Abstract
Human coxsackievirus (CV) belongs to the picornavirus family, which consists of over 200 medically relevant viruses. In picornavirus, a chymotrypsin-like protease (3Cpro) is required for viral replication by processing the polyproteins, and thus it is regarded as an antiviral drug target. A 3C-like protease (3CLpro) also exists in human coronaviruses (CoV) such as 229E and the one causing severe acute respiratory syndrome (SARS). To combat SARS, we previously had developed peptidomimetic and zinc-coordinating inhibitors of 3CLpro. As shown in the present study, some of these compounds were also found to be active against 3Cpro of CV strain B3 (CVB3). Several crystal structures of 3Cpro from CVB3 and 3CLpro from CoV-229E and SARS-CoV in complex with the inhibitors were solved. The zinc-coordinating inhibitor is tetrahedrally coordinated to the His40-Cys147 catalytic dyad of CVB3 3Cpro. The presence of specific binding pockets for the residues of peptidomimetic inhibitors explains the binding specificity. Our results provide a structural basis for inhibitor optimization and development of potential drugs for antiviral therapies.
Coxsackieviruses (CVs)4 are positive sense, single-stranded RNA viruses, which belong to the Picornaviridae family (1). Symptoms of infection with CV B-group include fever, headache, sore throat, gastrointestinal distress, as well as chest and muscle pain, known as pleurodynia or Bornholme disease in many areas. In some cases, the symptoms progress to myocarditis or pericarditis, which can result in permanent heart damage or death. Coxsackie B virus infection may also induce aseptic meningitis. As a group, CV are the most common cause of unexpected sudden death and may account for up to 50% of such cases (2). The various members of the coxsackievirus B-group were discovered almost entirely in the United States, although a sixth member of the group has been found in the Philippines (1).
To date, no specific therapy for diseases caused by picornaviruses is available, although some general purpose antiviral drugs are in use. In Picornaviridae, a virally encoded chymotrypsin-like protease (3Cpro) is required for the proteolytic processing of the large polyproteins translated from the viral RNA genomes and thus is essential for viral replication (1, 3). The 3Cpro in rhinovirus (RV), another member of the Picornaviridae family, has been used as a drug target to develop the peptidomimetic inhibitor AG7088, aiming for treatment of the common cold. This inhibitor contains a lactam ring to mimic Gln at the P1 position and an α,β-unsaturated ester at P1′ as a Michael acceptor to form a covalent bond with the active site Cys residue (4–6).
Analogous to picornaviruses, human coronaviruses (CoV) are also positive sense, single-stranded RNA viruses, which contain a 3C-like protease (3CLpro) for viral polyprotein processing. The virus of severe acute respiratory syndrome (SARS), which caused an outbreak in 2002–2003 that killed ∼800 patients, has been identified as a human CoV, named SARS-CoV (7–10). The other members of CoV include CoV-229E, CoV-OC43, CoV-HKU, and CoV-NL63 (11–13). Although sharing similar substrate specificity with 3Cpro in recognizing Gln as the P1 residue (14), the 3CLpro of SARS-CoV possess no sequence homology with those of the 3Cpro type. AG7088, a potent inhibitor of RV 3Cpro, was tried but failed to inhibit 3CLpro of SARS-CoV (15), indicating subtle structural differences in their active sites. However, AG7088 analogues and several classes of novel inhibitors have been discovered to combat SARS-CoV by targeting its 3CLpro (16, 17).
In an attempt to develop new inhibitors against CV, in this study we have prepared, characterized, and solved the crystal structure of the recombinant 3Cpro from CVB3. Two classes of SARS-CoV 3CLpro inhibitors, zinc-coordinating and peptidomimetic compounds (18–20), were tested and found active against the recombinant 3Cpro of CVB3. We also determined the structures of 3Cpro and 3CLpro complexes with these inhibitors to elucidate the binding specificity. Our results could serve as the structural basis for further inhibitor optimization and development of potential drugs for antiviral therapies.
EXPERIMENTAL PROCEDURES
Preparation of SARS-CoV 3CLpro, CoV-229E 3CLpro, and CVB3 3Cpro—SARS-CoV 3CLpro was prepared as described previously (21). The gene encoding CoV-229E 3CLpro was cloned from viral cDNA obtained from Tri-Service General Hospital (Taipei, Taiwan) using PCR. Primers (forward, 5′-CGCGGATCCATCGAAGGTCGTGCTGGTTTGCGCAAAATGGCAC-3′ (encoding Factor Xa cleavage site); and reverse, (5′-CCGCTCGAGTCATTGCAGGTTAACACCAAACATTTGTTTG-3′) containing BamHI and XhoI restriction sites (underlined) were used. The PCR product was digested by BamHI and XhoI and then ligated into pGEX-6p-1 vector (GE Healthcare), which contains a glutathione S-transferase tag at the N terminus.
The gene encoding CVB 3Cpro was cloned from viral cDNA obtained from Chang-Gung Memorial Hospital (Tao-Yuan, Taiwan) by PCR. Primers (forward, 5′-CATGCCATGGGCCCTGCCTTTGAGTT-3′; and reverse, 5′-GCGCTCGAGTCAATGATGATGATGATGATGTTGCTCATCATTGAAGTAGTG-3′) containing NcoI and XhoI restriction sites (underlined) were used for C-terminal His6 tag construction. The PCR products were digested with NcoI and XhoI, and the DNA fragment were cloned into pET16b (Novagen). The correct constructs were subsequently transformed to Escherichia coli BL21 (Novagen) for protein expression. Overnight culture (5 ml) of a single transformation was used to inoculate 500 ml of fresh LB medium containing 100 μg/ml ampicillin. The cells were grown to A600 = 0.6 and induced with 1 mm isopropyl-β-thiogalactopyranoside at 20 °C. After 16 h, the cells were harvested by centrifugation at 7000 × g for 15 min.
Purification of the proteases was conducted at 4 °C. His-tagged CVB3 3Cpro was purified using a nickel-nitrilotriacetic acid column and eluted with 25 mm Tris-HCl, pH 7.5, 120 mm NaCl, and 300 mm imidazole. The protein solution was dialyzed against 2× 2L buffer (containing 12 mm Tris-HCl, pH 7.5, 120 mm NaCl, and 0.1 mm EDTA, 7.5 mm β-mercaptoethanol, and 1 mm dithiothreitol). The 229E 3CLpro was purified using a glutathione S-transferase column and then treated with Factor Xa to remove the fusion tag. For further purity improvement and buffer exchange of both enzymes, a HiTrap™ 26/10 QFF column (GE Healthcare) was used with the buffers, which contained 50 mm Tris-HCl, pH 7.5, 1 mm EDTA, and 1 mm dithiothreitol (for CVB3 3Cpro) and 1 m NaCl and 50 mm Tris-HCl, pH 8.0 (for 229E 3CLpro). The purified proteins were concentrated for crystallization.
Peptide Synthesis and Examination of Substrate Specificity—The peptides used for testing as protease substrates were synthesized by using a 433A peptide synthesizer (Applied Biosystems). Starting with 0.10 mmol (0.101 g) of p-hydroxymethylphenoxymethyl polystyrene resin (1.01 mmol/g), synthesis was performed using a stepwise FastMoc protocol (Applied Biosystems). The amino acids were introduced using the manufacturer's prepacked cartridges (1 mmol each). Mass spectra of the synthesized peptides were obtained using the Finnigan LCQ mass spectrometer with an electrospray ion source.
For examining the substrate specificity of the protease, each peptide at 50 μm was incubated with 1 μm protease for 12 h, and the resulting mixture was analyzed by C-18 reverse-phase HPLC using the above mentioned conditions. The peak areas of the product were integrated to calculate the reaction rate of each peptide substrate under catalysis of the protease. The optimal peptide substrate was labeled with a fluorescence quenching pair; thereby the fluorogenic peptide (Dabcyl-KTSAVLQSGFRKME-Edans) could be used to measure the protease activity.
Protease Activity Assays—By using the fluorogenic peptide, the kinetic measurements were performed in 10 mm MES (pH 6.5, the optimal pH for protease activity) at 25 °C. Enhanced fluorescence due to cleavage of the peptide was monitored at 538 nm with excitation at 355 nm using a fluorescence plate reader (Fluoroskan Ascent, Thermo Scientific). The enzyme concentration used in measuring Km and kcat values was 0.5 μm, and the substrate concentrations were 0.5–5-fold the Km value. Substrate concentration was determined by using the extinction coefficients 5438 m–1 cm–1 at 336 nm (Edans) and 15,100 m–1 cm–1 at 472 nm (Dabcyl). The initial rate, within 10% of the substrate consumption, was used to calculate the kinetic parameters using Michaelis-Menten equation fitting with the KaleidaGraph computer program (Synergy Software).
Enzyme Inhibition Assay—For inhibition constant measurements of CVB3 3Cpro, reactions were performed with 0.5 μm protease and 10 μm fluorogenic peptide in a buffer of 10 mm MES, pH 6.5, and 25 °C. The fluorescence change resulting from the reaction was followed with time using a 96-well fluorescence plate reader. To determine the inhibitory effect of various compounds, the compound and the enzyme were preincubated at 25 °C for 20 min prior to the addition of the substrate. The inhibition constant of SARS 3CLpro was analyzed with reverse-phase HPLC using a C-18 column. Ki was calculated as described previously (18, 20).
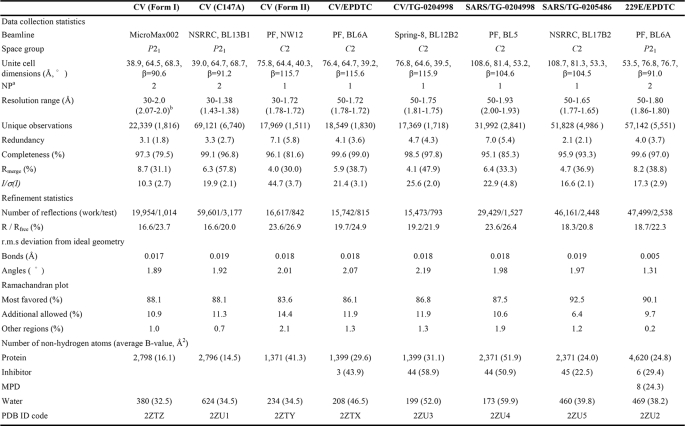
Crystallization—All inhibitors, including TG-0204998, TG-0205486, and zinc N-ethyl-N-phenyldithiocarbamate (EPDTC), were dissolved in DMSO. Using the sitting-drop vapor diffusion method, the enzyme solution was mixed with inhibitor solutions at a molar ratio of 1:5 for 20 min before being combined with equal amounts of reservoir. 3CLpro-EPDTC crystal of CoV-229E was obtained using a reservoir of 18% PEG 6000, 10% DMSO, 14% methyl-2,4-pentanediol (MPD), and 0.12 m Tris-HCl, pH 8.5. The crystals were flash-frozen with 20–25% ethylene glycol (v/v) as a cryoprotectant. For CVB3 3Cpro and 3Cpro-EPDTC crystals, a solution of 24% PEG 4000 and 0.1 m Tris-HCl, pH 8.0, was used as the reservoir. Native CVB3 3Cpro was crystallized using 25% PEG 4000, 0.2 m MgCl2, and 0.1 m Tris-HCl, pH 8.5, to give two monoclinic forms with space groups P21 (form I) and C2 (form II). The second crystal form was obtained when the enzyme solution was mixed with the reservoir at a volume ratio of 3:2. The other two CVB3 3Cpro crystals, the TG-0204998 complex and the inactive C147A mutant, were obtained using a reservoir solution of 24–30% PEG 4000, 0.2 m MgCl2, and 0.1 m Tris-HCl, pH 7.5–8.5. These crystals were rinsed with 26% glycerol as a cryoprotectant prior to data collection. Crystals of SARS 3CLpro complexed with TG-0204998 and TG-0205486 were obtained using a reservoir solution of 3–6% PEG 6000, 4–6% DMSO or MPD, 1 mm dithiothreitol, 0.1 m MES, pH 6.5, and a cryoprotectant of 25–30% glycerol or ethylene glycol. All diffraction data were collected at cryogenic temperatures and processed and scaled using the program HKL2000 (22).
Structure Determination—All crystal structures were determined by molecular replacement using the program AMoRe (23). Using the SWISS-MODEL server (24) and the human RV 3Cpro (1CQQ; 4) structure as a template, a CV3B 3Cpro model was generated to solve its EPDTC complex structure. The remaining CV 3Cpro crystal structures were solved using the partially refined 3Cpro-EPDTC complex structure as a search model. For SARS 3CLpro and CoV-229E 3CLpro complex structures, 1Z1I (25) and 1P9S (26) were used as the search models for molecular replacement, respectively.
The program CNS (Crystallography and NMR System) (27) was used for structure refinement. All manual modifications of the models were performed using the program XtalView (28). Difference Fourier (Fo – Fc) maps were calculated to locate the inhibitors and solvent molecules. Data collection and final model statistics are shown in Table 1. The atomic coordinates and structure factors of the various structures have been deposited in the Protein Data Bank (see Table 1).
TABLE 1.
Data collection and refinement statistics for CVB3 3Cpro, SARS-CoV 3CLpro, and CoV-229E 3CLpro crystals
a Number of protein molecules per asymmetric unit.
b Numbers in parentheses are for the outermost resolution shell.
Detection of Coxsackievirus B3 by RT-PCR—The confluent human embryonal rhabdomyosarcoma cells in 12-well plates were infected or mock-infected by coxsackievirus B3 at a multiplicity of infection of 0.01, with treatment of compound TG-0204998 at 0, 0.5, 1, 2, and 3 μm or compound TG-0205221 at 0, 1, 2, 5, 10, and 50 μm. Forty-eight hours after infection, total culture supernatants were harvested from the culture medium, and the supernatants of cell platelets were disrupted by freeze-thaw method. Viral RNA, extracted from 150 μl of total culture supernatant using the viral RNA extraction mini-prep system kit (Viogene, Sunnyvale, CA) with the protocol recommended by the manufacturer, was eluted in 50 μl of diethylpyrocarbonate-treated water. RT-PCR amplifications were performed using a Reverse iT™ one-step RT-PCR kit (Abgene, Epsom, Surrey, UK) using the primer set specific for pan-enterovirus detection (29). RT-PCR was carried out in a 25-μl reaction mixture containing 11 μl of RNA template, 0.5 μl of 10 μm forward and reverse primers, 0.5 μl of kit-supplied enzyme mixture, and 12.5 μl of 2× RT-PCR Master Mix. The RT step included incubation at 42 °C for 1 h and then at 95 °C for 5 min followed by 25 cycles of 95 °C for 30 s, 50 °C for 30 s, and 72 °C for 1 min and a final elongation step of 72 °C for 5 min. PCR products were analyzed by gel electrophoresis on 1% agarose gel containing 2 μg/ml ethidium bromide. The DNA bands were visualized and photographed through UV transillumination.
RESULTS
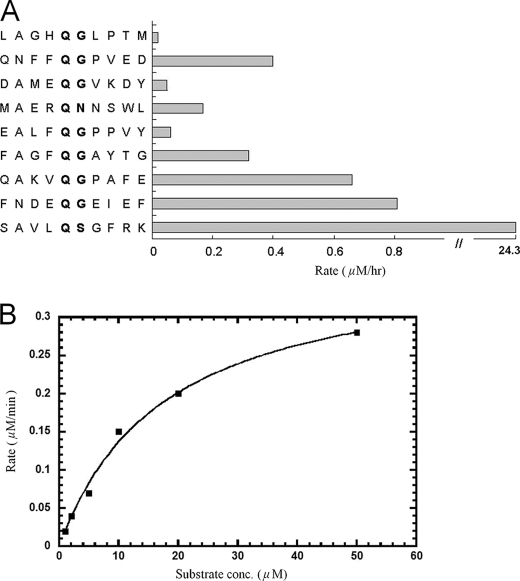
Substrate Specificity and Kinetics of CVB3 3Cpro—The peptides corresponding to the autoprocessing sites of CVB3 3Cpro included LAGHQ ↓ GLPTM between vp2 and vp3, QNFFQ ↓ GPVED between vp3 and vp1, DAMEQ ↓ GVKDY between P2A and P2B, MAERQ ↓ NNSWL between P2B and P2C, EALFQ ↓ GPPVY between P2C and P3A, FAGFQ ↓ GAYTG between P3A and VPg, QAKVQ ↓ GPAFE between VPg and 3C, FNDEQ ↓ GEIEF between 3C and RNA-dependent RNA polymerase, and SAVLQ ↓ SGFRK derived from the N-terminal autoprocessing site of 3CLpro from SARS-CoV (almost all of which contained a Gln followed by a small amino acid (Gly or Ser) to serve as the cleavage site) were tested as substrates for the protease (↓ indicates the cleavage site). Surprisingly, CVB3 3Cpro showed substantially better activity (30-fold) against SAVLQ ↓ SGFRK, the substrate of SARS-CoV 3CLpro, than its own cleavage sites based on the HPLC assay (Fig. 1A). Therefore, the peptide Dabcyl-KTSAVLQSGFRKME-Edans, with the fluorescence quenching pair Dabcyl-Edans, was chosen for CVB3 protease activity assay by monitoring the fluorescence increase due to peptide bond cleavage in real time. The kcat (Vmax/[E]) and Km values of the protease using this fluorogenic substrate were determined to be 6.2 × 10–3 s–1 and 9.2 ± 1.7 μm, respectively (Fig. 1B).
FIGURE 1.
Substrate specificity and kinetics of CVB3 3Cpro. A, compared with the peptides derived from its processing sites, CVB3 3Cpro showed the best activity toward the preferred substrate, SAVLQSGFRK, of SARS-CoV 3CLpro. B, by using the fluorogenic substrate Dabcyl-KTSAVLQSGFRKME-Edans, the initial reaction rates of the protease under a variety of different substrate concentrations were plotted against substrate concentrations to obtain the Vmax and Km values of the enzyme. The kinetic data were fitted with the Michaelis-Menten equation and kcat (Vmax/[E]) and Km were determined to be 6.2 × 10–3 s–1 and 9.2 ± 1.7 μm, respectively.
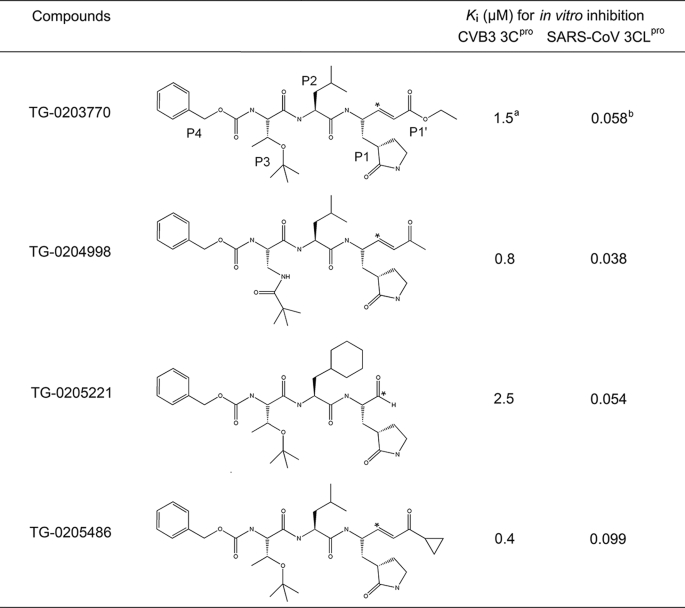
Evaluation of Inhibitors—Because CVB3 3Cpro showed substrate specificity similar to that of SARS-CoV 3CLpro, our reported 3CLpro inhibitors were tested against the recombinant 3Cpro of CVB3 by using the fluorogenic substrate to determine their inhibitory activities. EPDTC, which inhibited SARS protease with a Ki of 1 μm (19), showed lower inhibition against CVB3 3Cpro (Ki = 4.4 ± 0.7 μm) (Fig. S1). From the inhibition pattern, it was shown to be competitive with respect to the substrate. For the peptidomimetic inhibitors, the Ki values of TG-0203770, TG-0204998, TG-0205221, and TG-0205486 against SARS-CoV 3CLpro were 0.058, 0.038, 0.054, and 0.099 μm, respectively. The Ki values of TG-0203770 and TG-0205221 are reported elsewhere (20). Larger Ki values of 1.5, 0.8, 2.5, and 0.4 μm for the four compounds against CVB3 3Cpro were observed, indicating their lower affinity toward CVB3 3Cpro (Table 2). TG-0204998 and TG-0205486 have similar structures, differing only by an extra cyclopropyl group at the P1′ residue, and they also possess similar potency with a Ki of 0.8 and 0.4 μm, respectively, against CVB3 3Cpro.
TABLE 2.
Peptidomimetic inhibitors against CVB3 3Cpro and SARS-CoV 3CLpro (Ref. 22)
* Michael addition site.
a Analyzed using fluorescence.
b Analyzed using reverse-phase HPLC.
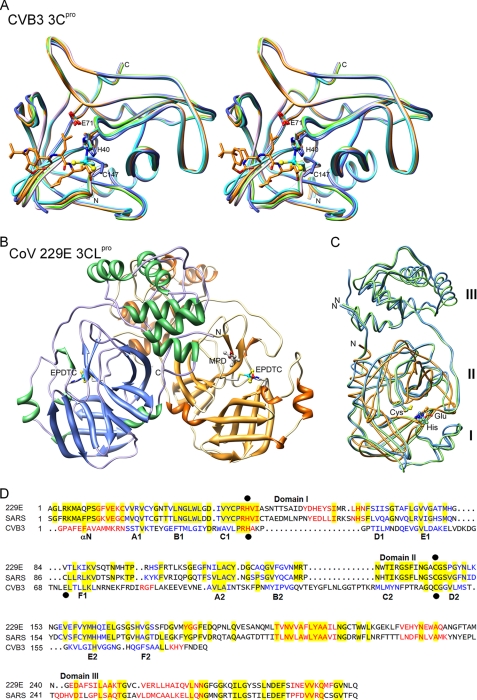
Structures of CVB3 3Cpro and CoV 3CLpro—Two crystal forms belonging to the monoclinic space groups P21 (form I) and C2 (form II), of CVB3 3C protease were obtained under similar crystallization conditions (Table 1). Form I crystal has two molecules per asymmetric unit related by a pseudo-dyad; form II crystal has only one molecule. As shown in Fig. 2A, the structure of CVB3 3Cpro adopts a chymotrypsin protein fold similar to those of RV 3Cpro (4) and other viral 3C proteases. The root-mean-square deviation between CVB3 3Cpro and RV 3Cpro is 0.73 Å for 166 Cα atoms. The N terminus starts with an α-helix of residues 1–14 and is followed by two topologically equivalent β-barrel domains comprising residues 15–77 and 99–173, which pack together to form an extended shallow groove for substrate binding. The catalytic triad of Cys147, His40, and Glu71 is located in the cleft between the two β-barrel domains. The protease structures in the two crystal forms are very similar, except that the loop of residues 143–146 is disordered, the side chain of Cys147 has a different orientation, and His40 is slightly shifted in the form II crystal (Fig. 2A). This loop is flexible on the protein surface, which may regulate the substrate binding. Such variable loop conformation was also observed in the 3Cpro structure of foot-and-mouth disease virus (30). The C147A mutant of CVB3 3Cpro crystallized in a unit cell similar to that of the wild-type form I crystal and diffracted to 1.38 Å (Table 1 and Fig. S2). Both complexes of CVB3 3Cpro with EPDTC and TG-0204998 crystallized in the form II unit cell, with only a slight variation in the protein conformation (Fig. 2A).
FIGURE 2.
Comparison of 3Cpro and 3CLpro structures. A, stereo view of five CVB3 3Cpro structures (form I, form II, C147A, and complexed with EPDTC and TG-0204998) superimposed and colored in pink, marine, green, cyan, and orange, respectively. EPDTC, TG-0204998, and the catalytic residues are shown as sticks, and the triad catalytic residues His40, Glu71, and Cys147 are labeled. The disordered residues 143–146 in the form II crystal are indicated by dashed lines. B, overall three-dimensional structure of CoV-229E 3CLpro with bound EPDTC and MPD, colored by secondary structure elements. C, crystal structures of CVB3 3Cpro, SARS-CoV 3CLpro (Protein Data Bank code 2Z9J), and CoV-229E 3CLpro are superimposed and colored in orange, light blue, and green, respectively. Their catalytic residues are indicated. The labels I, II, and III (on the right) denote the three domains of 3CLpro. D, structure-based sequence alignment of CoV-229E 3CLpro, SARS-CoV 3CLpro, and CVB3 3Cpro. Residues of α-helices are shown in red and those of β-strands in blue, and the identical residues are highlighted in yellow. Catalytic residues are marked with black dots. The three domains according to the 3CLpro structures are shown above the sequence, and the secondary structure elements of CVB3 3Cpro are shown below.
The complex crystals of SARS-CoV 3CLpro/TG-0204998 and 3CLpro/TG-0205486 belong to the C2 space group with one complex model per asymmetric unit (Table 1). In these structures, the inhibitors were well defined in the Fourier maps. CoV-229E 3CLpro-EPDTC complex crystallized in a P21 unit cell with a dimer as its asymmetric unit (Fig. 2B). As reported previously, both CoV-229E and SARS-CoV 3CLpro are homodimers with three domains in each monomer. The catalytic dyad of His-Cys is located in the active site in the cleft between domains I and II, whereas domain III participates in the protease dimerization (26, 31). In contrast, CVB3 3Cpro is a monomer, and it does not contain the dimerization domain III. By employing the program O (32), CVB3 3Cpro monomer can be superimposed onto SARS 3CLpro with a root-mean-square deviation of 2.11 Å for 121 matched Cα atoms or 1.06 Å for 48 Cα if more stringent criteria were used. His40 and Cys147 in the catalytic triad of CVB3 3Cpro coincide almost perfectly with the His-Cys dyads of the CoV proteases (Fig. 2C). Although the two types of proteases share very little sequence homology, structure-based sequence comparison reveals some similarity in the regions of domains I and II (Fig. 2D), which explains the similar substrate specificity and catalytic mechanism. However, the different affinities of the protein for the inhibitors encouraged further analysis of their interactions.
Interactions of 3Cpro and 3CLpro with the Inhibitors—The structure of EPDTC is shown in Fig. 3A, and those of the peptide inhibitors are shown in Table 1. The local structures of CVB3 3Cpro and CoV-229E 3CLpro in complex with EPDTC are shown in Fig. 3, B and C, and the previously determined structure of SARS-CoV 3CLpro in complex with EPDTC is shown in Fig. 3D for comparison. The environments of the peptide inhibitors bound to the proteases are shown in Fig. 4. Omit maps for the bound inhibitors are shown in Fig. 5.
FIGURE 3.
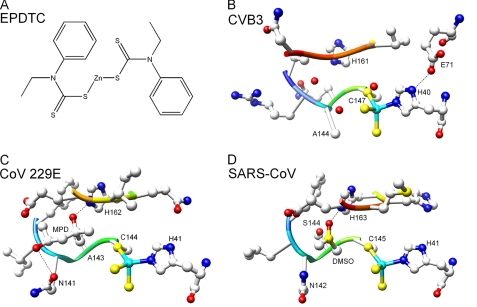
EPDTC bound to the three different proteases. A, schematic drawing of the chemical structure of zinc-coordinating inhibitor EPDTC. B–D, the inhibitor is coordinated to the catalytic dyad residues His and Cys of CVB3 3Cpro, CoV-229E 3CLpro, and SARS-CoV 3CLpro with a zinc-centered tetrahedral geometry. A MPD molecule located in the S1 pocket of CoV-229E 3CLpro and a DMSO molecule in SARS-CoV 3CLpro are shown in addition to the inhibitor. The oxygen atoms are shown in red, nitrogen in blue, sulfur in orange, and carbon in gray. The zinc ions are depicted as a cyan sphere.
FIGURE 4.
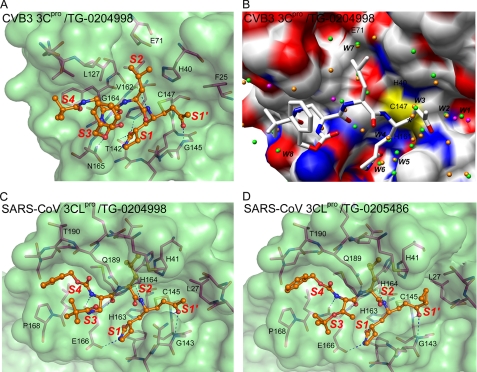
Peptidomimetic inhibitors bound to the proteases. A, the active site of CVB3 3Cpro with the bound TG-0204998. Protein is rendered as a semitransparent solvent-accessible surface with associated protein backbone and side chain atoms. The oxygen atoms are shown in red, nitrogen in blue, sulfur in yellow, and protein carbon atoms in magenta. The inhibitor atoms are shown as ball-and-stick form and colored in orange for carbon. The sulfur atom of Cys147 is covalently attached to the inhibitor carbon. The substrate binding subsites are designated as S1′, S1, S2, S3, and S4. B, conserved water molecules, identified by superimposing the two molecules of CVB3 3Cpro C147A mutant on the TG-0204998 complex of CVB3 3Cpro. The water molecules are shown here as spheres colored green/orange (C147A) and magenta (complex). The protein model of the TG-0204998 complex structure is shown as an electrostatic surface and TG-0204998 as sticks. The conserved water molecules of the C147A mutant structure in the active site are marked. The black star indicates the Michael addition site. C and D, the active site of SARS 3CLpro with bound TG-0204998 (C) and TG-0205486 (D), colored as in A. The substrate-binding subsites are also indicated.
FIGURE 5.
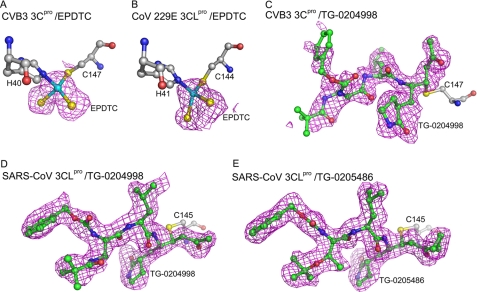
Omit maps of inhibitors. Fo – Fc omit maps (magenta) were calculated for the five inhibitors and contoured at the 2.0 σ level. The inhibitor and inhibitor-binding residues are shown as ball-and-stick models with carbon atoms in green and gray. The sulfur, nitrogen, and oxygen atoms are colored in yellow, blue, and red, respectively. A and B, EPDTC bound to CVB3 3Cpro and CoV-229E 3CLpro. The zinc ion (cyan sphere) of EPDTC was coordinated to the Cys and His residues. C and D, TG-0204998 in covalent bond with the Cys of CVB3 3Cpro and SARS-CoV 3CLpro. E, TG-0205486 in covalent bond with the Cys of SARS-CoV 3CLpro.
The zinc sites were confirmed by anomalous difference Fourier maps (not shown). The binding mode of EPDTC in the CVB3 and CoV-229E proteases are similar to that observed in SARS-CoV 3CLpro, where the Zn2+ binds to the two catalytic residues Cys and His. The zinc and two sulfur atoms of EPDTC were clearly seen in every subunit, but the bulky substituent groups in both crystals were invisible. In the zinc center, the S—Zn—S bond angle is 103.1° for CVB3 3Cpro, similar to the 107.2° observed in SARS-CoV 3CLpro structure; but a distorted tetrahedral geometry was observed for CoV-229E 3CLpro (Fig. 5B), with an S—Zn—S angle of 85.25°, despite the restraints to an ideal angle of 109.5° employed in the refinement.
In addition to Zn2+, an MPD molecule from the crystallization reservoir was found in one subunit of CoV-229E 3CLpro, which is located in the S1 pocket. The two oxygen atoms of MPD form two H-bonds with the side chain of His162 and Gln141 with distances of 2.66 and 3.15 Å, respectively. MPD binds in a similar position to that of DMSO (coming from the solvent to dissolve the inhibitor) in SARS-CoV 3CLpro complexed structure. No such organic molecule was observed in the complex structure of CVB3 3Cpro with EPDTC.
In the CVB3 3Cpro/TG-0204998 structure (Fig. 4A), the α,β-unsaturated ethyl ester occupies the S1′ site, which is in close proximity to the catalytic center. The Michael acceptor forms a covalent C–S bond with the Cys147. The lactam ring occupies the S1 site, as it is a Gln mimic and forms two H-bonds with Thr142 and His161. The P2 Leu side chain occupies the S2 site, a large hydrophobic pocket. Interestingly, the P3 group of TG-0204998 is actually located in the S4 site. The P4 benzoxy ester group lacks strong electron densities (Fig. 5C) because it is exposed to the bulk solvent. In Fig. 4B, two protein molecules in the C147A structure are superimposed, along with the TG-0204998 complex and eight conserved water molecules with distances of less than 0.4 Å between the equivalents. Some of these waters occupied the inhibitor binding sites, whereas others may help improve the drug-protein interactions (see below).
In the SARS-CoV 3CLpro/TG-0204998 structure (Fig. 4C), Cys145 attacks the α-carbon of the α,β-unsaturated ketone at the P1′ position to form a covalent C–S bond of 1.99 Å and induces hydrogen bonding between the ketone oxygen and the N-H groups of Gly143 and Cys145 itself in the S1′ pocket. In the S1 site, the carbonyl oxygen and the nitrogen of the five-member lactam ring at the P1 position form hydrogen bonds with His163 and Glu166, respectively. The following P2 Leu side chain binds into a hydrophobic S2 pocket. The P3 group is directed toward the bulk solvent. The benzoxy group at the P4 position is bound in the S4 pocket with its phenyl ring aligned parallel to a flat surface near Ala191. Most of the carbon atoms (4 of 6) in the phenyl ring show hydrophobic contacts with the protein.
In the structure of SARS-CoV 3CLpro/TG-0205486 (Fig. 4D), the inhibitor adopts a similar binding mode to that in the above structure. In both structures, the peptide N-H groups of residues P1 to P3 form four hydrogen bonds with the backbone CO groups of His164 and Glu166, and the side chain of Gln189. In addition, the CO group of P3 interacts with the N-H groups of Glu166.
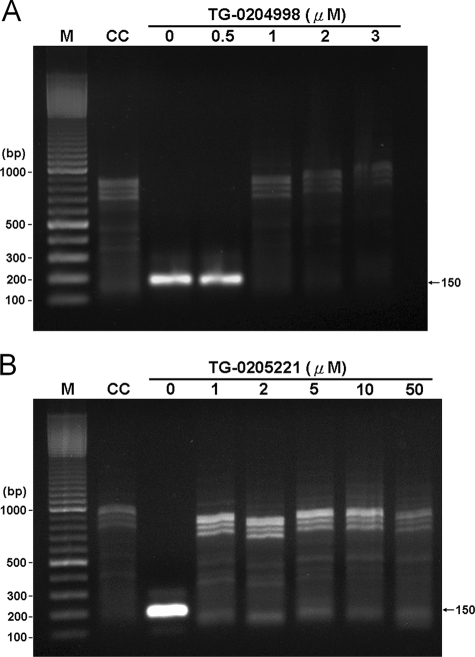
Antiviral Compounds TG-0204998 and TG-0205221 Can Effectively Inhibit Replication of CVB3—The critical and essential step in coxsackievirus replication is polyprotein cleavage by 3Cpro after viral protein translation, which is the potential target for antiviral drugs TG-0204998 and TG-0205221 (33). To observe the effect of compounds TG-0204998 and TG-0205221 on virus replication, RT-PCR was applied to detect the coxsackievirus genome in the infected cells. As shown in Fig. 6, a 150-bp DNA fragment could be detected in virus-infected cells either in the absence or presence of 0.5 μm TG-0204998. However, no specific DNA fragment could be found when the concentration of compound TG-0204998 or TG-0205221 was increased to 1 μm, indicating that both compounds could effectively block viral replication in the host cells.
FIGURE 6.
CVB3 replication in rhabdomyosarcoma cells detected by RT-PCR. RNAs extracted from mock-infected (CC, lane 2) or CVB3-infected rhabdomyosarcoma cells in the presence of compound TG-0204998 at 0, 0.5, 1, 2, and 3 μm (A, lanes 3–7) or compound TG-0205221 at 0, 1, 2, 5, 10 and 50 μm (B, lanes 3–8) were amplified by RT-PCR as described under “Experimental Procedures” and resolved in 1% agarose gel. The expected size (150 bp) of the RT-PCR product is indicated with an arrow. The 100-bp DNA marker shows the molecular size (M, lane 1).
DISCUSSION
Two kinds of proteases, 3Cpro and 3CLpro, serve as drug targets for developing inhibitors against picornaviruses and coronaviruses, respectively. Although these two proteases do not share sequence homology, they share similar substrate specificity in recognizing Gln as the P1 residue, a hydrophobic residue at the P2 position, and a small amino acid residue at the P1′ position. In this study, we found that CVB3 3Cpro actually prefers the substrate of SARS-CoV 3CLpro derived from its N-terminal processing site. However, the inhibitors could not inhibit both proteases equally (Table 2), suggesting some structural differences in the active site. To understand the structural basis of the specificity, we solved the structures of 3Cpro from CVB3 and 3CLpro from CoV-229E and SARS-CoV with the same inhibitors to compare their binding modes. The information obtained here is useful for designing specific inhibitors against each kind of the proteases.
For the zinc-coordinating inhibitor EPDTC, similar to the previously determined binding mode in the structure of a complex with SARS-CoV 3CLpro, the Zn2+ cation is tetrahedrally coordinated by the His-Cys catalytic residues of CVB3 3Cpro (Fig. 3B). The binding is facilitated by the suitable distances from the nitrogen atom of His and the S– anion of Cys to the zinc center in both 3C and 3CL proteases. However, the EPDTC inhibition of CVB3 3Cpro is 4.4-fold weaker (Fig. S1); this is probably because the side chain of Glu71 is hydrogen-bonded to the Nδ1 atom of His40 to favor the protonation of Nε2. Therefore the Nε2 atom must be deprotonated before EPDTC binding takes place. The lack of electron density for the bulky substituent groups might suggest that they are disordered. According to a computer model of the inhibitor with SARS-CoV 3CLpro (21), the entire EPDTC could be accommodated in the active site with sufficient room for the bulky side groups to rotate about. On the other hand, a molecule of DMSO (SARS-CoV) or MPD (CoV-229E) is bound in the S1 site of 3CLpro. Although the DMSO molecule forms a hydrogen bond with Ser144 of the former enzyme, the smaller side chain of Ala143 in the latter case provides hydrophobic interaction and larger space for MPD bonding (Fig. 3, C and D). No such solvent molecule was seen in the complex structure of CVB3 3Cpro with EPDTC, because its more open S1 pocket fails to trap small molecules.
Unlike 3CLpro, which is dimeric and in which each subunit is composed of three domains, 3Cpro is a monomer with only the two catalytic domains. Structure-based sequence alignment shows that 3CLpro has a large loop between β-strands C1 and D1, whereas 3Cpro has smaller loops inserted between E1 and F1 and between B2 and C2 (Fig. 2D). The C1–D1 loop of SARS-CoV 3CLpro secures the S2 hydrophobic pocket for the P2 side chain. The two loops E1–F1 and B2–C2 of CVB3 3Cpro are also adjacent to the active site, and they modulate the binding of the P3 and P4 residues. The four peptidomimetic compounds have a higher Ki for CVB3 3Cpro than for SARS-CoV 3CLpro in general, not only because it has a more open active site but also because the binding sites are different.
For the peptidomimetic inhibitors, both TG-0204998 and TG-0205486 bind to the active site of SARS-CoV 3CLpro in similar modes, whereas TG-0204998 binds differently to CVB3 3Cpro. The P2 cyclohexyl side chain of TG0205221 fits well in the S2 site of SARS-CoV 3CLpro but is too bulky for CVB3 3Cpro, whereas the P2 Leu of TG0204998 fits well in both. The E1–F1 loop of CVB3 3Cpro makes the S2 site shallow and open (Fig. 4B), which is consistent with its 46-fold higher Ki of TG-0205221 (Table 2). On the other hand, our CVB3 3Cpro structure is analogous to that of RV 3Cpro, and a P2 phenyl side chain may be preferred by CVB3 3Cpro as evidenced by the tight binding of AG7088 to RV 3Cpro (4). Moreover, the t-butyl group of P3 is favored for tight binding to the S3 site of SARS-CoV 3CLpro, which enhances the inhibition by more than 10-fold (20). Conversely, the P3 Val in AG7088 fits RV 3Cpro very well, but the additional t-butyl group makes our compounds weaker inhibitors of CVB3 3Cpro. With the t-butyl group, the bulky P3 residue is actually relocated to the hydrophobic environment in the S4 site formed by the B2–C2 loop of CVB3 3Cpro, leaving the unbound P4 benzoxy group facing the bulk solvent (Figs. 2A and 4B). This may also contribute to the higher Ki. Removal of the P3 t-butyl group or the entire P4 residue may improve the inhibitors against CVB3 3Cpro.
In these inhibitors, the P1 site favors Gln or its mimicking lactam ring, and the lactam ring provides 15-fold better inhibitory activity than Gln, as revealed by the previously reported structure-activity relationships (4). The strong binding of the lactam ring to the proteases is evidenced by the multiple hydrogen bond formations in the crystal structures (Fig. 4). The addition of a cyclopropyl group to the P1′ residue of TG-0205486 enhances the inhibition against CVB3 3Cpro by almost 4-fold as compared with TG-0203770, but it becomes weaker against SARS-CoV 3CLpro (Table 2). As shown in Fig. 4D, the triangular group tends to clash with the protein atoms because of the more limited space of the S1′ site adjacent to loop C1–D1. In CVB3 3Cpro, the S1′ site is more open, yet still flanked by the hydrophobic side chain of Phe25.
The conserved water molecules in the active site, as revealed by the high-resolution crystal structure of the C147A mutant of CVB3 3Cpro (Fig. 4B), may also offer some trends for optimizing the inhibitors. If the water model is superimposed on the bound TG-0204998 molecules, W3 and W4 are equivalent to two carbon atoms in the P1′ and P1 residues of TG-0204998. W5 and W6 are located in the S1 pocket, forming hydrogen bonds to the protein including His161. These are to be displaced by the P1 lactam ring with a similar bonding pattern. W7 is located in the S2 pocket with a distance of about 1.85 Å to the side chain carbon of P2 Leu and is hydrogen-bonded to the side chain of Glu71 at a distance of 2.98 Å. Based on these observations, a new drug can have the W4-equivalent carbon atom replaced by a nitrogen or sulfur, but the W3-equivalent carbon cannot be changed because it is part of the α,β-unsaturated aldehyde for the Michael addition. The P2 Leu may be replaced by an Arg or a Lys residue, which can interact with Glu71.
In summary, we have shown here that the antiviral compounds TG-0204998 and TG-0205221 inhibit the viral protease, thus preventing CVB3 genome replication. Our study reveals the key interactions in determining the binding specificity of the inhibitors against 3C and 3CL proteases. The information will be useful for further inhibitor optimization and drug discovery.
Supplementary Material
Acknowledgments
We are grateful to the National Synchrotron Radiation Research Center of Taiwan, SPring-8, and Photon Factory of Japan for beam time allocations. We also thank Chi-Shen Wu and Li-Wen Chang of TaiGen Biotechnology, Taipei, for kindly providing peptidomimetic inhibitor compounds.
This work was supported by grants from Academia Sinica and the National Science Council, Taiwan (NSC-95-3112-B-001-015-Y to A. H.-J. W.) for the National Core Facility of High-Throughput Protein Crystallography at Academia Sinica, Taiwan. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
The atomic coordinates and structure factors (codes 2ZTZ, 2ZU1, 2ZTY, 2ZTX, 2ZU2, 2ZU3, 2ZU4, and 2ZU5) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).
Footnotes
The abbreviations used are: CV, coxsackievirus; 3Cpro, 3C protease; RV, rhinovirus; 3CLpro, 3CL protease; CoV, coronavirus; SARS, severe acute respiratory syndrome; EPDTC, N-ethyl-N-phenyldithiocarbamate; Dabcyl, 4-(4-dimethylaminophenylazo)benzoic acid; Edans, 5-[(2-aminoethyl)amino]naphthalene-1-sulfonic acid; MES, 2-(N-morpholino)ethanesulfonic acid; MPD, methyl-2,4-pentanediol; HPLC, high pressure liquid chromatography; DMSO, dimethyl sulfoxide; PEG, polyethylene glycol; RT, reverse transcription.
References
- 1.David, M. K., Peter, M. H., Diane, E. G., Robert, A. L., Malcolm, A. M., Bernard, R., and Stephen, E. S. (2007) Fields Virology, 5th Ed., pp. 795–893, Wolters Kluwer Health-Lippincott Williams and Wilkins, New York
- 2.Maze, S. S., and Adolph, R. J. (1990) Clin. Cardiol. 13 69–79 [DOI] [PubMed] [Google Scholar]
- 3.Porter, A. G. (1993) J. Virol. 67 6917–6921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matthews, D. A., Dragovich, P. S., Webber, S. E., Fuhrman, S. A., Patick, A. K., Zalman, L. S., Hendrickson, T. F., Love, R. A., Prins, T. J., Marakovits, J. T., Zhou, R., Tikhe, J., Ford, C. E., Meador, J. W., Ferre, R. A., Brown, E. L., Binford, S. L., Brothers, M. A., DeLisle, D. M., and Worland, S. T. (1999) Proc. Natl. Acad. Sci. U. S. A. 96 11000–11007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson, T. O., Hua, Y., Luu, H. T., Brown, E. L., Chan, F., Chu, S. S., Dragovich, P. S., Eastman, B. W., Ferre, R. A., Fuhrman, S. A., Hendrickson, T. F., Maldonado, F. C., Matthews, D. A., Meador, J. W., Patick, A. K., Reich, S. H., Skalitzky, D. J., Worland, S. T., Yang, M., and Zalman, L. S. (2002) J. Med. Chem. 45 2016–2023 [DOI] [PubMed] [Google Scholar]
- 6.Binford, S. L., Maldonado, F., Brothers, M. A., Weady, P. T., Zalman, L. S., Meador, J. W., Matthews, D. A., and Patick, A. K. (2005) Antimicrob. Agents Chemother. 49 619–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peiris, J. S., Lai, S. T., Poon, L. L., Guan, Y., Yam, L.Y., Lim, W., Nicholls, J., Yee, W. K., Yan, W. W., Cheung, M. T., Cheng, V. C., Chan, K. H., Tsang, D. N., Yung, R. W., Ng, T. K., and Yuen, K. Y. (2003) Lancet 361 1319–1325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ksiazek, T. G., Erdman, D., Goldsmith, C. S., Zaki, S. R., Peret, T., Emery, S., Tong, S., Urbani, C., Comer, J. A., Lim, W., Rollin, P. E., Dowell, S. F., Ling, A. E., Humphrey, C. D., Shieh, W. J., Guarner, J., Paddock, C. D., Rota, P., Fields, B., DeRisi, J., Yang, J. Y., Cox, N., Hughes, J. M., LeDuc, J. W., Bellini, W. J., and Anderson, L. J. (2003) N. Engl. J. Med. 348 1953–1966 [DOI] [PubMed] [Google Scholar]
- 9.Fouchier, R. A., Kuiken, T., Schutten, M., van Amerongen, G., van Doornum, G. J., van den Hoogen, B. G., Peiris, M., Lim, W., Stohr, K., and Osterhaus, A. D. (2003) Nature 423 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drosten, C., Gunther, S., Preiser, W., van der Werf, S., Brodt, H. R., Becker, S., Rabenau, H., Panning, M., Kolesnikova, L., Fouchier, R. A., Berger, A., Burguiere, A. M., Cinatl, J., Eickmann, M., Escriou, N., Grywna, K., Kramme, S., Manuguerra, J. C., Muller, S., Rickerts, V., Sturmer, M., Vieth, S., Klenk, H. D., Osterhaus, A. D., Schmitz, H., and Doerr, H. W. (2003) N. Engl. J. Med. 348 1967–1976 [DOI] [PubMed] [Google Scholar]
- 11.Bradburne, A. F., Bynoe, M. L., and Tyrrell, D. A. (1967) Br. Med. J. 3 767–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woo, P. C., Lau, S. K., Chu, C. M., Chan, K. H., Tsoi, H. W., Huang, Y., Wong, B. H., Poon, R. W., Cai, J. J., Luk, W. K., Poon, L. L., Wong, S. S., Guan, Y., Peiris, J. S., and Yuen, K. Y. (2005) J. Virol. 79 884–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van der Hoek, L., Pyrc, K., and Berkhout, B. (2006) FEMS Microbiol. Rev. 30 760–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fan, K., Wei, P., Feng, Q., Chen, S., Huang, C., Ma, L., Lai, B., Pei, J., Liu, Y., Chen, J., and Lai, L. (2004) J. Biol. Chem. 279 1637–1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shie, J. J., Fang, J. M., Kuo, T. H., Kuo, C. J., Liang, P. H., Huang, H. J., Wu, Y. T., Jan, J. T., Cheng, Y.-S. E., and Wong, C. H. (2005) Bioorg. Med. Chem. 13 5240–5252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liang, P. H. (2006) Curr. Top. Med. Chem., 6 361–376 [DOI] [PubMed] [Google Scholar]
- 17.Wang, H. M., and Liang, P. H. (2007) Expert Opin. Ther. Pat. 17 533–546 [Google Scholar]
- 18.Hsu, J. T., Kuo, C. J., Hsieh, H. P., Wang, Y. C., Huang, K. K., Lin, C. P., Huang, P. F., Chen, X., and Liang, P. H. (2004) FEBS Lett. 574 116–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee, C. C., Kuo, C. J., Hsu, M. F., Liang, P. H., Fang, J. M., Shie, J. J., and Wang, A. H.-J. (2007) FEBS Lett. 581 5454–5458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang, S., Chen, S. J., Hsu, M. F., Wu, J. D., Tseng, C. T., Liu, Y. F., Chen, H. C., Kuo, C. W., Chang, L. W., Chen, W. C., Lio, S. Y., Chang, T. Y., Hung, H. H., Shr, H. L., Liu, C. Y., Huang, Y. A., Chang, L. Y., Hsu, J. C., Peters, C. J., Wang, A. H.-J., and Hsu, M. C. (2006) J. Med. Chem. 49 4971–4980 [DOI] [PubMed] [Google Scholar]
- 21.Kuo, C. J., Chi, Y. H., Hsu, T. A., and Liang, P. H. (2004) Biochem. Biophys. Res. Comm. 318 862–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Otwinowski, Z., and Minor, W. (1997) in Processing of X-ray Diffraction Data Collected in Oscillation Mode (Sweet, R. M., ed) pp. 307–326, Academic Press, New York [DOI] [PubMed]
- 23.Navaza, J. (1994) Acta Crystallogr. Sect. A 50 157–163 [Google Scholar]
- 24.Schwede, T., Kopp, J., Guex, N., and Peitsch, M. C. (2003) Nucleic Acids Res. 31 3381–3385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hsu, M. F., Kuo, C. J., Chang, K. T., Chang, H. C., Chou, C. C., Ko, T. P., Shr, H. L., Chang, G. G., Wang, A. H.-J., and Liang, P. H. (2005) J. Biol. Chem. 280 31257–31266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anand, K., Ziebuhr, J., Wadhwani, P., Mesters, J. R., and Hilgenfeld, R. (2003) Science 300 1763–1767 [DOI] [PubMed] [Google Scholar]
- 27.Brünger, A. T., Adams, P. D., Clore, G. M., Delano, W. L., Gros, P., Grosse-Kunstleve, R. W., Jiang, J. S., Kuszewski, J., Nilges, M., Pannu, N. S., Read, R. J., Rice, L. M., Simonson, T., and Warren, G. L. (1998) Acta Crystallogr. Sect. D 54 905–921 [DOI] [PubMed] [Google Scholar]
- 28.McRee, D. E. (1999) J. Struct. Biol. 125 156–165 [DOI] [PubMed] [Google Scholar]
- 29.Monpoeho, S., Dehee, A., Miqnotte, B., Schwartzbrod, L., Marechal, V., Nicolas, J. C., Billaudel, S., and Ferre, V. (2000) BioTechniques 29 88–93 [DOI] [PubMed] [Google Scholar]
- 30.Birtley, J. R., Knox, S. R., Jaulent, A. M., Brick, P., Leatherbarrow, R. J., and Curry, S. (2005) J. Biol. Chem. 280 11520–11527 [DOI] [PubMed] [Google Scholar]
- 31.Shi, J., Wei, Z., and Song, J. (2004) J. Biol. Chem. 279 24765–24773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones, T. A., Zou, J. Y., Cowan, S. W., and Kjeldgaard, M. (1991) Acta Crystallogr. Sect. A 47 392–400 [DOI] [PubMed] [Google Scholar]
- 33.Shih, S. R., Chiang, C., Chen, T. C., Wu, C. N., Hsu, J. T. A., Lee, J. C., Hwang, M. J., Li, M. L., Chen, G. W., and Ho, M. S. (2003) J. Biomed. Sci. 11 239–248 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.