Abstract
In this work, Photosystem I supercomplexes have been purified from Lhca-deficient lines of Arabidopsis thaliana using a mild detergent treatment that does not induce loss of outer antennas. The complexes have been studied by integrating biochemical analysis with electron microscopy. This allows the direct correlation of changes in protein content with changes in supramolecular structure of Photosystem I to get information about the position of the individual Lhca subunits, the association of the antenna to the core, and the influence of the individual subunits on the stability of the system. Photosystem I complexes with only two or three antenna complexes were purified, showing that the binding of Lhca1/4 and Lhca2/3 dimers to the core is not interdependent, although weak binding of Lhca2/3 to the core is stabilized by the presence of Lhca4. Moreover, Lhca2 and Lhca4 can be associated with the core in the absence of their “dimeric partners.” The structure of Photosystem I is very rigid, and the absence of one antenna complex leaves a “hole” in the structure that cannot be filled by other Lhcas, clearly indicating that the docking sites for the individual subunits are highly specific. There is, however, an exception to the rule: Lhca5 can substitute for Lhca4, yielding highly stable PSI supercomplexes with a supramolecular organization identical to the WT.
Photosystem I (PSI)2 operates in the light reactions of photosynthesis as a plastocyanin:ferredoxin oxidoreductase. In higher plants, it is embedded in the thylakoid membranes of chloroplasts. It can be divided into two moieties: (i) the core, harboring all the electron transport cofactors: 102 chlorophyll a (Chl a) molecules and ∼22 β-carotenes, and (ii) the peripheral light-harvesting complex (LHCI) (1–3). Under standard conditions, LHCI is composed of four pigment-protein complexes (Lhca1-Lhca4), coordinating Chl a, Chl b, lutein, violaxanthin, and β-carotene (4, 5) arranged on one side of the core (6, 7). In the crystal structure of PSI from pea (1), the Lhca order was assigned starting at the G-pole as Lhca1, Lhca4, Lhca2, Lhca3. These complexes are organized as dimers as shown by biochemical analysis (4). Studies of Lhca knock-out and antisense lines suggested that these subunits can only be associated with the core in their dimeric form. One of the dimers, which has been purified from plants and refolded in vitro (8, 9), is composed of Lhca1 and Lhca4. The second dimer is believed to be formed by Lhca2 and Lhca3, although the data are not conclusive because of the impossibility of purifying it from plants and refolding it in vitro. Moreover, fluorescence data suggested that part of the Lhca2 and Lhca3 population can also form homodimers (10).
A fifth pigment-protein complex, Lhca5, is present in non-stoichiometric amounts in wild type (WT) plants. Its expression level is higher in mutant plants depleted in Lhca1 and Lhca4; however no corresponding polypeptide could be detected on SDS-PAGE, indicating that even in these mutants, Lhca5 is not present in stoichiometric amounts with the core (11). Based on cross-linking studies, Lhca5 has been suggested to form homodimers (12), but in vitro reconstitution has shown that it can form heterodimers with Lhca1 (13, 14).
Lhca proteins are encoded by nuclear genes belonging to the Lhc multigene family, which also encodes for the Lhcb proteins of Photosystem II (PSII) (15). Judged from the crystal structure of PSI (1) and LHCII (16), the Chl organization of the members of the family is rather similar, although their spectroscopic properties differ substantially. Lhca complexes absorb more to the red than Lhcb complexes and while Lhcb complexes have low temperature fluorescence emission maxima at 680 nm (17), Lhca complexes have red-shifted maxima at 702 nm (Lhca1 and Lhca2), 725 nm (Lhca3), and 733 nm (Lhca4) (4, 5, 9, 10). It was shown that Asn as a ligand for Chl A5 is required for the strong red-shifted absorption and emission of Lhca3 and Lhca4 (18). In Lhca5 the ligand for Chl A5 is a His, as in Lhca1 and Lhca2, and accordingly it does not contain low-energy Chls (13).
The flexibility of the LHCI composition is a point of discussion. Ballottari et al. (19) showed that the Lhca stoichiometry is not affected by growth conditions. Furthermore, studies on Lhca knock-out and antisense plants showed that the lack of one antenna subunit is not compensated by increased levels of other antenna subunits, thus suggesting a rigid LHCI organization (20–22). However, it has been reported that the level of individual Lhca antenna is significantly changed under different light conditions (23, 24).
To clarify this picture, we combined biochemical analysis and electron microscopy (EM) single particle analysis to study PSI-LHCI complexes from Lhca-deficient lines (Δa) of Arabidopsis thaliana. We show that the individual antennas (Lhca1–4) are not interchangeable, and that the absence of an antenna results in a “hole” in the PSI structure. There is one exception to this rule: Lhca4 can be replaced by Lhca5 to yield PSI-LHCI particles with the same supramolecular organization as WT plants.
EXPERIMENTAL PROCEDURES
Plant Material—T-DNA knock-out (Δa1, Δa4) and antisense (Δa2 and Δa3) A. thaliana (WT-col-0) plants (as described before (20, 25)) were grown in a growth chamber at a day/night regime of 22 °C/19 °C, a photoperiod of 8 h with a light intensity of 4000 lux and 70% relative humidity. Information about the physiological effect of these mutations has been presented previously (25, 26).
Isolation of PSI-LHCI—Thylakoids were isolated as described elsewhere (27). PSI-LHCI isolation was modified from Ref. 28. Thylakoid membranes were washed with 5 mm EDTA, pH 8.0 and centrifuged for 10 min at 12,000 × g, 4 °C. The pellet was solubilized with 0.6% dodecyl-α-d-maltoside (α-DM) or dodecyl-β-d-maltoside (β-DM, see text), 10 mm Tricine, pH 7.8 at a final concentration of 0.5 mg Chl/ml and vortexed for 1 min. Unsolubilized material was removed by centrifugation at 12,000 × g, 10 min, 4 °C. The sample was loaded onto a 0.1–1 m sucrose gradient, and centrifuged for 22 h at 41,000 rpm in a Beckman SW41 rotor. Fractions were harvested with a syringe; the lowest green band contained PSI-LHCI. This fraction was concentrated and diluted in a sucrose-free buffer and loaded on a second gradient. The gradient was obtained by freezing and thawing a solution of 0.5 m sucrose, 0.06% α-DM, and 10 mm Tricine pH 7.8.
Electrophoresis and Immunoblotting—A modified Laemmli SDS-PAGE system (29) was used, as described in Ref. 30. Non-denaturing Deriphat PAGE was modified from Ref. 31. The stacking gel contained 3.5% and the resolving gel 6% acrylamide (75:1 acrylamide/bisacrylamide), polymerization was started by adding 0.03% ammonium persulfate and 0.1% TEMED. The running buffer contained 0.1% Deriphat-160. To investigate the stability of PSI-LHCI, the particles were solubilized prior to loading with 0.5% β-DM or 0.5% β-DM and 0.2% ZWITTERGENT 3–16 (ZG-16, Calbiochem) at a final Chl concentration of 0.2 mg/ml, and samples were vortexed for 1 min.
For immunoblot analysis, proteins were transferred to a nitrocellulose membrane (0.45 μm, Millipore) using a Bio-Rad blot system. Primary antibodies against Lhca1–5 PsaD, PsbG, and PsbK were from Agrisera (Sweden). The level of antibody binding was monitored with secondary goat anti-rabbit IgG alkaline phosphatase antibody (Sigma-Aldrich) in combination with NBT/BCIP (AppliChem).
Electron Microscopy and Single Particle Analysis—Freshly prepared samples were dialyzed (Spectra/Por 12,000–14,000 MWCO dialysis membranes) for 2 to 4 h and negatively stained with 2% uranyl acetate on glow discharged carbon-coated copper grids. Electron microscopy was performed on a Philips CM120 electron microscope equipped with a LaB6 tip, operated at 120 kV. Images were recorded with a Gatan 4000 SP 4K slow-scan CCD camera at ×80,000 magnification with a pixel size of 0.375 nm at the specimen level after binning the images. “GRACE” software was used for semi-automated specimen selection and data acquisition (32). Single particles were selected with an 80 × 80 pixel frame and analyzed with the GRONINGEN IMAGE PROCESSING (GRIP) software package, with multi-reference procedures and multivariate statistical analysis and classification.
Pigment Analysis—For pigment analysis, a combined approach of HPLC and fitting the absorption spectra of the 80% acetone extract with the spectra of the individual pigments was used, as described before (33).
Absorption and Fluorescence Measurements—Absorption spectra were recorded at room temperature on a Varian Cary 4000 UV-Vis-spectrophotometer.
Fluorescence spectra were recorded at 77 K on a Fluorolog 3.22 spectrofluorimeter manufactured by Jobin Yvon-Spex. The bandwidth for excitation and emission was 3.5 nm. Spectra were corrected for wavelength-dependent sensitivity of the detector. Samples were diluted to an OD of 0.04 at the Qy maximum in 66.7% (w/v) glycerol, 10 mm Tricine pH 7.8, and 0.03% α-DM.
RESULTS
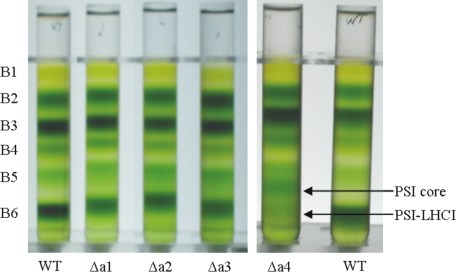
Purification of PSI-LHCI—To purify the PSI-LHCI complexes from Lhca-deficient plants, isolated thylakoids of WT and Δa1-Δa4 plants were mildly solubilized with 0.6% α-DM and subjected to sucrose density ultracentrifugation. Six pigment-containing bands were resolved (Fig. 1). The bands were identified by absorption spectroscopy and SDS-PAGE analysis (data not shown) as: free pigments (B1), monomeric light harvesting complexes (B2), trimeric LHCII (B3), LHCII-CP24-CP29 supercomplex (B4), and PSII core (B5). The lowest band (B6) was identified as PSI-LHCI with full (WT) or reduced (Δa1-Δa4) antenna size. In the gradient from Δa4 plants an additional band, identified as PSI core was present, showing that the Lhca polypeptide level in thylakoids of these plants is considerably reduced (11, 21).
FIGURE 1.
Sucrose gradient analysis of thylakoid membranes from WT, Δa1, Δa2, Δa3, and Δa4 plants solubilized with 0.6% α-DM. Six pigment-containing bands were resolved, identified as: B1, free pigments, B2, Lhc monomers, B3, LHCII trimers, B4, supercomplex of LHCII trimer with CP24 and CP29, B5, PSII core, and B6, PSI-LHCI,. In the Δa4 gradient, an additional band of PSI core was observed between the PSII core and PSI-LHCI.
The PSI-LHCI fractions from all plants were slightly contaminated with PSII components, because of the presence of PSII supercomplexes resistant to mild solubilization conditions. Therefore, the samples were concentrated and diluted in a sucrose-free buffer and loaded on a second gradient. This procedure resulted, for WT and Δa1-Δa3, in PSI preparations virtually free of PSII; only PSI-Δa4 remained slightly contaminated because of the low relative abundance of PSI-LHCI compared with PSII.
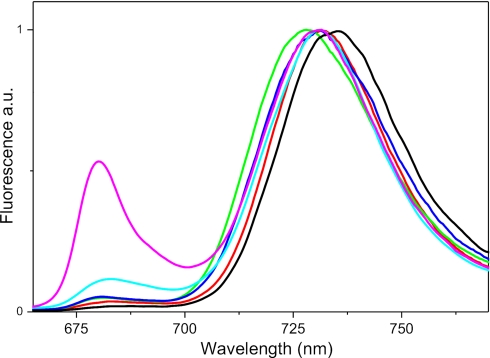
To check the integrity of the preparations the fluorescence emission spectra were measured at 77 K, and they are reported in Fig. 2. PSI is characterized by the presence of low energy absorption forms, which act as an energy sink and are responsible for the red-shifted fluorescence typical for the complex (34). The absence in the complexes of significant fluorescence emission at wavelengths shorter then 700 nm indicates that all Chls are connected to the red-forms, and thus that the complexes are functionally intact. Moreover, these data also confirm that the samples are not notably contaminated with PSII components, with the exception of PSI-Δa4, for which a relatively intense emission at 680 nm, typical for PSII, could be detected. After excitation at 475 nm, the fluorescence emission maxima were at 735 nm for PSI-WT, 731.5 nm for Δa1, 728 nm for Δa2, and 731 nm for Δa3 and Δa4. For PSI-Δa1 and Δa2 the values are similar to those previously reported (21), but for PSI-Δa3 and PSI-Δa4, the maxima are red-shifted by 4 and 11 nm, respectively, indicating a higher amount of antennas associated to the core.
FIGURE 2.
Low temperature fluorescence emission of PSI preparations. 77 K emission spectra of PSI-LHCI from WT (black), Δa1 (red), Δa2 (green), Δa3 (blue), and Δa4 (magenta) plants. Excitation was at 475 nm. Spectra are normalized to the red maximum. The fluorescence emission of PSI-Δa4 purified in β-DM is also reported (cyan).
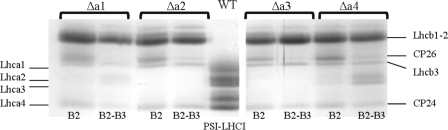
Are Lhca Antennas Released upon Thylakoid Solubilization?— It was previously shown that in PSI of all Lhca-deficient lines, the outer antenna is less strongly bound to the core compared with the WT, resulting in the loss of Lhca complexes upon solubilization of the thylakoid membranes, whereas WT plants retained their antenna (22). To investigate whether a similar effect was also induced by our solubilization conditions, the gradient fractions in which monomeric (B2) and dimeric antenna complexes (B2-B3) are expected to migrate were analyzed. The presence of Lhca complexes in these fractions, strongly enriched in Lhcb antennas, was detected by absorption spectroscopy, taking advantage of the red absorption (above 700 nm) that characterizes the Lhca complexes and is absent in Lhcb complexes (4). Increased absorption in the red compared with WT was observed for both fractions of the Δa4 lines and in small amounts in the dimeric fraction of Δa1, indicating the presence of some Lhca complexes. This was confirmed by SDS-PAGE (Fig. 3), which reveals the presence of “free” Lhca2 and Lhca3. No traces of Lhca complexes could be detected in the WT, Δa2, and Δa3 fractions.
FIGURE 3.
Analysis of B2 and B2-B3 from ΔLhca1–4 gradients. SDS-PAGE of monomeric antenna (B2) and the fraction between B2 and B3 (B2-B3) from the sucrose gradients of ΔLhca1–4 plants. The region of MW of Lhca and Lhcb antenna is shown. The identity of the bands is indicated.
The results indicate that under our solubilization conditions, in contrast to previous reports (21, 22), PSI complexes from Δa2 and Δa3 lines and in a first approximation from Δa1 can be purified without loss of external antenna components, as in the case of the WT. This allows using these preparations to get quantitative information about the binding of the individual complexes. On the other hand, the release of Lhca2/3 from PSI of Δa4 plants indicates a weak association of these complexes in this mutant. However, the amount of “free” Lhca2 and Lhca3 in the Δa4 gradient is too low to explain the presence of the PSI core band in the gradient as the result of dissociation of PSI-LHCI. This clearly indicates that in the membranes of this mutant, there is a population of PSI core, which does not coordinate antenna complexes.
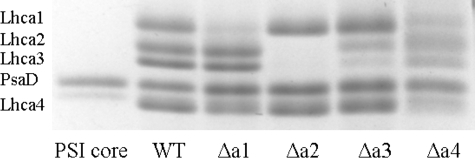
Lhca Composition of PSI Supercomplexes—To get quantitative information about the Lhca complexes coordinated to the core in the different PSI complexes, the samples were analyzed by SDS-PAGE (Fig. 4). In PSI from Δa1 plants (PSI-Δa1) a very small amount (<5%) of Lhca1 was still present, in agreement with the fact that the insertion is in the promotor region and not in the protein-encoding region. Lhca2 and Lhca3 were fully retained, while the amount of Lhca4 was reduced to ∼60% of the WT level. Thus, in a substantial part of the PSI population, Lhca4 is associated with the core in the absence of its partner, Lhca1. On the contrary, in Δa2 plants, not only the level of Lhca2 (absent in this line as also assessed by Western blotting), but also that of Lhca3 was reduced below the detection limits of the Coomassie Blue stain, suggesting that Lhca3 cannot be associated with the core in the absence of Lhca2. In this mutant, the levels of Lhca1 and Lhca4 were not affected, indicating that the Lhca2/3 dimer is not necessary for the association of the Lhca1/4 dimer to the core. This result was confirmed by the analysis of PSI from Δa3 plants, in which the levels of Lhca1 and Lhca4 were also not affected. In these PSI particles, there was some (<10%) residual amount of Lhca3 (probably due to re-expression in the antisense line used). However the level of Lhca2 was about 35% of the WT level, thus indicating that, as was the case of Lhca4, Lhca2 can also be bound to PSI without its partner. The analysis of PSI-LHCI from Δa4 plants was slightly complicated by the contamination of PSII components, because of the similar migration behavior of Lhcb3 and Lhca1 and CP24 and Lhca4, and thus quantitative data could not be obtained (but see below). However, SDS-PAGE shows the presence of Lhca2 and Lhca3 and of Lhca1, whereas Lhca4 was not detected. (The faint band at the level of Lhca4 in the gel belongs to one of the isoforms of PsaD as assessed by Western blotting.) This is in line with results from Western blots of the thylakoids, which shows the presence of Lhca1 in this mutant, although at levels lower than in the WT, and no traces of Lhca4 (data not shown).
FIGURE 4.
Analysis of Lhca content in PSI-LHCI of WT and ΔLhca1–4 plants. SDS-PAGE of PSI-LHCI purified from WT and ΔLhca1–4 plants, and PSI core. The region of the gel where Lhca1–4 and PsaD migrate is shown. The identity of the bands from top to bottom are: Lhca1, Lhca2, Lhca3, PsaD, and Lhca4. Note that one of the two isoforms of PsaD appears as a faint band at the level of Lhca4.
We also checked the presence of PsaG and PsaK, which have been suggested to interact and stabilize the binding of dimers Lhca1/4 and Lhca2/3, respectively (6, 35, 36). The immunoblotting shows that these two subunits are present at the same level as in the WT in the absence of their neighboring dimer (see supplemental Fig. S1).
Supramolecular Organization of Lhca-deficient PSI Particles— To reveal the supramolecular organization of the PSI complexes from WT and Δa1-Δa4 plants, electron microscopy was performed. Micrographs of negatively stained particles were recorded, and PSI projections were selected. The obtained data sets were analyzed by single particle analysis, including multi-reference alignments, multivariant statistical analysis, and classification.
In Fig. 5, the projection views are presented with the same orientations and handedness as PSI in the PSI-LHCII complex, based on the correlation with the specific features of the PSI core clearly visible in the projections as white features. In these projections, PSI is viewed from the stromal side, and PsaK is located on the right side of the complex (37).
FIGURE 5.
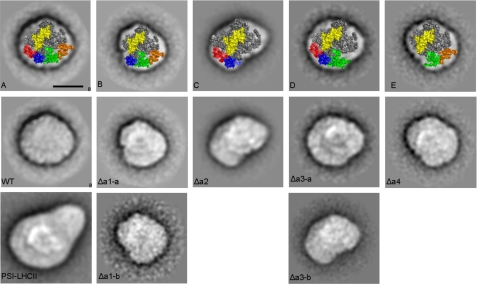
Electron microscopy analysis of PSI with different LHCI compositions. Fig. A–E are top views of the crystal structure (1) overlaying the projections of PSI-LHCI (A), PSI lacking Lhca1 (B), PSI lacking the Lhca2/3 dimer (C), PSI lacking Lhca3 (D), and PSI lacking the Lhca1/4 dimer (E). PsaC and PsaE are presented in yellow, Lhca1 in red, Lhca2 in green, Lhca3 in orange, and Lhca4 in blue. All projections are viewed from the stromal side. Averaged images of PSI from WT, Δa1-Δa4 plants are presented, and, for comparison, a PSI-LHCII complex (37). Images are sums of 765 (WT), 768 (WT PSI-LHCII), 775 (Δa1-a), 64 (Δa1-b), 1317 (Δa2), 212 (Δa3-a), 217 (Δa3-b), and 648 (Δa4) aligned projections. The scale bar equals 10 nm.
The top-view projection of WT PSI is in good agreement with the crystal structure (Fig. 5A and Ref. 1). For PSI-Δa1, two types of particles could be observed: Δa1-a, which was the most abundant, and Δa1-b. In the former, absence of a protein density is observed on the left side of the complex, and it can account for the lack of Lhca1. The low abundance of Δa1-b particles resulted in a low-resolution image, but still it is clear that the loss of protein density is more severe in these particles, accounting for the lack of the Lhca1/4 dimer in agreement with SDS-PAGE analysis, which revealed a decreased level of Lhca4 in this preparation.
The PSI-Δa2 particle shows a loss of density, with the size of two Lhca complexes at the right side of the complex. For PSI from ΔLhca3 plants, two particles were found with either three (Δa3-a) or two (Δa3-b) outer antenna complexes bound to the core. The density absent in both particles is assigned to Lhca3, in agreement with SDS-PAGE, while the one only present in Δa3-a is assigned to Lhca2. In the PSI-Δa4 preparation, a particle identical to Δa1-b was observed, corresponding to PSI supercomplexes lacking the Lhca1/4 dimer. Taken together, these results show that the order of the outer antenna complexes, starting from the G-pole, is Lhca1, Lhca4, Lhca2, Lhca3, confirming the assignment of Amunts et al. (1), see also Fig. 5, A–E. Furthermore, the EM image clearly shows the presence of Lhca2 and Lhca4 associated with the core in the absence of their dimeric partner, in agreement with SDS-PAGE results.
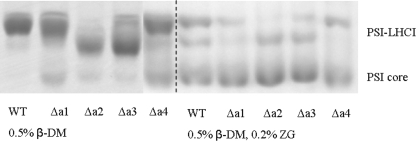
Stability of PSI-LHCI Complexes with Reduced Antenna Sizes—To compare the stability of PSI-LHCI from WT and Δa1-Δa4 lines and to assess the strength of the association to the core of the individual Lhca complexes, the particles were subjected to different detergent treatments and analyzed by non-denaturing PAGE (Fig. 6). Treatment of the PSI particles with β-DM had the smallest effect on the WT preparation. Only a small band of PSI with reduced antenna size was observed, but no PSI core was detected, indicating only limited and partial loss of the external antenna. A band corresponding to the PSI core could be detected for all mutants. This band was faint for PSI-Δa3, and its intensity was slightly higher for PSI-Δa2, increased for PSI-Δa1, and even more for PSI-Δa4. This indicates that the stability of PSI-LHCI is mostly affected when Lhca4 is lacking, as previously suggested (22). Under the strongest solubilization conditions (DM plus zwittergent), all PSI particles lost most of their antenna. However, the PSI-WT complex was slightly more resistant to the detergent treatment compared with the Lhca-deficient complexes. In summary, these results demonstrate that PSI-LHCI of Δa2 and Δa3 plants is almost as stable as that of the WT, while the stability of PSI from Δa1 plants is slightly more affected by the detergent; the strongest destabilization is observed for PSI-Δa4. This indicates that the Lhca1/4 dimer has a stronger association with the core than the Lhca2/3 dimer, in agreement with the release of this dimer after very mild solubilization of the thylakoids.
FIGURE 6.
Stability analysis of PSI-LHCI from WT and ΔLhca1–4 plants. Non-denaturing Deriphat-PAGE of PSI from WT and ΔLhca1–4 plants under two conditions: (i) solubilized with 0.5% β-DM or (ii) with 0.5% β-DM and 0.2% ZG-16 at a final Chl concentration of 0.2 mg/ml. The section of gel where PSI migrates is shown. The position of PSI with antenna (PSI-LHCI) and the PSI core are indicated.
Lhca5 Is Associated with PSI-LHCI in Δa4 Plants—Surprisingly, after detergent treatment, a green band with molecular weight similar to that of the WT complex was still present for PSI-Δa4 (Fig. 6), suggesting that part of the population of PSI-Δa4 is very resistant to detergent treatment.
To obtain a preparation enriched in these particles, we repeated the PSI-LHCI purification from Δa4 plants with a modified procedure using β-DM instead of the milder α-DM. This had the additional advantage that the preparation was free of PSII contamination, as the analysis of the fluorescence emission spectrum confirms (Fig. 2), permitting us to obtain quantitative data about the Lhca composition of this mutant, see Fig. 7A. SDS-PAGE shows that Lhca2 and Lhca3 are retained, and Lhca1 is present similar to the preparation in α-DM; but interestingly an additional band was observed inbetween Lhca1 and Lhca2. This band was identified by immunoblotting as Lhca5 (Fig. 7B). The fact that Lhca5 was detectable on a gel indicates that the β-DM PSI-Δa4 preparation is strongly enriched in this subunit. Further immunoblotting analysis showed that the level of Lhca5 in this preparation and in PSI-Δa1 is ∼200 and 10 times higher, respectively, compared with PSI-WT (see supplemental Fig. S2).
FIGURE 7.
Analysis of PSI-LHCI from Δa4 plants, isolated using β-DM. A and C, SDS-PAGE analysis of Lhca composition of PSI-LHCI from WT and Δa4 plants. B, immunoblot detection of PsaD (#) and Lhca5 (*) in PSI-LHCI of WT and Δa4 plants, respectively; 7 and 0.32 μg of Chl was loaded. D, top-view projection of PSI-LHCI particle from Δa4 plants. The image is the sum of 2783 aligned projections. The scale bar equals 10 nm.
Small variations in the gel preparation resulted in slight changes in the Lhca migration behavior. Fig. 7C shows the Lhca region of a second gel. Here Lhca5 overlaps with Lhca2, and Lhca1 is fully separated and can be quantified, showing that it is almost completely (>90%) retained in this preparation.
To determine the location of the Lhca5 complex, the β-DM PSI-Δa4 particles were analyzed by EM. The analysis revealed that the preparation was highly homogeneous and was composed of particles practically identical to those of PSI-WT (Fig. 5, WT), revealing that four Lhca antenna subunits were present. The total absence of Lhca4 in this line (as assessed by Western blotting) and the presence of high levels of Lhca1 and of Lhca5 in this preparation suggest that Lhca5 substitutes for Lhca4 in these complexes and forms a heterodimer with Lhca1.
Pigment Composition—The pigment composition of the purified PSI complexes was analyzed. Results are presented in Table 1. It is known that the Lhca complexes coordinate xanthophylls, β-carotene, Chl a, and Chl b (4, 5), whereas the PSI core coordinates only Chl a and β-carotene (3). In full agreement, the Lhca-depleted PSI complexes have decreased relative amounts of Chl b and xanthophylls, while the level of β-carotene is increased as compared with the WT. Note that the Chl a/b ratio of all Lhca-lacking PSI are lower than in a previous report (21) indicating that the amount of antenna in our preparations is higher. The difference is particularly large for PSI-Δa4, which has a Chl a/b ratio of 10.8 in our preparation, while it was 18 and 32 in previous reports (21, 22), indicating that these preparations contained mainly PSI core.
TABLE 1.
Pigment analysis of PSI-LHCl complexes differing in antenna composition For Δa4, the α-DM preparation is reported. The number of the carotenoids is normalized to 100 Chls. S.D. for four measurements is: Chl/Car < 0.1, Chl a/b < 0.4, and S.D. in the Cars (determined for two preparations) is less then 0.3.
| Chl a/b | Chl/Car | n. Vio | n. Lut | n. β-Car | |
|---|---|---|---|---|---|
| WT | 9.7 | 4.8 | 2.3 | 5.5 | 13.1 |
| Δa1 | 10.8 | 5.0 | 1.6 | 4.8 | 13.6 |
| Δa2 | 11.1 | 5.1 | 1.7 | 3.8 | 14.1 |
| Δa3 | 11.1 | 5.0 | 2.0 | 4.3 | 13.7 |
| Δa4 | 10.8 | 5.1 | 1.5 | 4.1 | 14.0 |
DISCUSSION
In this work, we analyzed the PSI-LHCI from A. thaliana lines depleted in individual Lhca proteins to investigate the role of the subunits in the supramolecular organization of PSI. The new purification procedure used and the possibility of performing biochemical analysis and electron microscopy on the same preparations allow us to relate variations in the protein composition to changes in the supramolecular organization and to obtain a comprehensive picture of the overall organization and stability of the PSI supercomplex.
Lhca1, -2, -3, and -4 Have a Fixed Position in the PSI Structure and Are Not Interchangeable—It has been suggested that the level of Lhca complexes (23) or at least of Lhca4 (24) changes depending on growth conditions, although a more recent report shows that the level of the individual Lhca complexes is fixed to 1 per core under different light conditions (19). A change in the antenna composition can occur in two ways. 1) Extra dimers can be associated with the supercomplex; this is clearly not the case under our growing conditions, but we cannot exclude the presence of extra dimers in other specific light conditions. 2) Different subunits can occupy the same position in the structure. This is apparently not the case. The EM analysis clearly shows that each Lhca complex (type 1–4) has a specific binding site. The absence of an antenna complex resulted in a “hole” in the structure of PSI at the position, which in the WT is occupied by this complex, while the supramolecular organization of the rest of the complex remained the same. This suggests a very rigid organization of LHCI, with specific interactions between the individual Lhca types and their partners in the core and in the antenna. This is unlike the situation in PSII for which it has been shown that in the absence of the two main subunits of the LHCII trimer (Lhcb1 and Lhcb2), CP26, which is monomeric in WT plants, forms trimers that perfectly substitute the LHCII trimers, leading to an overall organization of the PSII supercomplex that is virtually indistinguishable from that of the WT (38).
The results also suggest that Lhca complexes only form heterodimers. This was already clear for Lhca1/4 (9), but the impossibility of purifying Lhca2 and Lhca3 to homogeneity (4) and obtaining dimers by in vitro reconstitution (5) led to different suggestions about the organization of these two subunits (1, 10). In the case that Lhca2 and Lhca3 would be able to form homodimers, we would have expected to detect particles with the full antenna complement in Δa2 and Δa3 plants, although this was not the case.
However, Lhca5 Can Replace Lhca4 in the Supercomplex— Although the results clearly show that the different Lhca types (Lhca1–4) are not interchangeable, an exception to this rule exists. In the Δa4 line, totally lacking Lhca4, we were able to purify a small population (around 1%, normalized to the amount of PSI core) of PSI supercomplexes with the full antenna complement. These particles comprise Lhca1, Lhca2, Lhca3, and Lhca5, most likely as Lhca1/5 and Lhca2/3 dimers. This is supported by a mass spectrometry study where Lhca5 was detected in the LHCI-730 fraction, suggesting an interaction with Lhca1 or Lhca4 (14) and by the in vitro reconstitution results, which showed that the Lhca1/5 dimer can be obtained (13). This novel PSI-Lhca1/5-Lhca2/3 complex is as stable as the WT complex, indicating that Lhca5 is a perfect substitute for Lhca4 in the assembly of the system. It is thus likely that Lhca5 can also replace Lhca4 in PSI-LHCI from WT plants. The sequence identity between Lhca5 and Lhca4 is very high (15), suggesting that the regions necessary for the interaction with the core complex are conserved in these two proteins. Interestingly, Lhca5 does not contain red forms (15), which are present in Lhca4. Therefore, the tuning of Lhca5 expression could be important for optimal light-harvesting under varying light conditions. Previous studies have addressed the effect of light intensity on Lhca levels (11, 19, 23, 24). However, in light of the different absorption properties of Lhca4 and Lhca5, it would be interesting to study the effect of light quality on the Lhca5 expression and protein levels.
Role of the Individual Lhcas in the Assembly and Stability of the Supercomplex—The analysis of the structure of PSI-LHCI suggests that the interactions of Lhca complexes with the core are stronger at the G-pole than at the K-pole and that Lhca1 acts as an anchor point for facilitating the binding of other Lhca subunits (39). In contrast, biochemical analysis of Lhca-depleted plants suggested that Lhca4 is the key unit for the assembly of the antenna system around the core (21, 22). Indeed, PSI purified from Δa4 was shown to consist mainly of core complexes (21, 22, 40). It has been proposed that the low level of the other Lhca proteins in this mutant is influenced by post-translational events and is related to the stability of the complexes (21, 26). Our analysis shows that PSI core particles depleted in their outer antenna are present in the membranes of the Δa4 mutant. However, in contrast to previous results (21, 22), in addition to the core, two other PSI populations were observed: PSI-Lhca2/3 and PSI-Lhca1/5-Lhca2/3. PSI-Lhca2/3 complexes, although less stable than the WT, survive a purification step that induces dissociation of the PSII supercomplex. This indicates that the interactions of core/antenna in this complex are stronger than in PSII, which in the membrane is perfectly assembled, suggesting that the low amounts of PSI-LHCI found in the Δa4 mutant are probably not due to instability of the system. Moreover, in the Δa1 mutant, the levels of Lhca2 and Lhca3 are identical to those in the WT with a 1:1 ratio with the core, although in 40% of the complexes Lhca4 is absent. This indicates that Lhca4 is not strictly required for the assembly of Lhca2/3 onto the core. Thus, the possibility exists that the primary role of Lhca4 in the PSI organization is performed at a different level: the absence of Lhca4 produces a pleiotropic effect on the full set of Lhca proteins, with Lhca4 playing a “dominant” role, similar to what was observed for the chloroplastic subunits (41); most likely via a different mechanism.
The data also indicate a role for Lhca1. In the absence of Lhca1 the amount of Lhca4 is reduced, suggesting that Lhca1 is important for the stable association of Lhca4 with the core, as suggested by Nelson and co-workers based on the structural analysis (1, 39).
The data clearly show that the Lhca1/4 dimer is interacting with the core more strongly than the Lhca2/3 dimer. Moreover, in the Δa2 mutant the PSI population (PSI core-Lhca1/4) is highly homogeneous, indicating that Lhca1/4 does not need Lhca2/3 for the assembly. The same is true for the Lhca2/3 dimer, although in this case the presence of Lhca1/4 or at least of Lhca4 stabilizes the binding of Lhca2/3 to the core via interaction of Lhca2 with Lhca4. The same interactions are probably at the origin of the fact that, in contrast to a previous proposal (22), both these subunits (Lhca2 and -4) can be stably associated with the core in the absence of their “dimeric partners.” It seems to be a general rule that the stable association of an antenna complex in the photosynthetic supercomplexes needs interactions with at least two partners.3 This can explain why it is possible to observe particles with Lhca4 or Lhca2 in the absence of their dimeric partners, but never with Lhca1 and Lhca3, which are located at the periphery of the half ring, and already in the WT interact only with the core and one Lhca subunit.
Functional Organization of the Lhca Complexes—It is known that in PSI, there are at least three Chl clusters that emit in the red; two of them are associated with the outer antenna complexes, in particular to Lhca3 (725 nm), Lhca4 (733 nm), and to the core complex (720 nm) (4, 5, 9, 10, 42).
The fluorescence emission of PSI-WT has its maximum at 735 nm. Excitations at 440 nm and 475 nm, which lead to a different initial distribution of the excitation energy in the core and in the outer antenna (43), show no difference, indicating that even at low temperature the red forms of the core are transferring energy to the antenna. The emission spectrum of PSI-Δa4, which is highly enriched in PSI-Lhca2/3 dimer, shows no dependence on the excitation wavelength, indicating that the red forms of the core are still able to transfer to the antenna. As already reported (21), this is not the case for PSI-Δa2, which shows an emission maximum at 728 nm for 475-nm excitation and at 725 nm for 440-nm excitation. It was suggested that excited core pigments cannot efficiently transfer excitation energy to the red pigments of the antenna and thus that this spectrum is the sum of two contributions: the red forms of the core and the red forms of Lhca4. Indeed, the fluorescence spectra can be decomposed in the spectra of the PSI core and WT PSI-LHCI (data not shown). Based on these observations, it can be suggested that the red forms of the core can equilibrate with those of the Lhca2/3 dimer, but not with those of Lhca1/4. Considering (i) the large energetic distance between the red forms and the bulk Chls and (ii) at low temperature the thermal energy is not sufficient to drive the energy transfer from the red forms to the bulk, it is likely that the low energy forms of the core are located in close proximity to the red forms of Lhca3.
The analysis of PSI-Δa4 allows the determination of the emission maximum of native Lhca3 associated to the core. The emission maximum of PSI-Δa4 is at 731 nm, and it can be ascribed to Lhca3, which is the red-most emitter in this complex. However, based on the data of reconstituted complexes, the emission of Lhca3 is expected at 725 nm (10). Hence, the emission of native Lhca3 in the PSI complex is about 6 nm more to the red than for the in vitro refolded monomeric complex. This is supported by the analysis of absorption spectra, which suggests that the red forms of Lhca3 associated with PSI absorb more to the red than in the reconstituted sample (21). Indeed, the red forms are generated by interaction between two Chls (18), and a small change in the structure can easily account for a large change in the energy of these forms. Probably the docking of Lhca3 onto the core is stabilizing the most-red conformation.
The steady-state low temperature fluorescence emission experiments provided us with information about the properties of the individual Lhca antenna in the complex. A thorough time-resolved fluorescence study on these highly pure and well-characterized PSI particles promises to increase our understanding of the energy transfer processes taking place in PSI-LHCI.
Supplementary Material
Acknowledgments
We thank Dr. W. Keegstra for excellent technical support.
This work was supported by the Council for Earth and Life Sciences of the Nederlandse Organisatie voor Wetenschappelijk Onderzoek via a VIDI grant (to R. C.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
Footnotes
The abbreviations used are: PSI, Photosystem I; PSII, Photosystem II; WT, wild type; Chl, chlorophyll; DM, d-maltoside; Tricine, N-[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]glycine.
S. Caffarri, R. Kouril, S. Kereïche, E. J. Boekema, and R. Croce, in preparation.
References
- 1.Amunts, A., Drory, O., and Nelson, N. (2007) Nature 447 58-63 [DOI] [PubMed] [Google Scholar]
- 2.Fromme, P., Jordan, P., and Krauss, N. (2001) Bba-Bioenergetics 1507 5-31 [DOI] [PubMed] [Google Scholar]
- 3.Jordan, P., Fromme, P., Witt, H. T., Klukas, O., Saenger, W., and Krauss, N. (2001) Nature 411 909-917 [DOI] [PubMed] [Google Scholar]
- 4.Croce, R., Morosinotto, T., Castelletti, S., Breton, J., and Bassi, R. (2002) Bba-Bioenergetics 1556 29-40 [DOI] [PubMed] [Google Scholar]
- 5.Schmid, V. H. R., Potthast, S., Wiener, M., Bergauer, V., Paulsen, H., and Storf, S. (2002) J. Biol. Chem. 277 37307-37314 [DOI] [PubMed] [Google Scholar]
- 6.Ben-Shem, A., Frolow, F., and Nelson, N. (2003) Nature 426 630-635 [DOI] [PubMed] [Google Scholar]
- 7.Boekema, E. J., Jensen, P. E., Schlodder, E., van Breemen, J. F. L., van Roon, H., Scheller, H. V., and Dekker, J. P. (2001) Biochemistry 40 1029-1036 [DOI] [PubMed] [Google Scholar]
- 8.Knoetzel, J., Svendsen, I., and Simpson, D. J. (1992) Eur. J. Biochem. 206 209-215 [DOI] [PubMed] [Google Scholar]
- 9.Schmid, V. H. R., Cammarata, K. V., Bruns, B. U., and Schmidt, G. W. (1997) Proc. Natl. Acad. Sci. U. S. A. 94 7667-7672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castelletti, S., Morosinotto, T., Robert, B., Caffarri, S., Bassi, R., and Croce, R. (2003) Biochemistry 42 4226-4234 [DOI] [PubMed] [Google Scholar]
- 11.Ganeteg, U., Klimmek, F., and Jansson, S. (2004) Plant Mol. Biol. 54 641-651 [DOI] [PubMed] [Google Scholar]
- 12.Lucinski, R., Schmid, V. H. R., Jansson, S., and Klimmek, F. (2006) Febs Lett. 580 6485-6488 [DOI] [PubMed] [Google Scholar]
- 13.Storf, S., Jansson, S., and Schmid, V. H. R. (2005) J. Biol. Chem. 280 5163-5168 [DOI] [PubMed] [Google Scholar]
- 14.Storf, S., Stauber, E. J., Hippler, M., and Schmid, V. H. R. (2004) Biochemistry 43 9214-9224 [DOI] [PubMed] [Google Scholar]
- 15.Jansson, S. (1999) Trends Plant Science 4 236-240 [DOI] [PubMed] [Google Scholar]
- 16.Liu, Z., Yan, H., Wang, K., Kuang, T., Zhang, J., Gui, L., An, X., and Chang, W. (2004) Nature 428 287-292 [DOI] [PubMed] [Google Scholar]
- 17.Nussberger, S., Dekker, J. P., Kuhlbrandt, W., van Bolhuis, B. M., van Grondelle, R., and van Amerongen, H. (1994) Biochemistry 33 14775-14783 [DOI] [PubMed] [Google Scholar]
- 18.Morosinotto, T., Breton, J., Bassi, R., and Croce, R. (2003) J. Biol. Chem. 278 49223-49229 [DOI] [PubMed] [Google Scholar]
- 19.Ballottari, M., Dall'Osto, L., Morosinotto, T., and Bassi, R. (2007) J. Biol. Chem. 282 8947-8958 [DOI] [PubMed] [Google Scholar]
- 20.Ganeteg, U., Strand, A., Gustafsson, P., and Jansson, S. (2001) Plant Physiol. 127 150-158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klimmek, F., Ganeteg, U., Ihalainen, J. A., van Roon, H., Jensen, P. E., Scheller, H. V., Dekker, J. P., and Jansson, S. (2005) Biochemistry 44 3065-3073 [DOI] [PubMed] [Google Scholar]
- 22.Morosinotto, T., Ballottari, M., Klimmek, F., Jansson, S., and Bassi, R. (2005) J. Biol. Chem. 280 31050-31058 [DOI] [PubMed] [Google Scholar]
- 23.Bailey, S., Walters, R. G., Jansson, S., and Horton, P. (2001) Planta 213 794-801 [DOI] [PubMed] [Google Scholar]
- 24.Tikkanen, M., Piippo, M., Suorsa, M., Sirpio, S., Mulo, P., Vainonen, J., Vener, A. V., Allahverdiyeva, Y., and Aro, E. M. (2006) Plant Mol. Biol. 62 795-795 [DOI] [PubMed] [Google Scholar]
- 25.Ganeteg, U., Kulheim, C., Andersson, J., and Jansson, S. (2004) Plant Physiol. 134 502-509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ganeteg, U. (2004) The Light-harvesting Antenna of Higher Plant Photosystem I, Umea Plant Science Centre, PhD Thesis, Umea
- 27.Croce, R., Zucchelli, G., Garlaschi, F. M., Bassi, R., and Jennings, R. C. (1996) Biochemistry 35 8572-8579 [DOI] [PubMed] [Google Scholar]
- 28.Caffarri, S., Croce, R., Breton, J., and Bassi, R. (2001) J. Biol. Chem. 276 35924-35933 [DOI] [PubMed] [Google Scholar]
- 29.Laemmli, U. K. (1970) Nature 227 680-& [DOI] [PubMed] [Google Scholar]
- 30.Ballottari, M., Govoni, C., Caffarri, S., and Morosinotto, T. (2004) Eur. J. Biochem. 271 4659-4665 [DOI] [PubMed] [Google Scholar]
- 31.Peter, G. F., and Thornber, J. P. (1991) J. Biol. Chem. 266 16745-16754 [PubMed] [Google Scholar]
- 32.Oostergetel, G. T., Keegstra, W., and Brisson, A. (1998) Ultramicroscopy 74 47-59 [Google Scholar]
- 33.Croce, R., Canino, G., Ros, F., and Bassi, R. (2002) Biochemistry 41 7334-7343 [DOI] [PubMed] [Google Scholar]
- 34.Jennings, R. C., Zucchelli, G., Croce, R., and Garlaschi, F. M. (2003) Bba-Bioenergetics 1557 91-98 [DOI] [PubMed] [Google Scholar]
- 35.Jensen, P. E., Gilpin, M., Knoetzel, J., and Scheller, H. V. (2000) J. Biol. Chem. 275 24701-24708 [DOI] [PubMed] [Google Scholar]
- 36.Varotto, C., Pesaresi, P., Jahns, P., Lessnick, A., Tizzano, M., Schiavon, F., Salamini, F., and Leister, D. (2002) Plant Physiol. 129 616-624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kouril, R., Zygadlo, A., Arteni, A. A., de Wit, C. D., Dekker, J. P., Jensen, P. E., Scheller, H. V., and Boekema, E. J. (2005) Biochemistry 44 10935-10940 [DOI] [PubMed] [Google Scholar]
- 38.Ruban, A. V., Wentworth, M., Yakushevska, A. E., Andersson, J., Lee, P. J., Keegstra, W., Dekker, J. P., Boekema, E. J., Jansson, S., and Horton, P. (2003) Nature 421 648-652 [DOI] [PubMed] [Google Scholar]
- 39.Amunts, A., and Nelson, N. (2008) Plant Physiol. Biochem. 46 228-237 [DOI] [PubMed] [Google Scholar]
- 40.Ihalainen, J. A., Klimmek, F., Ganeteg, U., van Stokkum, I. H. M., van Grondelle, R., Jansson, S., and Dekker, J. P. (2005) Febs Lett. 579 4787-4791 [DOI] [PubMed] [Google Scholar]
- 41.Wollman, F. A., Minai, L., and Nechushtai, R. (1999) Biochim. Biophy. Acta-Bioenerg. 1411 21-85 [DOI] [PubMed] [Google Scholar]
- 42.Croce, R., Zucchelli, G., Garlaschi, F. M., and Jennings, R. C. (1998) Biochemistry 37 17355-17360 [DOI] [PubMed] [Google Scholar]
- 43.van Oort, B., Amunts, A., Borst, J. W., van Hoek, A., Nelson, N., van Amerongen, H., and Croce, R. (2008) Biophys. J. 95 5851-5861 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.