-κB activation.
We identified two pathways (TAK1/NIK and Src/Rac1/MKK3/6/p38) that contribute to SF-mediated NF-κB activation. To synthesize these pathways, we studied the role of Src in the TAK1/NIK/NF-κB pathway and potential interactions of the TAK1/NIK and MKK3/6/p38 pathways. Exogenous Src failed to rescue the inhibition of SF-stimulated NF-κB activity because of inhibition of TAK1 or NIK. Conversely, exogenous TAK1 or NIK failed to rescue the inhibition of SF-stimulated NF-κB activity by DN-Src. These findings suggest cross-talk between the Src/NF-κB and TAK1/NIK/NF-κB pathways so that Src, TAK1, and NIK are all required for SF-mediated NF-κB activation. One scenario consistent with our findings is that SF stimulates Src-dependent/TAK1/NIK-dependent and Src-dependent/TAK1/NIK-independent pathways of NF-κB activation. In this scenario, both pathways are required for full SF-stimulated NF-κB activation; and these pathways act synergistically, so that inactivation of either pathway can reduce NF-κB activity to levels only modestly greater than the basal activity.
In studies of the TAK1/NIK versus MKK3/6/p38 pathways, DN-MKK3 and DN-MKK6 each blocked the SF+WT-TAK1-stimulated NF-κB activation, although DN-MKK3 was more effective than DN-MKK6 in this respect. However, even in the presence of DN-MKK3, SF+WT-TAK1 and SF+WT-NIK were still able to stimulate NF-κB activity to some extent, consistent with a model in which SF/TAK1/NIK/NF-κB signaling occurs by MKK3-dependent and MKK3-independent mechanisms. Further studies revealed that inhibition of TAK1 or NIK by siRNAs caused only a modest reduction in the SF+WT-MKK3-induced NF-κB activity. The relative inability of TAK1- or NIK-siRNA to block SF+WT-MKK3-induced NF-κB activation suggests that for the most part, MKK3 is not upstream of TAK1/NIK in SF-mediated NF-κB activation.
Because NF-κB may not be the only Src target for SF protection against ADR, we tested the roles of these signaling pathways in the survival response to ADR. Inhibition of transducers for SF-mediated NF-κB activation (TAK1, NIK, and MKK3/6) abrogated SF protection against ADR, indicating that these proteins are required for protection. Conversely, overexpression of each wild-type protein enhanced SF protection. As with Src, overexpression of the wild-type proteins gave only modest increases in survival in the absence of SF, suggesting that these proteins alone cannot fully activate survival signaling. Unlike Src, TAK1, and NIK, inhibition of MKK3 or MKK6 reduced the survival of SF+ADR-treated cells to below that for ADR alone. Consistent with the NF-κB assays, DN-Src abrogated the ability of SF plus WT-TAK1 or WT-NIK to stimulate cell survival. But in contrast to NF-κB, WT-MKK3 and WT-MKK6 failed to rescue the inhibition of SF protection by DN-Src. DN-MKK3 and DN-MKK6 each reduced the survival of cells treated with SF+WT-Src+ADR to less than that of cells treated with ADR alone. Thus, whereas the survival assays were usually consistent with NF-κB assays, manipulation of Src may modulate survival in a manner is not always predicted by NF-κB assays. Our findings also suggest that unlike Src, MKK3/6 contribute significantly to the basal survival of DNA-damaged cells, although we cannot rule out the possibility that the DN-MKK3/6 proteins have off-target effects that enhance sensitivity to ADR.
Because other signals that cooperate with c-Met may modulate the biologic consequences of c-Met activation, we tested whether the signaling pathways for SF protection against DNA damage also mediate protection against anoikis (apoptosis because of loss of contact with the substratum) (3, 32). Protection against anoikis is due, in part, to an integrin-mediated survival signal via the PI 3-kinase/Akt pathway (56, 57). We found that some of the same signaling proteins involved in SF protection against ADR (Src, Rac1, p38, MKK3/6, NIK, and TAK1) contribute to SF protection against anoikis. However, some differences in survival outcomes were observed. Although Src contributed negligibly to survival of ADR-treated cells in the absence of SF, it contributed significantly to protection against anoikis in the absence of SF. In addition, the magnitude of SF protection against anoikis was greater than that of SF protection against ADR, suggesting that the survival signal because of attachment to the extracellular matrix cooperates with that due to c-Met ligation or endogenous Src activity.
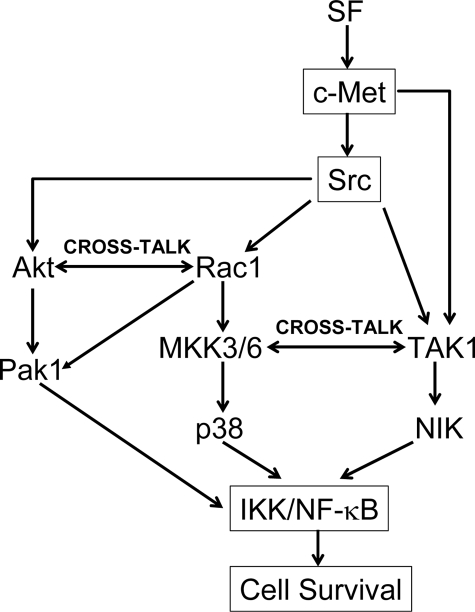
In toto, our studies suggest three pathways by which Src mediates SF stimulation of NF-κB activity as follows: 1) Src/Akt/IKK/NF-κB; 2) Src/Rac1/MKK3/6/p38/IKK/NF-κB; and 3) TAK1/NIK/NF-κB. Pathways 1 and 2 appear to exhibit cross-talk through Rac1, and pathways 2 and 3 appear to show cross-talk through MKK3 (see Fig. 13).
FIGURE 13.
Pathways by which Src may mediate SF stimulation of NF-κB activity and cell survival.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grants RO1-ES09169 (to E. M. R.) and RO1-NS43987 (to J. J. L. and E. M. R.) from USPHS. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1 and 2.
Footnotes
The abbreviations used are: SF, scatter factor; ADR, adriamycin; DMEM, Dulbecco's modified Eagle's medium; DN, dominant negative; IκB, inhibitor of κ light chain gene enhancer in B cells; IKK-β, IκB kinase-β; MDCK, Madin-Darby canine kidney; MKK, mitogen-activated protein kinase kinase; NF-κB, nuclear factor of κ light chain gene enhancer in B cells; NIK, NF-κB-inducing kinase; SH3, Src homology 3; TAK1, transforming growth factor-β activated kinase 1; PI, phosphatidylinositol; PIPES, 1,4-piperazinediethanesulfonic acid; PTEN, phosphatase and tensin homolog; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; siRNA, small interfering RNA; IP, immunoprecipitation; poly-HEMA, polyhydroxyethyl-methacrylate; MAPK, mitogen-activated protein kinase.
References
- 1.Rosen, E. M., Nigam, S. K., and Goldberg, I. D. (1994) J. Cell Biol. 127 1783–1787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Higashio, K., and Shima, N. (1993) EXS (Basel) 65 351–368 [PubMed] [Google Scholar]
- 3.Frisch, S. M., and Francis, H. (1994) J. Cell Biol. 124 619–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bardelli, A., Longatti, P., Albero, D., Goruppi, S., Schneider, C., Ponzetto, C., and Comoglio, P. M. (1996) EMBO J. 15 6205–6212 [PMC free article] [PubMed] [Google Scholar]
- 5.Bowers, D. C., Fan, S., Walter, K. A., Abounader, R., Williams, J. A., Rosen, E. M., and Laterra, J. (2000) Cancer Res. 60 4277–4283 [PubMed] [Google Scholar]
- 6.Fan, S., Ma, Y. X., Wang, J. A., Yuan, R. Q., Meng, Q., Cao, Y., Laterra, J. J., Goldberg, I. D., and Rosen, E. M. (2000) Oncogene 19 2212–2223 [DOI] [PubMed] [Google Scholar]
- 7.Fan, S., Ma, Y. X., Gao, M., Yuan, R. Q., Meng, Q., Goldberg, I. D., and Rosen, E. M. (2001) Mol. Cell. Biol. 21 4968–4984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosen, E. M., Lamszus, K., Laterra, J., Polverini, P. J., Rubin, J. S., and Goldberg, I. D. (1997) CIBA Found. Symp. 212 215–229 [DOI] [PubMed] [Google Scholar]
- 9.Hurle, R. A., Davies, G., Parr, C., Mason, M. D., Jenkins, S. A., Kynaston, H. G., and Jiang, W. G. (2005) 20 1339–1349 [DOI] [PubMed]
- 10.Yamashita, J., Ogawa, M., Yamashita, S., Nomura, K., Kuramoto, M., Saishoji, T., and Shin, S. (1994) Cancer Res. 54 1630–1633 [PubMed] [Google Scholar]
- 11.Kang, J. Y., Dolled-Filhart, M., Ocal, I. T., Singh, B., Lin, C. Y., Dickson, R. B., Rimm, D. L., and Camp, R. L. (2003) Cancer Res. 63 1101–1105 [PubMed] [Google Scholar]
- 12.Siegfried, J. M., Weissfeld, L. A., Singh-Kaw, P., Weyant, R. J., Testa, J. R., and Landreneau, R. J. (1997) Cancer Res. 57 433–439 [PubMed] [Google Scholar]
- 13.Tsukinoki, K., Yasuda, M., Mori, Y., Asano, S., Naito, H., Ota, Y., Osamura, R. Y., and Watanabe, Y. (2004) Oncol. Rep. 12 1017–1021 [PubMed] [Google Scholar]
- 14.Fan, S., Gao, M., Meng, Q., Laterra, J. J., Symons, M. H., Coniglio, S., Pestell, R. G., Goldberg, I. D., and Rosen, E. M. (2005) Oncogene 24 1749–1766 [DOI] [PubMed] [Google Scholar]
- 15.Frame, M. C. (2004) J. Cell Sci. 117 989–998 [DOI] [PubMed] [Google Scholar]
- 16.Liu, A. X., Testa, J. R., Hamilton, T. C., Jove, R., Nicosia, S. V., and Cheng, J. Q. (1998) Cancer Res. 58 2973–2977 [PubMed] [Google Scholar]
- 17.Hakak, Y., Hsu, Y. S., and Martin, G. S. (2000) Oncogene 19 3164–3171 [DOI] [PubMed] [Google Scholar]
- 18.Levin, V. A. (2004) Cancer Treat. Res. 119 89–119 [DOI] [PubMed] [Google Scholar]
- 19.Ponzetto, C., Bardelli, A., Zhen, Z., Maina, F., dalla Zonca, P., Giordano, S., Graziani, A., Panayotou, G., and Comoglio, P. M. (1994) Cell 77 261–271 [DOI] [PubMed] [Google Scholar]
- 20.Rahimi, N., Hung, W., Tremblay, E., Saulnier, R., and Elliott, B. (1998) J. Biol. Chem. 273 33714–33721 [DOI] [PubMed] [Google Scholar]
- 21.Popsueva, A., Poteryaev, D., Arighi, E., Meng, X., Angers-Loustau, A., Kaplan, D., Saarma, M., and Sariola, H. (2003) J. Cell Biol. 161 119–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wojcik, E. J., Sharifpoor, S., Miller, N. A., Wright, T. G., Watering, R., Tremblay, E. A., Swan, K., Mueller, C. R., and Elliott, B. E. (2006) Oncogene 25 2773–2784 [DOI] [PubMed] [Google Scholar]
- 23.Reddy, S., Mazzu, D., Mahan, D., and Shalloway, D. (1990) J. Virol. 64 3545–3550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cong, L. N., Chen, H., Li, Y., McGibbon, M. A., Taylor, S. I., and Quon, M. J. (1997) Mol. Endocrinol. 11 1881–1890 [DOI] [PubMed] [Google Scholar]
- 25.Yamauchi, J., Tsujimoto, G., Kaziro, Y., and Itoh, H. (2001) J. Biol. Chem. 276 23362–23372 [DOI] [PubMed] [Google Scholar]
- 26.Raingeaud, J., Gupta, S., Rogers, J. S., Dickens, M., Han, J., Ulevitch, R. J., and Davis, R. J. (1995) J. Biol. Chem. 270 7420–7426 [DOI] [PubMed] [Google Scholar]
- 27.Malinin, N. L., Boldin, M. P., Kovalenko, A. V., and Wallach, D. (1997) Nature 385 540–544 [DOI] [PubMed] [Google Scholar]
- 28.Yang, X., Kovalenko, D., Nadeau, R. J., Harkins, L. K., Mitchell, J., Zubanova, O., Chen, P. Y., and Friesel, R. (2004) J. Biol. Chem. 279 38099–38102 [DOI] [PubMed] [Google Scholar]
- 29.Alley, M. C., Scudiero, D. A., Monks, A., Hursey, M. L., Czerwinski, M. J., Fine, D. L., Abbott, B. J., Mayo, J. G., Shoemaker, R. H., and Boyd, M. R. (1988) Cancer Res. 48 589–601 [PubMed] [Google Scholar]
- 30.Manser, E., Leung, T., Salihuddin, H., Zhao, Z. S., and Lim, L. (1994) Nature 367 40–46 [DOI] [PubMed] [Google Scholar]
- 31.Ding, J., Knaus, U. G., Lian, J. P., Bokoch, G. M., and Badwey, J. A. (1996) J. Biol. Chem. 271 24869–24873 [DOI] [PubMed] [Google Scholar]
- 32.Zeng, Q., McCauley, L. K., and Wang, C.-Y. (2002) J. Biol. Chem. 277 50137–50142 [DOI] [PubMed] [Google Scholar]
- 33.Bae, I., Fan, S., Meng, Q., Rih, J. K., Kim, H. J., Kang, H. J., Xu, J., Goldberg, I. D., Jaiswal, A. K., and Rosen, E. M. (2004) Cancer Res. 64 7893–7909 [DOI] [PubMed] [Google Scholar]
- 34.Weng, Z., Rickles, R. J., Feng, S., Richard, S., Shaw, A. S., Schreiber, S. L., and Brugge, J. S. (1995) Mol. Cell. Biol. 15 5627–5634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zandi, E., Rothwarf, D. M., Delhase, M., Hayakawa, M., and Karin, M. (1997) Cell 91 243–252 [DOI] [PubMed] [Google Scholar]
- 36.Kmiecik, T. E., and Shalloway, D. (1987) Cell 49 65–73 [DOI] [PubMed] [Google Scholar]
- 37.Stambolic, V., Suzuki, A., de la Pompa, J. L., Brothers, G. M., Mirtsos, C., Sasaki, T., Ruland, J., Penninger, J. M., Siderovski, D. P., and Mak, T. W. (1998) Cell 95 29–39 [DOI] [PubMed] [Google Scholar]
- 38.Raingeaud, J., Whitmarsh, A. J., Barrett, T., Derijard, B., and Davis, R. J. (1996) Mol. Cell. Biol. 16 1247–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chini, C. C., Boos, M. D., Dick, C. J., Schoon, R. A., and Leibson, P. J. (2000) Eur. J. Immunol. 30 2791–2798 [DOI] [PubMed] [Google Scholar]
- 40.Ninomiya-Tsuji, J., Kishimoto, K., Hiyama, A., Inoue, J., Cao, Z., and Matsumoto, K. (1999) Nature 398 252–256 [DOI] [PubMed] [Google Scholar]
- 41.Kaminska, B., Wesolowska, A., and Danilkiewicz, M. (2005) Acta Biochim. Pol. 52 329–337 [PubMed] [Google Scholar]
- 42.Li, S. S. (2005) Biochem. J. 390 641–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang, W. C., Chen, J. J., and Chen, C. C. (2003) J. Biol. Chem. 278 9944–9952 [DOI] [PubMed] [Google Scholar]
- 44.Funakoshi-Tago, M., Tago, K., Andoh, K., Sonoda, Y., Tominaga, S., and Kasahara, T. (2005) J. Biochem. (Tokyo) 137 189–197 [DOI] [PubMed] [Google Scholar]
- 45.Shin, I., Kim, S., Song, H., Kim, H. R., and Moon, A. (2005) J. Biol. Chem. 280 14675–14683 [DOI] [PubMed] [Google Scholar]
- 46.Ozes, O. N., Mayo, L. D., Gustin, J. A., Pfeffer, S. R., Pfeffer, L. M., and Donner, D. B. (1999) Nature 401 82–85 [DOI] [PubMed] [Google Scholar]
- 47.Kane, L. P., Shapiro, V. S., Stokoe, D., and Weiss, A. (1999) Curr. Biol. 9 601–604 [DOI] [PubMed] [Google Scholar]
- 48.Fan, S., Meng, Q., Laterra, J. J., and Rosen, E. M. (2007) Oncogene 26 4774–4796 [DOI] [PubMed] [Google Scholar]
- 49.Schmeck, B., Zahlten, J., Moog, K., Van Laak, V., Huber, S., Hocke, A. C., Opitz, B., Hoffman, E., Kracht, M., Zerrahn, J., Hammerschmidt, S., Rosseau, S., Suttorp, N., and Hippenstiel, S. (2004) J. Biol. Chem. 279 53241–53247 [DOI] [PubMed] [Google Scholar]
- 50.Mikami, F., Gu, H., Jono, H., Andalibi, A., Kai, H., and Li, J. D. (2005) J. Biol. Chem. 280 36185–36194 [DOI] [PubMed] [Google Scholar]
- 51.Abu-Amer, Y., Ross, F. P., McHugh, K. P., Livolsi, A., Peyron, J. F., and Teitelbaum, S. L. (1998) J. Biol. Chem. 273 29417–29423 [DOI] [PubMed] [Google Scholar]
- 52.Han, Q., Leng, J., Bian, D., Mahanivong, C., Carpenter, K. A., Pan, Z. K., Han, J., and Huang, S. (2002) J. Biol. Chem. 277 48379–48385 [DOI] [PubMed] [Google Scholar]
- 53.Woronicz, J. D., Gao, X., Cao, Z., Rothe, M., and Goeddel, D. V. (1997) Science 278 866–869 [DOI] [PubMed] [Google Scholar]
- 54.Nakano, H., Shindo, M., Sakon, S., Nishinaka, S., Mihara, M., Yagita, H., and Okumura, K. (1998) Proc. Natl. Acad. Sci. U. S. A. 95 3537–3542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yin, L., Wu, L., Wesche, H., Arthur, C. D., White, J. M., Goeddel, D. V., and Schreiber, R. D. (2001) Science 291 2162–2165 [DOI] [PubMed] [Google Scholar]
- 56.Khwaja, A., Rodriguez-Viciana, P., Wennstrom, S., Warne, P. H., and Downward, J. (1997) EMBO J. 16 2783–2793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xia, H., Nho, R. S., Kahm, J., Kleidon, J., and Henke, C. A. (2004) J. Biol. Chem. 279 33024–33034 [DOI] [PubMed] [Google Scholar]
- 58.Janssens, S., Tinel, A., Lippens, S., and Tschopp, J. (2005) Cell 123 1079–1092 [DOI] [PubMed] [Google Scholar]
- 59.Bottero, V., Busuttil, V., Loubat, A., Magne, N., Fischel, J. L., Milano, G., and Peyron, J. F. (2001) Cancer Res. 61 7785–7791 [PubMed] [Google Scholar]
- 60.Kaltschmidt, B., Kaltschmidt, C., Hofmann, T. G., Hehner, S. P., Droge, W., and Schmitz, M. L. (2000) Eur. J. Biochem. 267 3828–3835 [DOI] [PubMed] [Google Scholar]
- 61.Zheng, Y., Ouaaz, F., Bruzzo, P., Singh, V., Gerondakis, S., and Beg, A. A. (2001) J. Immunol. 166 4949–4957 [DOI] [PubMed] [Google Scholar]
- 62.Liu, J., Yang, D., Minemoto, Y., Leitges, M., Rosner, M. R., and Lin, A. (2006) Mol. Cell 21 467–480 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.